Organizational Readiness to Implement Community Pharmacy-Based Opioid Counseling and Naloxone Services: A Scoping Review of Current Practice Models and Opportunities
Abstract
1. Introduction
2. Review Process
2.1. Data Sources and Search Terms
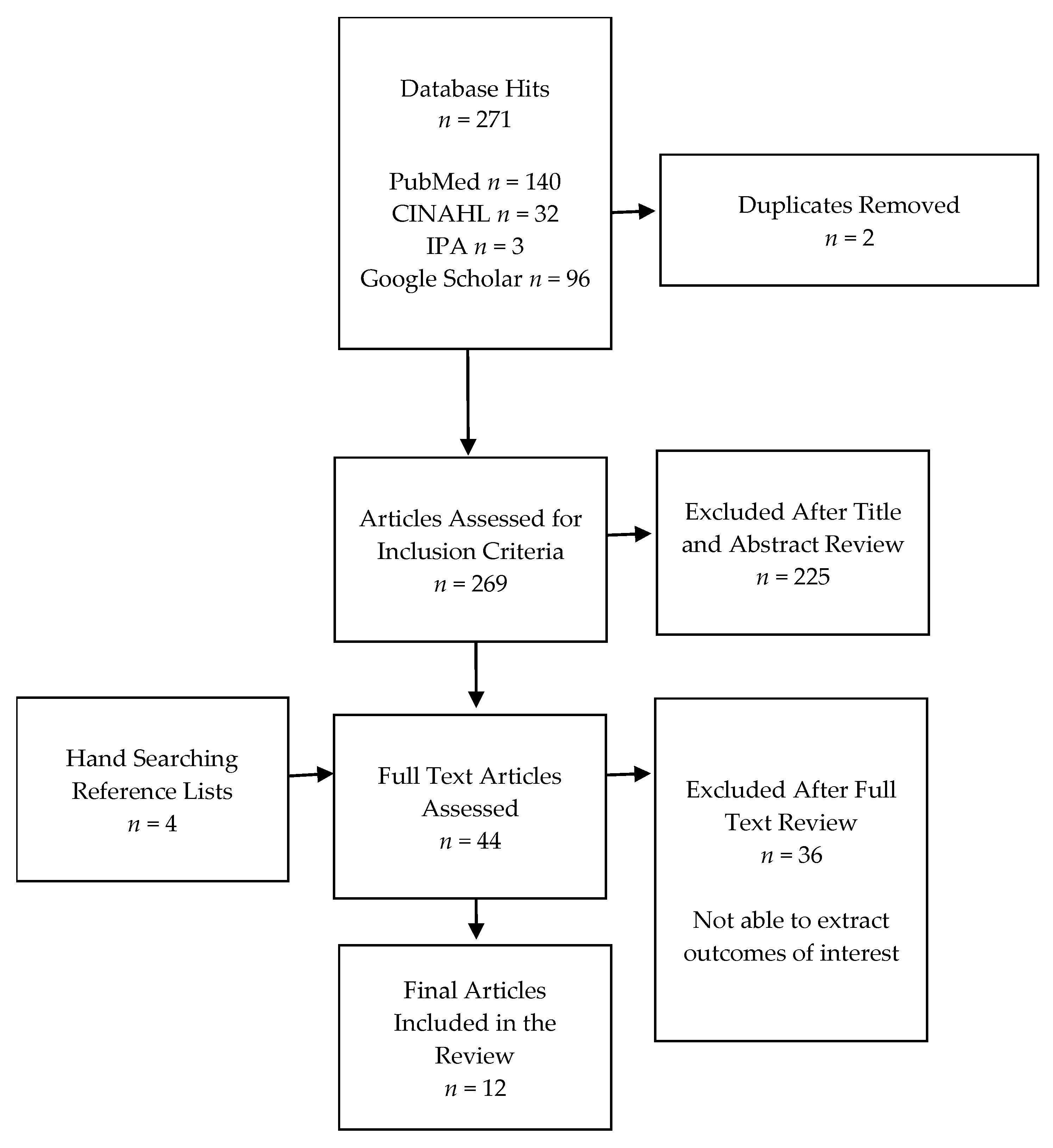
2.2. Study Selection, Outcome Measures, and Data Extraction
2.3. Data Synthesis
3. Results
3.1. Study Characteristics
3.2. Program Themes
3.2.1. Interprofessional Collaboration
3.2.2. Patient Education Format: One-on-One Patient Education versus Group Education Sessions
3.2.3. Non-Pharmacist Provider Education
3.2.4. Pharmacy Staff Education
3.2.5. Opioid Misuse Screening Tools
3.2.6. Naloxone Recommendation/Dispensing
3.2.7. Opioid Therapy and Pain Management
3.3. Program Inputs and Resources
3.4. Program Implementation Processes
3.4.1. Pharmacist Authority
3.4.2. Patient Identification
3.4.3. Pharmacist Interventions
3.4.4. Workflow
3.4.5. Business Operations
3.5. Programmatic Outcomes
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institute on Drug Abuse. Opioids. Available online: https://www.ncbi.nlm.nih.gov/pubmed/ (accessed on 15 February 2023).
- Centers for Disease Control and Prevention. Opioid Basics. Available online: https://www.cdc.gov/opioids/basics/index.html (accessed on 15 February 2023).
- Centers for Disease Control and Prevention. SUDORS Dashboard: Fatal Overdose Data. Available online: https://www.cdc.gov/drugoverdose/fatal/dashboard/index.html (accessed on 27 March 2023).
- US Department of Agriculture (USDA). Opioid Misuse in Rural America. Available online: https://www.usda.gov/topics/opioids (accessed on 15 February 2023).
- Luo, F.; Li, M.; Florence, C. State-level economic costs of opioid use disorder and fatal opioid overdose—United States, 2017. Morb. Mortal. Wkly. Rep. 2021, 70, 541. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.S.; Elliott, L. Naloxone’s role in the national opioid crisis—Past struggles, current efforts, and future opportunities. Transl. Res. 2021, 234, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Carr, D. State legal innovations to encourage naloxone dispensing. J. Am. Pharm. Assoc. 2017, 57, S180–S184. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.S.; Carr, D. Legal changes to increase access to naloxone for opioid overdose reversal in the United States. Drug Alcohol Depend. 2015, 157, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hines, K.; Garofoli, G.; Garofoli, M.; Elswick, B.; Winstanley, E. Impact of naloxone education for patients receiving buprenorphine-containing prescriptions indicated for opioid use disorder at an independent community pharmacy. J. Am. Pharm. Assoc. 2020, 60, e205–e214. [Google Scholar] [CrossRef]
- Akers, J.L.; Hansen, R.N.; Oftebro, R.D. Implementing take-home naloxone in an urban community pharmacy. J. Am. Pharm. Assoc. 2017, 57, S161–S167. [Google Scholar] [CrossRef]
- Cariveau, D.; Fay, A.; Baker, D.; Fagan, E.; Wilson, C. Evaluation of a pharmacist-led naloxone coprescribing program in primary care. J. Am. Pharm. Assoc. 2019, 59, 867–871. [Google Scholar] [CrossRef]
- Napoli, K.; Grant, M.; Remines, J.; Nadpara, P.; Goode, J.-V.R. Impact of pharmacist counseling to enhance the accessibility of naloxone nasal spray to patients in a community pharmacy setting. J. Am. Pharm. Assoc. 2021, 61, S127–S134. [Google Scholar] [CrossRef]
- Han, J.K.; Hill, L.G.; Koenig, M.E.; Das, N. Naloxone Counseling for Harm Reduction and Patient Engagement. Fam. Med. 2017, 49, 730–733. [Google Scholar]
- Teeter, B.; Thannisch, M.; Martin, B.; Zaller, N.; Jones, D.; Mosley, C.; Curran, G. Opioid overdose counseling and prescribing of naloxone in rural community pharmacies: A pilot study. Explor. Res. Clin. Soc. Pharm. 2021, 2, 100019. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). FDA Approves First Over-the-Counter Naloxone Nasal Spray. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray (accessed on 29 March 2023).
- Hohmann, L.A.; Fox, B.I.; Garza, K.B.; Wang, C.-H.; Correia, C.; Curran, G.M.; Westrick, S.C. Impact of a Multicomponent Educational Intervention on Community Pharmacy–Based Naloxone Services Implementation: A Pragmatic Randomized Controlled Trial. Ann. Pharmacother. 2023, 57, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.M.; Wyant, S.L. Pain management content in curricula of US schools of pharmacy. J. Am. Pharm. Assoc. 2003, 43, 34–40. [Google Scholar]
- Davenport, E.S.; Arnett, S.J.; Nichols, M.A.; Miller, M.L. Indiana community pharmacist preceptors’ knowledge and perceptions of medication-assisted treatment. J. Am. Pharm. Assoc. 2020, 60, S20–S28.e24. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, D.M.; Groves, B.K.; Mehta, B.H. Implementation of a naloxone dispensing program in a grocery store–based community pharmacy. Am. J. Health-Syst. Pharm. 2020, 77, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 1–9. [Google Scholar] [CrossRef]
- Hohmann, L.A.; Hastings, T.J.; Qian, J.; Curran, G.M.; Westrick, S.C. Medicare Annual Wellness Visits: A Scoping Review of Current Practice Models and Opportunities for Pharmacists. J. Pharm. Pract. 2020, 33, 666–681. [Google Scholar] [CrossRef]
- Donabedian, A. Evaluating the quality of medical care. Milbank Q. 2005, 83, 691–729. [Google Scholar] [CrossRef]
- ACT Academy. Quality, Service Improvement and Redesign Tools: A Model for Measuring Quality Care. NHS Improvement Brief 2023. Available online: https://www.med.unc.edu/ihqi/wp-content/uploads/sites/463/2021/01/A-Model-for-Measuring-Quality-Care-NHS-Improvement-brief.pdf (accessed on 24 April 2023).
- Gunter, M.J. The role of the ECHO model in outcomes research and clinical practice improvement. Am. J. Manag. Care 1999, 5, S217–S224. [Google Scholar]
- Nielsen, S.; Van Hout, M.C. What is known about community pharmacy supply of naloxone? A scoping review. Int. J. Drug Policy 2016, 32, 24–33. [Google Scholar] [CrossRef]
- Cochran, G.; Chen, Q.; Field, C.; Seybert, A.L.; Hruschak, V.; Jaber, A.; Gordon, A.J.; Tarter, R. A community pharmacy-led intervention for opioid medication misuse: A small-scale randomized clinical trial. Drug Alcohol Depend. 2019, 205, 107570. [Google Scholar] [CrossRef] [PubMed]
- Manzur, V.; Mirzaian, E.; Huynh, T.; Lien, A.; Ly, K.; Wong, H.; Wang, M.; Lou, M.; Durham, M. Implementation and assessment of a pilot, community pharmacy-based, opioid pain medication management program. J. Am. Pharm. Assoc. JAPhA 2020, 60, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Skoy, E.; Eukel, H.; Werremeyer, A.; Strand, M.; Frenzel, O.; Steig, J. Implementation of a statewide program within community pharmacies to prevent opioid misuse and accidental overdose. J. Am. Pharm. Assoc. 2020, 60, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Skoy, E.; Werremeyer, A.; Steig, J.; Eukel, H.; Frenzel, O.; Strand, M. Patient acceptance of naloxone resulting from targeted intervention from community pharmacists to prevent opioid misuse and accidental overdose. Subst. Abus. 2021, 42, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.A.; Eukel, H.; Frenzel, O.; Skoy, E.; Steig, J.; Werremeyer, A. Program evaluation of the Opioid and Naloxone Education (ONE Rx) program using the RE-AIM model. Res. Soc. Adm. Pharm. 2020, 16, 1248–1254. [Google Scholar] [CrossRef]
- Sexton, S.M.; Armstrong, A.; Gatton, O.; Rhodes, L.A.; Marciniak, M.W. A standardized team-based approach for identifying naloxone-eligible patients in a grocery store pharmacy. J. Am. Pharm. Assoc. 2019, 59, S95–S100. [Google Scholar] [CrossRef]
- Santa, H.M.; Amirova, S.G.; Ventricelli, D.J.; Downs, G.E.; Nowalk, A.A.; Pringle, J.L.; Aruru, M. Preparing pharmacists to increase naloxone dispensing within community pharmacies under the Pennsylvania standing order. Am. J. Health-Syst. Pharm. AJHP 2021, 78, 327–335. [Google Scholar] [CrossRef]
- Strand, M.A.; Eukel, H.; Burck, S. Moving opioid misuse prevention upstream: A pilot study of community pharmacists screening for opioid misuse risk. Res. Soc. Adm. Pharm. 2019, 15, 1032–1036. [Google Scholar] [CrossRef]
- Dowell, D. CDC Clinical Practice Guideline for Prescribing Opioids for Pain—United States, 2022. MMWR Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef]
- Substance Abuse and Mental Health Services Administration (SAMHSA). Opioid Overdose Prevention Toolkit. Available online: https://www.samhsa.gov/resource/ebp/opioid-overdose-prevention-toolkit (accessed on 16 November 2022).
- Prescribe to Prevent. Prescribe Naloxone, Save a Life. Available online: https://www.prescribetoprevent.com/ (accessed on 16 November 2022).
- Green, T. Maximizing OpiOid Safety with Naloxone (MOON) Study. Boston Medical Center. Available online: https://www.bmc.org/research/maximizing-opioid-safety-naloxone-moon-study (accessed on 7 February 2023).
- Washington State Agency Medical Directors Group. Interagency Guideline on Prescribing Opioids for Pain. Available online: https://www.agencymeddirectors.wa.gov/Files/2015AMDGOpioidGuideline.pdf (accessed on 8 February 2023).
- Knisely, J.S.; Wunsch, M.J.; Cropsey, K.L.; Campbell, E.D. Prescription Opioid Misuse Index: A brief questionnaire to assess misuse. J. Subst. Abus. Treat. 2008, 35, 380–386. [Google Scholar] [CrossRef]
- Webster, L.R.; Webster, R.M. Predicting aberrant behaviors in opioid-treated patients: Preliminary validation of the Opioid Risk Tool. J. Pain Med. 2005, 6, 432–442. [Google Scholar] [CrossRef] [PubMed]
- OneRx. Opioid and Naloxone Education. Available online: https://one-program.org/ (accessed on 8 February 2023).
- Shonesy, B.C.; Williams, D.; Simmons, D.; Dorval, E.; Gitlow, S.; Gustin, R.M. Screening, Brief Intervention, and Referral to Treatment (SBIRT) in a retail pharmacy setting: The pharmacist’s role in identifying and addressing risk of substance use disorder. J. Addict. Med. 2019, 13, 403. [Google Scholar] [CrossRef]
- Krebs, E.E.; Lorenz, K.A.; Bair, M.J.; Damush, T.M.; Wu, J.; Sutherland, J.M.; Asch, S.M.; Kroenke, K. Development and Initial Validation of the PEG, a Three-item Scale Assessing Pain Intensity and Interference. J. Gen. Intern. Med. 2009, 24, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Cid, A.; Daskalakis, G.; Grindrod, K.; Beazely, M.A. What is known about community pharmacy-based take-home naloxone programs and program interventions? A scoping review. Pharmacy 2021, 9, 30. [Google Scholar] [CrossRef]
- Weiss, R.D.; Jaffee, W.B.; de Menil, V.P.; Cogley, C.B. Group therapy for substance use disorders: What do we know? Harv. Rev. Psychiatry 2004, 12, 339–350. [Google Scholar] [CrossRef]
- Bastien, C.H.; Morin, C.M.; Ouellet, M.-C.; Blais, F.C.; Bouchard, S. Cognitive-behavioral therapy for insomnia: Comparison of individual therapy, group therapy, and telephone consultations. J. Consult. Clin. Psychol. 2004, 72, 653. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.S. Building a Legally Compliant Referral Network. Pharmacy Times 2018. Available online: https://www.pharmacytimes.com/view/building-a-legally-compliant-referral-network- (accessed on 4 April 2023).
- McKeirnan, K.C.; Frazier, K.R.; Nguyen, M.; MacLean, L.G. Training pharmacy technicians to administer immunizations. J. Am. Pharm. Assoc. 2018, 58, 174–178.e171. [Google Scholar] [CrossRef] [PubMed]
- Hohmeier, K.C.; McDonough, S.L.; Rein, L.J.; Brookhart, A.L.; Gibson, M.L.; Powers, M.F. Exploring the expanded role of the pharmacy technician in medication therapy management service implementation in the community pharmacy. J. Am. Pharm. Assoc. 2019, 59, 187–194. [Google Scholar] [CrossRef]
- Fadare, O.O.; Doucette, W.R.; Gaither, C.A.; Schommer, J.C.; Arya, V.; Bakken, B.K.; Kreling, D.H.; Mott, D.A.; Witry, M.J. Exploring the moderating role of job resources in how job demands influence burnout and professional fulfillment among US pharmacists. Res. Soc. Adm. Pharm. 2022, 18, 3821–3830. [Google Scholar] [CrossRef]
- Rudolph, S.E.; Branham, A.R.; Rhodes, L.A.; Moose, J.S.; Marciniak, M.W. Identifying barriers to dispensing naloxone: A survey of community pharmacists in North Carolina. J. Am. Pharm. Assoc. 2018, 58, S55–S58.e53. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.D.; Lyvers, E.; Scott, V.G.; Dwibedi, N. Pharmacists’ readiness to provide naloxone in community pharmacies in West Virginia. J. Am. Pharm. Assoc. 2017, 57, S12–S18.e14. [Google Scholar] [CrossRef] [PubMed]
- Gattiker, T.F.; Carter, C.R. Understanding project champions’ ability to gain intra-organizational commitment for environmental projects. J. Oper. Manag. 2010, 28, 72–85. [Google Scholar] [CrossRef]
- Waltz, T.J.; Powell, B.J.; Matthieu, M.M.; Damschroder, L.J.; Chinman, M.J.; Smith, J.L.; Proctor, E.K.; Kirchner, J.E. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: Results from the Expert Recommendations for Implementing Change (ERIC) study. Implement. Sci. 2015, 10, 109. [Google Scholar] [CrossRef]
- Barnes, B.; Hincapie, A.L.; Luder, H.; Kirby, J.; Frede, S.; Heaton, P.C. Appointment-based models: A comparison of three model designs in a large chain community pharmacy setting. J. Am. Pharm. Assoc. 2018, 58, 156–162.e151. [Google Scholar] [CrossRef]
- Thakur, T.; Frey, M.; Chewning, B. Pharmacist roles, training, and perceived barriers in naloxone dispensing: A systematic review. J. Am. Pharm. Assoc. 2020, 60, 178–194. [Google Scholar] [CrossRef]
- Hohmann, L.A.; Krauss, Z.; Patel, J.; Marley, G.T. Public Perceptions of Community Pharmacy-Based Naloxone Services: A National Cross-Sectional Survey. Pharmacy 2022, 10, 171. [Google Scholar] [CrossRef]
- Green, T.C.; Case, P.; Fiske, H.; Baird, J.; Cabral, S.; Burstein, D.; Schwartz, V.; Potter, N.; Walley, A.Y.; Bratberg, J. Perpetuating stigma or reducing risk? Perspectives from naloxone consumers and pharmacists on pharmacy-based naloxone in 2 states. J. Am. Pharm. Assoc. 2017, 57, S19–S27.e14. [Google Scholar] [CrossRef]
- McMahan, R. Operationalizing MTM through the use of health information technology. J. Manag. Care Pharm. 2008, 14, S18. [Google Scholar]
- Soriano, F.I. Conducting Needs Assessments: A Multidisciplinary Approach; SAGE Publications: Thousand Oaks, CA, USA, 2012; Volume 68. [Google Scholar]
- Joseph, K.; Udogwu, U.N.; Manson, T.T.; Ludwig, S.C.; Banagan, K.E.; Baker, M.; Yousaf, I.S.; Yousaf, O.; Demyanovich, H.; Pollak, A.N.; et al. Patient Satisfaction after Discharge Is Discordant with Reported Inpatient Experience. Orthopedics 2021, 44, e427–e433. [Google Scholar] [CrossRef]
- Sanyal, C. Economic burden of opioid crisis and the role of pharmacist-led interventions. J. Am. Pharm. Assoc. 2021, 61, e70–e74. [Google Scholar] [CrossRef] [PubMed]
- Acharya, M.; Chopra, D.; Hayes, C.J.; Teeter, B.; Martin, B.C. Cost-Effectiveness of Intranasal Naloxone Distribution to High-Risk Prescription Opioid Users. Value Health 2020, 23, 451–460. [Google Scholar] [CrossRef] [PubMed]
| Database a | Key Words |
|---|---|
| PubMed | (pharmacist or pharmacy) and ((opioid or opiate) or naloxone) and (counseling or service or program) and (resource or input or personnel or process or workflow or intervention or financial or economic or satisfaction) and ((develop or development) or (uptake or delivery) or planning) and (implement or implementation) |
| No. | Study | Study Design | Setting | Study Period | Study Population |
|---|---|---|---|---|---|
| 1 | Akers et al., 2017 [10] | Program evaluation, single-arm retrospective cohort | Community pharmacy. Kelley-Ross Pharmacy Group, Seattle, WA. | 2012–2016 | Bystanders (family and friends), median age 57 years |
| 2 | Cochran et al., 2019 [27] | Randomized controlled trial (RCT) | Two community pharmacies located in southwestern Pennsylvania, one associated with an academic medical center and the other an independent pharmacy in a rural county. | 2017–2018 | Adults aged 18 or older receiving prescription opioid therapy. |
| 3 | Manzur et al., 2020 [28] | Program evaluation, single-arm retrospective cohort | Community pharmacy in an academic medical center, CA. | 2016–2018 | Patients enrolled were prescribed opioids for chronic pain by a rheumatology clinic and at a high risk of an opioid overdose |
| 4a | Skoy et al., 2020a [29] | Program evaluation, one-group pretest–posttest (pre–post intervention) | Community pharmacy in North Dakota. | 2018–2019 | All patients prescribed opioids |
| 4b | Skoy et al., 2020b [30] | Program evaluation, one-group pretest–posttest (pre–post intervention) | Community pharmacy in North Dakota. | 2018–2019 | All patients prescribed opioids |
| 4c | Strand et al., 2020 [31] | Program evaluation, one-group pretest–posttest (pre–post intervention) | A total of 149 community pharmacies in North Dakota. | 2018–2019 | All patients prescribed opioids |
| 5 | Strand et al., 2019 [34] | Program evaluation, one-group posttest (post-intervention) | A total of 11 independent community pharmacies in North Dakota. | 2017–2018 | All patients prescribed opioids |
| 6 | Wilkerson et al., 2020 [19] | Program evaluation, single-arm retrospective cohort | Kroger community pharmacies, Ohio. A total of 114 pharmacies in the Columbus Division and 102 pharmacies in the Cincinnati Division. | 2016–2018 | Individuals prescribed opioids and at a high risk of an opioid overdose, or those who request naloxone. |
| 7 | Hines et al., 2020 [9] | Program evaluation, one-group pretest–posttest (pre–post intervention) | Independent community pharmacy in West Virginia. | 2 January 2019 to 15 February 2019 | Patients receiving buprenorphine-containing prescriptions for opioid use disorder (OUD). |
| 8 | Sexton et al., 2019 [32] | Two-group non-randomized controlled trial | Two Kroger community pharmacies, North Carolina. | 2017–2018 | Individuals prescribed opioids and at a high risk of an opioid overdose. |
| 9 | Teeter et al., 2021 [14] | Two-group non-randomized controlled trial (explanatory sequential mixed-methods) | Two intervention pharmacies and two rural pharmacies within the Harps community pharmacy chain, Arkansas | 2019–2020 | Individuals prescribed opioids and at a high risk of an opioid overdose. |
| 10 | Santa et al., 2021 [33] | One-group pretest–posttest (pre–post educational intervention) | A total of 11 community pharmacies (chain and independent) in Philadelphia | July 2019–December 2019 | All patients prescribed opioids |
| Study | Interprofessional Collaboration | Patient Education Format | Non-Pharmacist Provider Education | Pharmacy Staff Education | Opioid Misuse Screening Tool | Naloxone Recommendation/Dispensing | Opioid Therapy and Pain Management | |
|---|---|---|---|---|---|---|---|---|
| One-on-One Patient Education | Group Education Sessions | |||||||
| Akers et al., 2017 [10] | x | x | x | x | x | x | ||
| Cochran et al., 2019 [27] | x | x | x | |||||
| Manzur et al., 2020 [28] | x | x | x | x | x | x | ||
| Skoy et al., 2020a [29] | x | x | x | x | ||||
| Skoy et al., 2020b [30] | x | x | x | x | ||||
| Strand et al., 2020 [31] | x | x | x | x | ||||
| Strand et al., 2019 [34] | x | x | x | x | ||||
| Wilkerson et al., 2020 [19] | x | x | x | |||||
| Hines et al., 2020 [9] | x | x | ||||||
| Sexton et al., 2019 [32] | x | x | x | |||||
| Teeter et al., 2021 [14] | x | x | ||||||
| Santa et al., 2021 [33] | x | x | x | x | ||||
| Study | Inputs and Resources | ||
|---|---|---|---|
| OCN Personnel | Pharmacist FTEs for OCN | OCN Facilities and Expenses | |
| Akers et al., 2017 [10] | Pharmacist plus technicians and assistants | One | Patients were “roomed”; additional information not reported |
| Cochran et al., 2019 [27] | Staff pharmacist, study pharmacist, and navigator (researcher) | Two | Not reported |
| Manzur et al., 2020 [28] | Clinical pharmacist, pharmacy resident | Not reported | Patients seen in a private exam room in adjacent clinical suites of a community pharmacy |
| Skoy et al., 2020a [29] | Pharmacist | Not reported | Not reported |
| Skoy et al., 2020b [30] | Pharmacist | Not reported | Not reported |
| Strand et al., 2020 [31] | Pharmacist | Not reported | Not reported |
| Strand et al., 2019 [34] | Pharmacist | Not reported | Not reported |
| Wilkerson et al., 2020 [19] | Pharmacists, interns, technicians | Not reported | Note reported |
| Hines et al., 2020 [9] | Pharmacy resident | Not reported | Private counseling area |
| Sexton et al., 2019 [32] | Pharmacist, student pharmacist, technician | Not reported | Not reported |
| Teeter et al., 2021 [14] | Pharmacist | Not reported | Not reported |
| Santa et al., 2021 [33] | Pharmacist | Not reported | Not reported |
| Study | Pharmacist Authority | Patient Identification | Pharmacist Interventions | Workflow | Business Operations |
|---|---|---|---|---|---|
| Akers et al., 2017 [10] |
|
|
|
|
|
| Cochran et al., 2019 [27] |
|
|
|
|
|
| Manzur et al., 2020 [28] |
|
|
|
|
|
| Skoy et al., 2020a a [29] |
|
|
|
|
|
| Skoy et al., 2020b a [30] |
|
|
|
|
|
| Strand et al., 2020 a [31] |
|
|
|
|
|
| Strand et al., 2019 a [34] |
|
|
|
|
|
| Wilkerson et al., 2020 [19] |
|
|
|
|
|
| Hines et al., 2020 [9] |
|
|
|
|
|
| Sexton et al., 2019 [32] |
|
|
|
|
|
| Teeter et al., 2021 [14] |
|
|
|
|
|
| Santa et al., 2021 [33] |
|
|
|
|
|
| Study | Uptake and Delivery | Intervention Outcomes | Satisfaction |
|---|---|---|---|
| Akers et al., 2017 [10] |
|
|
|
| Cochran et al., 2019 [27] |
|
|
|
| Manzur et al., 2020 [28] |
|
|
|
| Skoy et al., 2020a [29] |
|
|
|
| Skoy et al., 2020b [30] |
|
|
|
| Strand et al., 2020 [31] |
|
|
|
| Strand et al., 2019 [34] |
|
|
|
| Wilkerson et al., 2020 [19] |
|
|
|
| Hines et al., 2020 [9] |
|
|
|
| Sexton et al., 2019 [32] |
|
|
|
| Teeter et al., 2021 [14] |
|
|
|
| Santa et al., 2021 [33] |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohmann, L.; Harris, K.; Zhao, Y.; Marlowe, K.; Phillippe, H.; Correia, C.; Fox, B. Organizational Readiness to Implement Community Pharmacy-Based Opioid Counseling and Naloxone Services: A Scoping Review of Current Practice Models and Opportunities. Pharmacy 2023, 11, 99. https://doi.org/10.3390/pharmacy11030099
Hohmann L, Harris K, Zhao Y, Marlowe K, Phillippe H, Correia C, Fox B. Organizational Readiness to Implement Community Pharmacy-Based Opioid Counseling and Naloxone Services: A Scoping Review of Current Practice Models and Opportunities. Pharmacy. 2023; 11(3):99. https://doi.org/10.3390/pharmacy11030099
Chicago/Turabian StyleHohmann, Lindsey, Klaudia Harris, Yi Zhao, Karen Marlowe, Haley Phillippe, Chris Correia, and Brent Fox. 2023. "Organizational Readiness to Implement Community Pharmacy-Based Opioid Counseling and Naloxone Services: A Scoping Review of Current Practice Models and Opportunities" Pharmacy 11, no. 3: 99. https://doi.org/10.3390/pharmacy11030099
APA StyleHohmann, L., Harris, K., Zhao, Y., Marlowe, K., Phillippe, H., Correia, C., & Fox, B. (2023). Organizational Readiness to Implement Community Pharmacy-Based Opioid Counseling and Naloxone Services: A Scoping Review of Current Practice Models and Opportunities. Pharmacy, 11(3), 99. https://doi.org/10.3390/pharmacy11030099





