Generating Practice-Based Evidence in the Use of Guideline-Recommended Combination Therapy for Secondary Prevention of Acute Myocardial Infarction
Abstract
:1. Introduction
2. Methods
2.1. Data, Cohort Selection, and Model Covariates
2.1.1. Treatment Measures
2.1.2. Outcome Measures
2.1.3. Model Covariates
2.2. Modeling Framework and Statistical Analyses
2.2.1. Assumptions and Specifications of Estimation Framework
2.2.2. Statistical Analyses
2.3. Evaluating IV Assumptions Regarding Unmeasured Confounders
3. Results
3.1. Study Population: Characteristics, Treatment, and Outcomes
3.2. Instrumental Variables (IV) Analyses
3.3. Evaluating IV Assumptions Regarding Unmeasured Confounders
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krumholz, H.M.; Normand, S.T.; Wang, Y. Twenty-Year Trends in Outcomes for Older Adults With Acute Myocardial Infarction in the United States. JAMA Netw. Open 2019, 2, e191938. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, E.D.; Shah, B.R.; Parsons, L.; Pollack, C.V., Jr.; French, W.J.; Canto, J.G.; Gibson, C.M.; Rogers, W.J. Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am. Heart J. 2008, 156, 1045–1055. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Benjamin, E.J.; Bonow, R.O.; Braun, L.T.; Creager, M.A.; Franklin, B.A.; Gibbons, R.J.; Grundy, S.M.; Hiratzka, L.F.; Jones, D.W. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: A guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. J. Am. Coll. Cardiol. 2011, 58, 2432–2446. [Google Scholar]
- Anderson, J.L.; Adams, C.D.; Antman, E.M.; Bridges, C.R.; Califf, R.M.; Casey, D.E.; Chavey, W.E.; Fesmire, F.M.; Hochman, J.S.; Levin, T.N. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, e179–e347. [Google Scholar]
- Arnold, S.V.; Spertus, J.A.; Tang, F.; Krumholz, H.M.; Borden, W.B.; Farmer, S.A.; Ting, H.H.; Chan, P.S. Statin use in outpatients with obstructive coronary artery disease. Circulation 2011, 124, 2405–2410. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.; Arbogast, P.G.; BeLue, R.; Daugherty, J.; Jain, M.K.; Ray, W.A.; Griffin, M.R. Outpatient adherence to beta-blocker therapy after acute myocardial infarction. J. Am. Coll. Cardiol. 2002, 40, 1589–1595. [Google Scholar] [CrossRef] [Green Version]
- Choudhry, N.K.; Setoguchi, S.; Levin, R.; Winkelmayer, W.C.; Shrank, W.H. Trends in adherence to secondary prevention medications in elderly post-myocardial infarction patients. Pharmacoepidemiol. Drug Saf. 2008, 17, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Lauffenburger, J.C.; Robinson, J.G.; Oramasionwu, C.; Fang, G. Racial/Ethnic and gender gaps in the use of and adherence to evidence-based preventive therapies among elderly Medicare Part D beneficiaries after acute myocardial infarction. Circulation 2014, 129, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Zeymer, U.; Berkenboom, G.; Coufal, Z.; Belger, M.; Sartral, M.; Norrbacka, K.; Bakhai, A. Predictors, cost, and outcomes of patients with acute coronary syndrome who receive optimal secondary prevention therapy: Results from the antiplatelet treatment observational registries (APTOR). Int. J. Cardiol. 2013, 170, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Hoedemaker, N.P.G.; Damman, P.; Ottervanger, J.P.; Dambrink, J.H.E.; Gosselink, A.T.M.; Kedhi, E.; Kolkman, E.; de Winter, R.J.; van’t Hof, A.W.J. Trends in Cardiovascular and Bleeding Outcomes in Acute Coronary Syndrome Patients Treated With or Without Proton Pump Inhibitors During the Introduction of Novel P2Y12 Inhibitors: A Five-Year Experience From a Single-Centre Observational Registry. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 5, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, M.J.; Robinson, J.G.; Annis, I.E.; Hickson, R.P.; Bell, J.S.; Hartikainen, J.; Fang, G. Adherence Tradeoff to Multiple Preventive Therapies and All-Cause Mortality After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 70, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Kirchmayer, U.; Di Martino, M.; Agabiti, N.; Bauleo, L.; Fusco, D.; Belleudi, V.; Arca, M.; Pinnarelli, L.; Perucci, C.A.; Davoli, M. Effect of evidence-based drug therapy on long-term outcomes in patients discharged after myocardial infarction: A nested case-control study in Italy. Pharmacoepidemiol. Drug Saf. 2013, 22, 649–657. [Google Scholar] [CrossRef]
- Kuepper-Nybelen, J.; Hellmich, M.; Abbas, S.; Ihle, P.; Griebenow, R.; Schubert, I. Association of long-term adherence to evidence-based combination drug therapy after acute myocardial infarction with all-cause mortality. A prospective cohort study based on claims data. Eur. J. Clin. Pharmacol. 2012, 68, 1451–1460. [Google Scholar] [CrossRef]
- Van der Elst, M.E.; Bouvy, M.L.; de Blaey, C.J.; de Boer, A. Effect of Drug Combinations on Admission for Recurrent Myocardial Infarction. Heart 2009, 93, 1226–1230. [Google Scholar] [CrossRef] [Green Version]
- Bezin, J.; Klungel, O.H.; Lassalle, R.; Dureau-Pournin, C.; Moore, N.; Pariente, A. Medications Recommended for Secondary Prevention After First Acute Coronary Syndrome: Effectiveness of Treatment Combinations in a Real-Life Setting. Clin. Pharmacol. Ther. 2018, 103, 1038–1046. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Cong, H.; Lu, C.; Wu, J. Impact of Optimal Medical Therapy at Discharge on One-year Direct Medical Costs in Patients with Acute Coronary Syndromes: A Retrospective, Observational Database Analysis in China. Clin. Ther. 2019, 41, 456–465.e452. [Google Scholar] [CrossRef]
- Kumar, A.; Fonarow, G.C.; Eagle, K.A.; Hirsch, A.T.; Califf, R.M.; Alberts, M.J.; Boden, W.E.; Steg, P.G.; Shao, M.; Bhatt, D.L.; et al. Regional and practice variation in adherence to guideline recommendations for secondary and primary prevention among outpatients with atherothrombosis or risk factors in the United States: A report from the REACH Registry. Crit. Pathw. Cardiol. 2009, 8, 104–111. [Google Scholar] [CrossRef]
- Kasargod, C.; Devlin, G.; Lee, M.; White, H.D.; Kerr, A.J. Prescribing Performance Post-Acute Coronary Syndrome Using a Composite Medication Indicator: ANZACS-QI 24. Heart Lung Circ. 2019, 29, 824–834. [Google Scholar] [CrossRef]
- Hickson, R.P.; Robinson, J.G.; Annis, I.E.; Killeya-Jones, L.A.; Fang, G. It’s Not Too Late to Improve Statin Adherence: Association Between Changes in Statin Adherence from Before to After Acute Myocardial Infarction and All-Cause Mortality. J. Am. Heart Assoc. 2019, 8, e011378. [Google Scholar] [CrossRef] [Green Version]
- Ferrieres, J.; Lautsch, D.; Ambegaonkar, B.M.; De Ferrari, G.M.; Vyas, A.; Baxter, C.A.; Bash, L.D.; Velkovski-Rouyer, M.; Horack, M.; Almahmeed, W.; et al. Use of guideline-recommended management in established coronary heart disease in the observational DYSIS II study. Int. J. Cardiol. 2018, 270, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Bramlage, P.; Messer, C.; Bitterlich, N.; Pohlmann, C.; Cuneo, A.; Stammwitz, E.; Tebbenjohanns, J.; Gohlke, H.; Senges, J.; Tebbe, U. The effect of optimal medical therapy on 1-year mortality after acute myocardial infarction. Heart 2010, 96, 604–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- β-Blocker Heart Attack Study Group. The β-blocker heart attack trial. JAMA 1981, 246, 2073–2074. [Google Scholar] [CrossRef]
- Dahl Aarvik, M.; Sandven, I.; Dondo, T.B.; Gale, C.P.; Ruddox, V.; Munkhaugen, J.; Atar, D.; Otterstad, J.E. Effect of oral β-blocker treatment on mortality in contemporary post-myocardial infarction patients: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Peto, R. Long-term and short-term beta-blockade after myocardial infarction. Lancet 1982, 319, 1159–1161. [Google Scholar] [CrossRef]
- ACE Inhibitor Myocardial Infarction Collaborative Group. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: Systematic overview of individual data from 100,000 patients in randomized trials. Circulation 1998, 97, 2202–2212. [Google Scholar] [CrossRef] [Green Version]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef]
- Cannon, C.P.; Braunwald, E.; McCabe, C.H.; Rader, D.J.; Rouleau, J.L.; Belder, R.; Joyal, S.V.; Hill, K.A.; Pfeffer, M.A.; Skene, A.M. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 2004, 350, 1495–1504. [Google Scholar] [CrossRef]
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N. Engl. J. Med. 1996, 335, 1001–1009. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Olsson, A.G.; Ezekowitz, M.D.; Ganz, P.; Oliver, M.F.; Waters, D.; Zeiher, A.; Chaitman, B.R. Atorvastatin for acute coronary syndromes. JAMA 2001, 286, 533–535. [Google Scholar] [PubMed]
- Gurwitz, J.H.; Col, N.F.; Avorn, J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. JAMA 1992, 268, 1417–1422. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Alexander, K.P.; Hammill, B.G.; Pasquali, S.K.; Peterson, E.D. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA 2001, 286, 708–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, K.S.; Saczynski, J.S.; Zhao, Y.; Goldberg, R.J.; Gurwitz, J.H. Exclusion of older adults and women from recent trials of acute coronary syndromes. J. Am. Geriatr. Soc. 2011, 59, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Uijen, A.A.; Bakx, J.C.; Mokkink, H.G.; van Weel, C. Hypertension patients participating in trials differ in many aspects from patients treated in general practices. J. Clin. Epidemiol. 2007, 60, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Dionne, J.; Pinho, G.; Gignac, J.; Almirall, J.; Lapointe, L. Randomized controlled trials: Do they have external validity for patients with multiple comorbidities? Ann. Fam. Med. 2006, 4, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Peters, E.; Wassef, A.; Desmarais, P.; Rémillard-Labrosse, D.; Tremblay-Gravel, M. Evolution of Age and Female Representation in the Most-Cited Randomized Controlled Trials of Cardiology of the Last 20 Years. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004713. [Google Scholar] [CrossRef]
- Boyd, C.M.; Vollenweider, D.; Puhan, M.A. Informing evidence-based decision-making for patients with comorbidity: Availability of necessary information in clinical trials for chronic diseases. PLoS ONE 2012, 7, e41601. [Google Scholar] [CrossRef]
- Swisher, A.K. Practice-based evidence. Cardiopulm. Phys. Ther. J. 2010, 21, 4. [Google Scholar]
- Ammerman, A.; Smith, T.W.; Calancie, L. Practice-based evidence in public health: Improving reach, relevance, and results. Annu. Rev. Public Health 2014, 35, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Green, L.W. Public health asks of systems science: To advance our evidence-based practice, can you help us get more practice-based evidence? Am. J. Public Health 2006, 96, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Berwick, D.M. Broadening the view of evidence-based medicine. Qual. Saf. Health Care 2005, 14, 315–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, L.W.; Allegrante, J.P. Practice-Based Evidence and the Need for More Diverse Methods and Sources in Epidemiology, Public Health and Health Promotion. Am. J. Health Promot. 2020, 34, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.D.; Gassaway, J. Practice based evidence: Incorporating clinical heterogeneity and patient-reported outcomes for comparative effectiveness research. Med. Care 2010, 48, S17–S22. [Google Scholar] [CrossRef]
- Mays, G.P.; Hogg, R.A. Expanding delivery system research in public health settings: Lessons from practice-based research networks. J. Public Health Manag. Pract. 2012, 18, 485–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, S.D.; Gassaway, J.; Pentz, L.; James, R. Practice-based evidence for clinical practice improvement: An alternative study design for evidence-based medicine. In Health Informatics; Studies in Health Technology and Informatics Series; IOS Press: Amsterdam, The Netherlands, 2010; Volume 151, pp. 446–460. [Google Scholar]
- Bezin, J.; Groenwold, R.H.; Ali, M.S.; Lassalle, R.; Robinson, P.; de Boer, A.; Moore, N.; Klungel, O.H.; Pariente, A. Comparative effectiveness of recommended versus less intensive drug combinations in secondary prevention of acute coronary syndrome. Pharmacoepidemiol. Drug Saf. 2017, 26, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.T.; Wong, I.C.K.; Man, K.K.C.; Chen, Y.; Crake, T.; Ozkor, M.A.; Ding, L.Q.; Wang, Z.X.; Zhang, L.; Wei, L. Effect of evidence-based therapy for secondary prevention of cardiovascular disease: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0210988. [Google Scholar] [CrossRef]
- Brookhart, M.A.; Rassen, J.A.; Schneeweiss, S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol. Drug Saf. 2010, 19, 537–554. [Google Scholar] [CrossRef] [Green Version]
- Schneeweiss, S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol. Drug Saf. 2006, 15, 291–303. [Google Scholar] [CrossRef]
- Haukoos, J.S.; Lewis, R.J. The Propensity Score. JAMA 2015, 314, 1637–1638. [Google Scholar] [CrossRef]
- Bucholz, E.M.; Krumholz, H.A.; Krumholz, H.M. Underweight, Markers of Cachexia, and Mortality in Acute Myocardial Infarction: A Prospective Cohort Study of Elderly Medicare Beneficiaries. PLoS Med. 2016, 13, e1001998. [Google Scholar] [CrossRef] [PubMed]
- McAlister, F.A.; Oreopoulos, A.; Norris, C.M.; Graham, M.M.; Tsuyuki, R.T.; Knudtson, M.; Ghali, W.A. Exploring the treatment-risk paradox in coronary disease. Arch. Intern. Med. 2007, 167, 1019–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozcan, C.; Deleskog, A.; Schjerning Olsen, A.M.; Nordahl Christensen, H.; Lock Hansen, M.; Hilmar Gislason, G. Coronary artery disease severity and long-term cardiovascular risk in patients with myocardial infarction: A Danish nationwide register-based cohort study. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, M.L.; Brookhart, M.A. Using Instrumental Variables to Address Bias from Unobserved Confounders. JAMA 2019, 321, 2124–2125. [Google Scholar] [CrossRef]
- Garabedian, L.F.; Chu, P.; Toh, S.; Zaslavsky, A.M.; Soumerai, S.B. Potential bias of instrumental variable analyses for observational comparative effectiveness research. Ann. Intern. Med. 2014, 161, 131–138. [Google Scholar] [CrossRef]
- Zhang, X.; Faries, D.E.; Li, H.; Stamey, J.D.; Imbens, G.W. Addressing unmeasured confounding in comparative observational research. Pharmacoepidemiol. Drug Saf. 2018, 27, 373–382. [Google Scholar] [CrossRef]
- Brooks, J.M.; Cook, E.; Chapman, C.G.; Schroeder, M.C.; Chrischilles, E.; Schneider, K.M.; Kulchaitanaroaj, P.; Robinson, J. Statin use after acute myocardial infarction by patient complexity: Are the rates right? Med. Care 2015, 53, 324–331. [Google Scholar] [CrossRef]
- Brooks, J.M.; Chapman, C.G.; Suneja, M.; Schroeder, M.C.; Fravel, M.A.; Schneider, K.M.; Wilwert, J.; Li, Y.J.; Chrischilles, E.A.; Brenton, D.W.; et al. Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers for Geriatric Ischemic Stroke Patients: Are the Rates Right? J. Am. Heart Assoc. 2018, 7, e009137. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, P.; Nagendran, M.; Campbell, W.B.; Price, A.; Jani, A.; Birkmeyer, J.D.; Gray, M. Strategies to reduce variation in the use of surgery. Lancet 2013, 382, 1130–1139. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Reames, B.N.; McCulloch, P.; Carr, A.J.; Campbell, W.B.; Wennberg, J.E. Understanding of regional variation in the use of surgery. Lancet 2013, 382, 1121–1129. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, J.N.; Bronner, K.K.; Morgan, T.S.; Wennberg, J.E. Trends and geographic variations in major surgery for degenerative diseases of the hip, knee, and spine. Health Aff. 2004, 23, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Lurie, J.D.; Olson, P.R.; Bronner, K.K.; Fisher, E.S. United States’ trends and regional variations in lumbar spine surgery: 1992-2003. Spine (Phila Pa 1976) 2006, 31, 2707–2714. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, J.E.; Cooper, M.M. The Dartmouth Atlas of Health Care; American Hospital Association Press: Chicago, IL, USA, 1996. [Google Scholar]
- Wennberg, J.E.; Fisher, E.S.; Skinner, J.S. Geography and the debate over Medicare reform. Health Aff. 2002, 21, W96–W114. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.A.; Schneider, K.M.; Robinson, J.; Wilwert, J.; Chrischilles, E.; Pendergast, J.; Brooks, J. Field methods in medical record abstraction: Assessing the properties of comparative effectiveness estimates. BMC Health Serv. Res. 2014, 14, 391. [Google Scholar] [CrossRef] [Green Version]
- Cook, E.A.; Schneider, K.M.; Chrischilles, E.; Brooks, J.M. Accounting for unobservable exposure time bias when using Medicare prescription drug data. Medicare Medicaid Res. Rev. 2013, 3, E1–E18. [Google Scholar] [CrossRef]
- Angrist, J.D.; Imbens, G.W.; Rubin, D.B. Identification of Causal Effects Using Instrumental Variables. J. Am. Stat. Assoc. 1996, 91, 444–455. [Google Scholar] [CrossRef]
- Angrist, J.D. Treatment Effect Heterogeneity in Theory and Practice. Econ. J. 2004, 114, C52–C83. [Google Scholar] [CrossRef] [Green Version]
- Heckman, J.J.; Urzua, S.; Vytlacil, E. Understanding Instrumental Variables in Models with Essential Heterogeneity. Rev. Econ. Stat. 2006, 88, 389–432. [Google Scholar] [CrossRef] [Green Version]
- McClellan, M.; McNeil, B.J.; Newhouse, J.P. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. JAMA 1994, 272, 859–866. [Google Scholar] [CrossRef]
- Newhouse, J.P.; McClellan, M. Econometrics in outcomes research: The use of instrumental variables. Annu. Rev. Public Health 1998, 19, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Harris, K.M.; Remler, D.K. Who Is the Marginal Patient? Understanding Instrumental Variables Estimates of Treatment Effects. Health Serv. Res. 1998, 33, 1337–1360. [Google Scholar] [PubMed]
- Brooks, J.M.; McClellan, M.; Wong, H.S. The marginal benefits of invasive treatments for acute myocardial infarction: Does insurance coverage matter? Inq. J. Med. Care Organ. Provis. Financ. 2000, 37, 75–90. [Google Scholar]
- Angrist, J.D. Estimation of Limited Dependent Variable Models With Dummy Endogenous Regressors: Simple Strategies for Empirical Practice. J. Bus. Econ. Stat. 2001, 19, 2–16. [Google Scholar] [CrossRef]
- Lumley, T.; Diehr, P.; Emerson, S.; Chen, L. The Importance of the Normality Assumption in Large Public Health Data Sets. Annu. Rev. Public Health 2002, 23, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Coe, N.B.; Chapman, C.G. 2SLS versus 2SRI: A ppropriate methods for rare outcomes and/or rare exposures. Health Econ. 2018, 27, 937–955. [Google Scholar] [CrossRef]
- Fang, G.; Brooks, J.M.; Chrischilles, E.A. A new method to isolate local-area practice styles in prescription use as the basis for instrumental variables in comparative effectiveness research. Med. Care 2010, 48, 710–717. [Google Scholar] [CrossRef]
- Brooks, J.M.; Chapman, C.G.; Cozad, M.J. The Identification Process Using Choice Theory Is Needed to Match Design With Objectives in CER. Med. Care 2017, 55, 91–93. [Google Scholar] [CrossRef]
- Fang, G.; Brooks, J.M.; Chrischilles, E.A. Apples and oranges? Interpretations of risk adjustment and instrumental variable estimates of intended treatment effects using observational data. Am. J. Epidemiol. 2012, 175, 60–65. [Google Scholar] [CrossRef]
- Fang, G.; Brooks, J.M.; Chrischilles, E.A. Comparison of instrumental variable analysis using a new instrument with risk adjustment methods to reduce confounding by indication. Am. J. Epidemiol. 2012, 175, 1142–1151. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.M.; Chrischilles, E.A. Heterogeneity and the interpretation of trea tment effect estimates from risk adjustment and instrumental variable methods. Med. Care 2007, 45, S123–S130. [Google Scholar] [CrossRef]
- Brookhart, M.A.; Wang, P.S.; Solomon, D.H.; Schneeweiss, S. Evaluating short-term drug effects using a physician-specific prescribing preference as an instrumental variable. Epidemiology 2006, 17, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Stukel, T.A.; Fisher, E.S.; Wennberg, D.E.; Alter, D.A.; Gottlieb, D.J.; Vermeulen, M.J. Analysis of observational studies in the presence of treatment selection bias: Effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 2007, 297, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Rassen, J.A.; Brookhart, M.A.; Glynn, R.J.; Mittleman, M.A.; Schneeweiss, S. Instrumental variables II: Instrumental variable application-in 25 variations, the physician prescribing preference generally was strong and reduced covariate imbalance. J. Clin. Epidemiol. 2009, 62, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.M.; Chrischilles, E.A.; Scott, S.D.; Chen-Hardee, S.S. Was breast conserving surgery underutilized for early stage breast cancer? Instrumental variables evidence for stage II patients from Iowa. Health Serv. Res. 2003, 38, 1385–1402. [Google Scholar] [CrossRef]
- Schroeder, M.C.; Tien, Y.Y.; Wright, K.; Halfdanarson, T.R.; Abu-Hejleh, T.; Brooks, J.M. Geographic variation in the use of adjuvant therapy among elderly patients with resected non-small cell lung cancer. Lung Cancer 2016, 95, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.X.; Brooks, J.M.; Wetmore, J.B.; Shireman, T.I. Association between higher rates of cardioprotective drug use and survival in patients on dialysis. Res. Soc. Adm. Pharm. 2015, 11, 824–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polgreen, L.A.; Cook, E.A.; Brooks, J.M.; Tang, Y.X.; Polgreen, P.M. Increased Statin Prescribing Does Not Lower Pneumonia Risk. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60, 1760–1766. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.M.; Chrischilles, E.A.; Landrum, M.B.; Wright, K.B.; Fang, G.; Winer, E.P.; Keating, N.L. Survival implications associated with variation in mastectomy rates for early-staged breast cancer. Int. J. Surg. Oncol. 2012, 2012, 127854. [Google Scholar] [CrossRef] [Green Version]
- Brooks, J.M.; Cook, E.A.; Chapman, C.G.; Kulchaitanaroaj, P.; Chrischilles, E.A.; Welch, S.; Robinson, J. Geographic variation in statin use for complex acute myocardial infarction patients: Evidence of effective care? Med. Care 2014, 52 (Suppl. 3), S37–S44. [Google Scholar] [CrossRef] [Green Version]
- Floyd, S.B.; Thigpen, C.; Kissenberth, M.; Brooks, J.M. Association of Surgical Treatment With Adverse Events and Mortality Among Medicare Beneficiaries With Proximal Humerus Fracture. JAMA Netw. Open 2020, 3, e1918663. [Google Scholar] [CrossRef] [Green Version]
- Floyd, S.B.; Campbell, J.; Chapman, C.G.; Thigpen, C.A.; Kissenberth, M.J.; Brooks, J.M. Geographic variation in the treatment of proximal humerus fracture: An update on surgery rates and treatment consensus. J. Orthop. Surg. Res. 2019, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Chapman, C.G.; Floyd, S.B.; Thigpen, C.A.; Tokish, J.M.; Chen, B.; Brooks, J.M. Treatment for Rotator Cuff Tear Is Influenced by Demographics and Characteristics of the Area Where Patients Live. JB JS Open Access 2018, 3, e0005. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.M.; Tang, Y.; Chapman, C.G.; Cook, E.A.; Chrischilles, E.A. What is the Effect of Area Size When Using Local Area Practice Style as an Instrument. J. Clin. Epidemiol. 2012, 66, S69–S83. [Google Scholar] [CrossRef] [Green Version]
- Goedken, A.M.; Brooks, J.M.; Milavetz, G.; Rudzianski, N.J.; Chrischilles, E.A. Geographic variation in inhaled corticosteroid use for children with persistent asthma in Medicaid. J. Asthma Off. J. Assoc. Care Asthma 2018, 55, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Ellerbeck, E.F.; Jencks, S.F.; Radford, M.J.; Kresowik, T.F.; Craig, A.S.; Gold, J.A.; Krumholz, H.M.; Vogel, R.A. Quality of care for Medicare patients with acute myocardial infarction. A four-state pilot study from the Cooperative Cardiovascular Project. JAMA 1995, 273, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control. Clin. Trials 1998, 19, 61–109. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged. The Index of ADL: A Standardized Measure of Biological and Psychosocial Function. JAMA 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Katz, S.; Downs, T.D.; Cash, H.R.; Grotz, R.C. Progress in development of the index of ADL. Gerontologist 1970, 10, 20–30. [Google Scholar] [CrossRef]
- Washington University School of Medicine. Adult Comorbidity Evaluation-27. Available online: http://otooutcomes.wustl.edu/portals/otooutcomes/PDFs/.pdf (accessed on 6 August 2022).
- Kallogjeri, D.; Piccirillo, J.F.; Spitznagel, E.L., Jr.; Steyerberg, E.W. Comparison of Scoring Methods for ACE-27: Simpler Is Better. J. Geriatr. Oncol. 2012, 3, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Piccirillo, J.F.; Tierney, R.M.; Costas, I.; Grove, L.; Spitznagel, J.; Edward, L. Prognostic Importance of Comorbidity in a Hospital-Based Cancer Registry. JAMA 2004, 291, 2441–2447. [Google Scholar] [CrossRef] [Green Version]
- Ertefaie, A.; Small, D.S.; Flory, J.H.; Hennessy, S. A tutorial on the use of instrumental variables in pharmacoepidemiology. Pharmacoepidemiol. Drug Saf. 2017, 26, 357–367. [Google Scholar] [CrossRef]
- Staiger, D.; Stock, J.H. Instrumental Variables Regression with Weak Instruments. Econometrica 1997, 65, 557–586. [Google Scholar] [CrossRef]
- Schroeder, M.C.; Robinson, J.G.; Chapman, C.G.; Brooks, J.M. Use of statins by medicare beneficiaries post myocardial infarction: Poor physician quality or patient-centered care? Inq. J. Med. Care Organ. Provis. Financ. 2015, 52, 0046958015571131. [Google Scholar] [CrossRef] [PubMed]
- Cziraky, M.J.; Willey, V.J.; McKenney, J.M.; Kamat, S.A.; Fisher, M.D.; Guyton, J.R.; Jacobson, T.A.; Davidson, M.H. Statin safety: An assessment using an administrative claims database. Am. J. Cardiol. 2006, 97, 61C–68C. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C. Unintended effects of statins in men and women in England and Wales: Population based cohort study using the QResearch database. Br. Med. J. 2010, 340, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Saran, R.; Erickson, S.R.; Hirth, R.A.; He, K.; Balkrishnan, R. Environmental and individual predictors of medication adherence among elderly patients with hypertension and chronic kidney disease: A geospatial approach. Res. Soc. Adm. Pharm. 2020, 16, 422–430. [Google Scholar] [CrossRef]
- Han, E.; Suh, D.C.; Lee, S.M.; Jang, S. The impact of medication adherence on health outcomes for chronic metabolic diseases: A retrospective cohort study. Res. Soc. Adm. Pharm. 2014, 10, e87–e98. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, Y.; Tang, F.; Jones, P.G.; Nambi, V.; Bittner, V.A.; Hira, R.S.; Nasir, K.; Chan, P.S.; Maddox, T.M.; Oetgen, W.J.; et al. Adoption of the 2013 American College of Cardiology/American Heart Association Cholesterol Management Guideline in Cardiology Practices Nationwide. JAMA Cardiol. 2017, 2, 361–369. [Google Scholar] [CrossRef]
- Bittner, V.; Colantonio, L.D.; Dai, Y.; Woodward, M.; Mefford, M.T.; Rosenson, R.S.; Muntner, P.; Monda, K.L.; Kilgore, M.L.; Jaeger, B.C.; et al. Association of Region and Hospital and Patient Characteristics With Use of High-Intensity Statins After Myocardial Infarction Among Medicare Beneficiaries. JAMA Cardiol. 2019, 4, 865–872. [Google Scholar] [CrossRef]
- Booth, J.N., 3rd; Colantonio, L.D.; Rosenson, R.S.; Safford, M.M.; Chen, L.; Kilgore, M.L.; Brown, T.M.; Taylor, B.; Dent, R.; Monda, K.L.; et al. Healthcare Utilization and Statin Re-Initiation Among Medicare Beneficiaries With a History of Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008462. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.A.E.; Colantonio, L.D.; Zhao, H.; Bittner, V.; Dai, Y.; Farkouh, M.E.; Monda, K.L.; Safford, M.M.; Muntner, P.; Woodward, M. Sex Differences in High-Intensity Statin Use Following Myocardial Infarction in the United States. J. Am. Coll. Cardiol. 2018, 71, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Ngo-Metzger, Q.; Zuvekas, S.; Shafer, P.; Tracer, H.; Borsky, A.E.; Bierman, A.S. Statin Use in the U.S. for Secondary Prevention of Cardiovascular Disease Remains Suboptimal. J. Am. Board Fam. Med. 2019, 32, 807–817. [Google Scholar] [CrossRef]
- Mathews, R.; Wang, W.; Kaltenbach, L.A.; Thomas, L.; Shah, R.U.; Ali, M.; Peterson, E.D.; Wang, T.Y. Hospital Variation in Adherence Rates to Secondary Prevention Medications and the Implications on Quality. Circulation 2018, 137, 2128–2138. [Google Scholar] [CrossRef]
- Figueroa, J.F.; Blumenthal, D.M.; Feyman, Y.; Frakt, A.B.; Turchin, A.; Doros, G.; Gao, Q.; Song, Y.; Joynt Maddox, K.E. Differences in Management of Coronary Artery Disease in Patients With Medicare Advantage vs Traditional Fee-for-Service Medicare Among Cardiology Practices. JAMA Cardiol. 2019, 4, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Fanaroff, A.C.; Chen, A.Y.; van Diepen, S.; Peterson, E.D.; Wang, T.Y. Association Between Intensive Care Unit Usage and Long-Term Medication Adherence, Mortality, and Readmission Among Initially Stable Patients With Non-ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e015179. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Shah, N.D.; Gersh, B.J.; Lopez-Jimenez, F.; Noseworthy, P.A. Assessment of Trends in Statin Therapy for Secondary Prevention of Atherosclerotic Cardiovascular Disease in US Adults From 2007 to 2016. JAMA Netw. Open 2020, 3, e2025505. [Google Scholar] [CrossRef]
- Arnold, S.V.; de Lemos, J.A.; Liu, Y.; Mues, K.E.; Bhatt, D.L.; Cannon, C.P.; Kosiborod, M. Adherence to Guideline Medication Recommendations to Prevent Atherosclerotic Cardiovascular Disease Progression Among Adults With Prior Myocardial Infarction. JAMA Netw. Open 2020, 3, e203032. [Google Scholar] [CrossRef] [Green Version]
- Bezin, J.; Pariente, A.; Lassalle, R.; Dureau-Pournin, C.; Abouelfath, A.; Robinson, P.; Moore, N.; Droz-Perroteau, C.; Fourrier-Reglat, A. Use of the recommended drug combination for secondary prevention after a first occurrence of acute coronary syndrome in France. Eur. J. Clin. Pharmacol. 2014, 70, 429–436. [Google Scholar] [CrossRef]
- Kugathasan, P.; Horsdal, H.T.; Aagaard, J.; Jensen, S.E.; Laursen, T.M.; Nielsen, R.E. Association of Secondary Preventive Cardiovascular Treatment After Myocardial Infarction With Mortality Among Patients With Schizophrenia. JAMA Psychiatry 2018, 75, 1234–1240. [Google Scholar] [CrossRef] [Green Version]
- Hoedemaker, N.P.G.; Damman, P.; Ottervanger, J.P.; Dambrink, J.H.E.; Gosselink, A.T.M.; Kedhi, E.; Kolkman, E.; de Winter, R.J.; van’t Hof, A.W.J. Trends in optimal medical therapy prescription and mortality after admission for acute coronary syndrome: A 9-year experience in a real-world setting. Eur. Heart J. Cardiovasc. Pharmacother. 2018, 4, 102–110. [Google Scholar] [CrossRef]
- Fried, T.R.; Mecca, M.C. Medication Appropriateness in Vulnerable Older Adults: Healthy Skepticism of Appropriate Polypharmacy. J. Am. Geriatr. Soc. 2019, 67, 1123–1127. [Google Scholar] [CrossRef]
- Wright, P.G. Moore’s Economic Cycles. Q. J. Econ. 1915, 29, 631–641. [Google Scholar] [CrossRef]
- McClellan, M.; Newhouse, J.P. Instrumental Variables Analysis Applications in Health Services Research—A Special Supplement to HSR-Overview of Supplement Issue. Health Serv. Res. 2000, 35, 1061–1069. [Google Scholar] [PubMed]
- Newhouse, J.P. Instrumental Variables in Health Services Research; Wiley StatsRef: Statistics Reference Online; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- McDowell, B.D.; Chapman, C.G.; Smith, B.J.; Button, A.M.; Chrischilles, E.A.; Mezhir, J.J. Pancreatectomy predicts improved survival for pancreatic adenocarcinoma: Results of an instrumental variable analysis. Ann. Surg. 2015, 261, 740–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angrist, J.D.; Krueger, A.B. Instrumental variables and the search for identification: From supply and demand to natural experiments. J. Econ. Perspect. 2001, 15, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Greenland, S.; Morgenstern, H. Confounding in health research. Annu. Rev. Public Health 2001, 22, 189–212. [Google Scholar] [CrossRef]
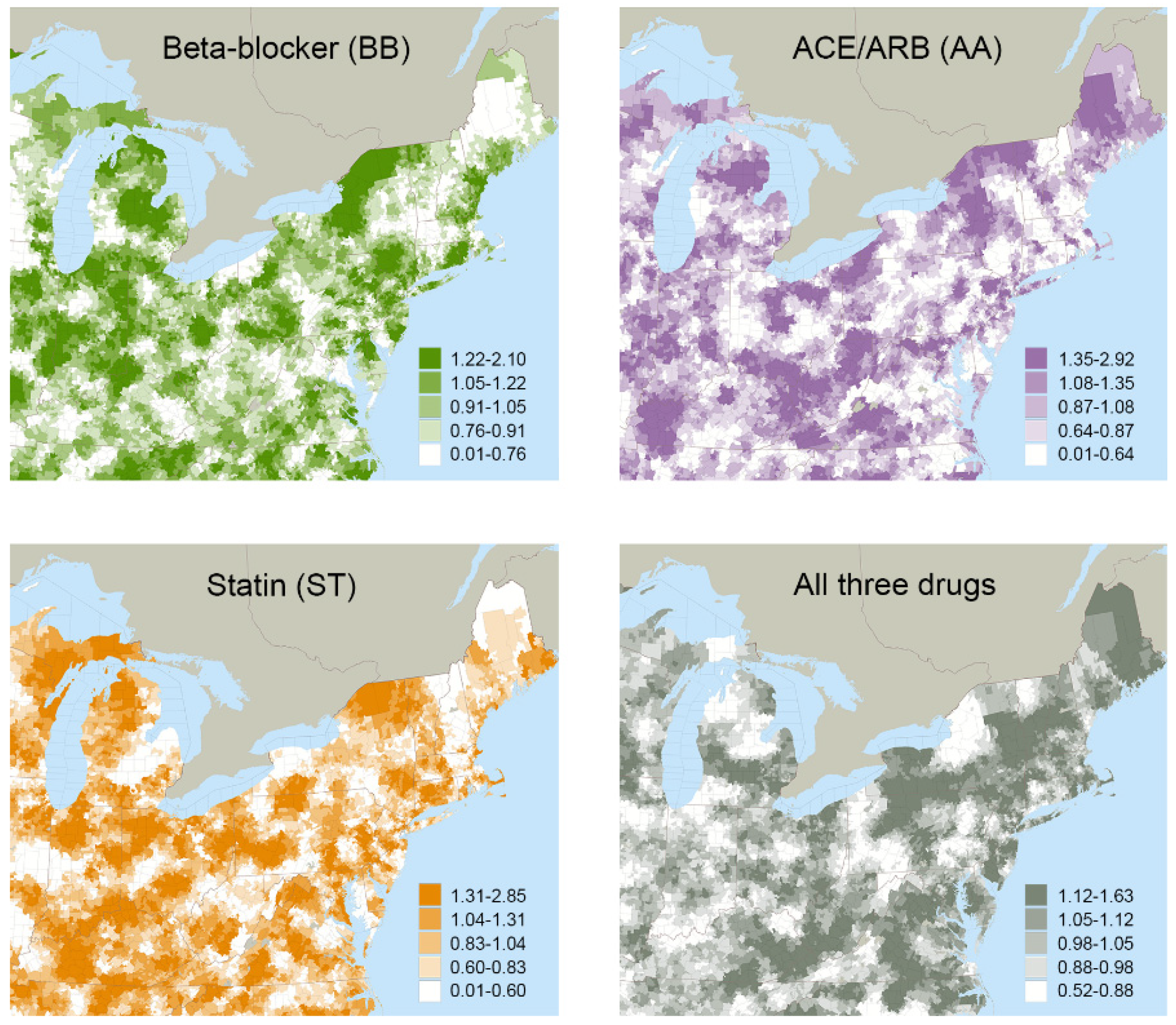
| Total | None | BB | AA | ST | BB+AA | BB+ST | AA+ST | BB+AA+ST | |
|---|---|---|---|---|---|---|---|---|---|
| Sample size (N) | 124,695 | 16,807 | 12,104 | 4790 | 4768 | 15,357 | 20,142 | 6187 | 44,540 |
| Percent of full sample * | 13.5% | 9.7% | 3.8% | 3.8% | 12.3% | 16.2% | 5.0% | 35.7% | |
| Female | 57.3% | 60.3% | 58.8% | 63.1% | 53.9% | 62.5% | 53.5% | 57.5% | 55.3% |
| Median age (years) | 78 | 81 | 80 | 80 | 78 | 79 | 77 | 77 | 76 |
| Age (years) | |||||||||
| 66–70 | 20.4% | 14.0% | 15.8% | 15.7% | 21.1% | 18.2% | 22.5% | 20.2% | 24.2% |
| 71–75 | 19.9% | 14.8% | 18.0% | 17.6% | 20.9% | 18.3% | 21.3% | 20.9% | 22.4% |
| 76–80 | 20.5% | 18.7% | 19.4% | 19.7% | 20.6% | 19.5% | 21.3% | 22.5% | 21.2% |
| 81–85 | 18.6% | 21.2% | 19.1% | 19.5% | 18.9% | 19.3% | 17.9% | 18.9% | 17.5% |
| 86+ | 20.6% | 31.3% | 27.6% | 27.4% | 18.5% | 24.8% | 17.1% | 17.6% | 14.7% |
| Race | |||||||||
| White | 83.1% | 81.8% | 84.4% | 80.5% | 84.1% | 83.8% | 84.8% | 81.4% | 82.8% |
| Black | 7.9% | 10.3% | 7.5% | 9.5% | 7.3% | 8.0% | 6.9% | 7.8% | 7.3% |
| Hispanic | 5.9% | 5.2% | 5.4% | 7.0% | 5.7% | 5.7% | 5.2% | 6.8% | 6.5% |
| Other | 3.1% | 2.7% | 2.7% | 3.1% | 2.9% | 2.5% | 3.1% | 4.0% | 3.4% |
| Number of CC | |||||||||
| 0 | 34.5% | 28.3% | 29.6% | 26.2% | 30.6% | 31.5% | 38.3% | 29.9% | 39.5% |
| 1 | 23.3% | 21.5% | 21.9% | 25.0% | 23.0% | 24.4% | 21.1% | 25.5% | 24.5% |
| 2 | 14.2% | 15.6% | 14.7% | 15.6% | 13.9% | 15.4% | 13.4% | 16.0% | 13.2% |
| 3+ | 28.0% | 34.6% | 33.8% | 33.1% | 32.5% | 28.7% | 27.2% | 28.5% | 22.8% |
| Chronic condition ** | |||||||||
| IHD | 56.5% | 57.7% | 60.4% | 60.2% | 60.8% | 59.2% | 55.9% | 59.6% | 53.1% |
| Heart failure | 30.6% | 39.5% | 35.8% | 39.1% | 32.6% | 35.6% | 26.8% | 31.4% | 24.6% |
| Comp hypertensn | 6.3% | 6.5% | 6.9% | 7.6% | 6.6% | 6.9% | 5.5% | 7.4% | 5.8% |
| unComp hypertensn | 81.5% | 80.4% | 81.7% | 88.0% | 78.8% | 86.0% | 77.8% | 85.9% | 81.0% |
| Hyperlipidemia | 66.9% | 55.8% | 61.9% | 62.2% | 73.4% | 63.7% | 70.4% | 74.9% | 70.7% |
| Diabetes mellitus | 36.9% | 37.4% | 35.2% | 40.5% | 36.9% | 39.2% | 33.4% | 41.3% | 37.0% |
| COPD | 25.9% | 31.7% | 29.0% | 33.3% | 33.4% | 25.6% | 24.7% | 31.5% | 21.2% |
| Atrial fibrillation | 13.0% | 16.4% | 16.9% | 17.0% | 14.8% | 15.0% | 11.5% | 13.4% | 9.8% |
| Unstable angina | 9.2% | 8.6% | 9.7% | 9.7% | 10.6% | 9.4% | 9.7% | 9.9% | 8.6% |
| NSTEMI | 73.6% | 77.7% | 78.6% | 78.8% | 77.1% | 74.9% | 74.3% | 75.5% | 68.8% |
| Median LOS | 6 | 7 | 6 | 6 | 6 | 6 | 6 | 6 | 5 |
| Part D insurance | |||||||||
| Dual eligible | 33.7% | 43.2% | 30.9% | 36.1% | 32.7% | 32.1% | 30.5% | 35.9% | 32.4% |
| Low-income subsidy | 6.2% | 5.5% | 5.9% | 5.9% | 6.2% | 6.5% | 6.1% | 6.2% | 6.4% |
| One-year outcomes | |||||||||
| Overall survival | 84.3% | 71.4% | 78.9% | 79.1% | 83.7% | 82.8% | 87.3% | 86.2% | 90.1% |
| CVE-free survival | 75.4% | 64.0% | 70.5% | 69.8% | 75.4% | 73.2% | 78.7% | 76.2% | 80.8% |
| 90-day outcome | |||||||||
| Adverse events | 5.4% | 6.7% | 5.7% | 6.6% | 5.6% | 5.9% | 4.9% | 5.3% | 4.7% |
| Treatment Group | Full Sample | Areas in Q1 (Lowest Use) | Areas in Q2 | Areas in Q3 | Areas in Q4 | Areas in Q5 (Highest Use) | %Δ | F-Statistic * |
|---|---|---|---|---|---|---|---|---|
| None | 13.5% | 9.7% | 12.2% | 13.4% | 14.7% | 17.4% | 80 | 30.5 (p < 0.001) |
| BB | 9.7% | 6.1% | 8.3% | 9.6% | 11.0% | 13.6% | 124 | 29.9 (p < 0.001) |
| AA | 3.8% | 1.6% | 2.9% | 3.8% | 4.6% | 6.3% | 289 | 29.0 (p < 0.001) |
| ST | 3.8% | 1.6% | 2.8% | 3.9% | 4.4% | 6.4% | 296 | 29.6 (p < 0.001) |
| BB+AA | 12.3% | 8.0% | 10.6% | 11.9% | 14.2% | 16.8% | 110 | 34.5 (p < 0.001) |
| BB+ST | 16.2% | 11.4% | 14.2% | 16.1% | 17.9% | 21.2% | 86 | 32.4 (p < 0.001) |
| AA+ST | 5.0% | 2.4% | 3.9% | 4.9% | 6.1% | 7.6% | 220 | 29.7 (p < 0.001) |
| BB+AA+ST | 35.7% | 27.7% | 32.9% | 36.4% | 38.9% | 42.8% | 54 | reference group |
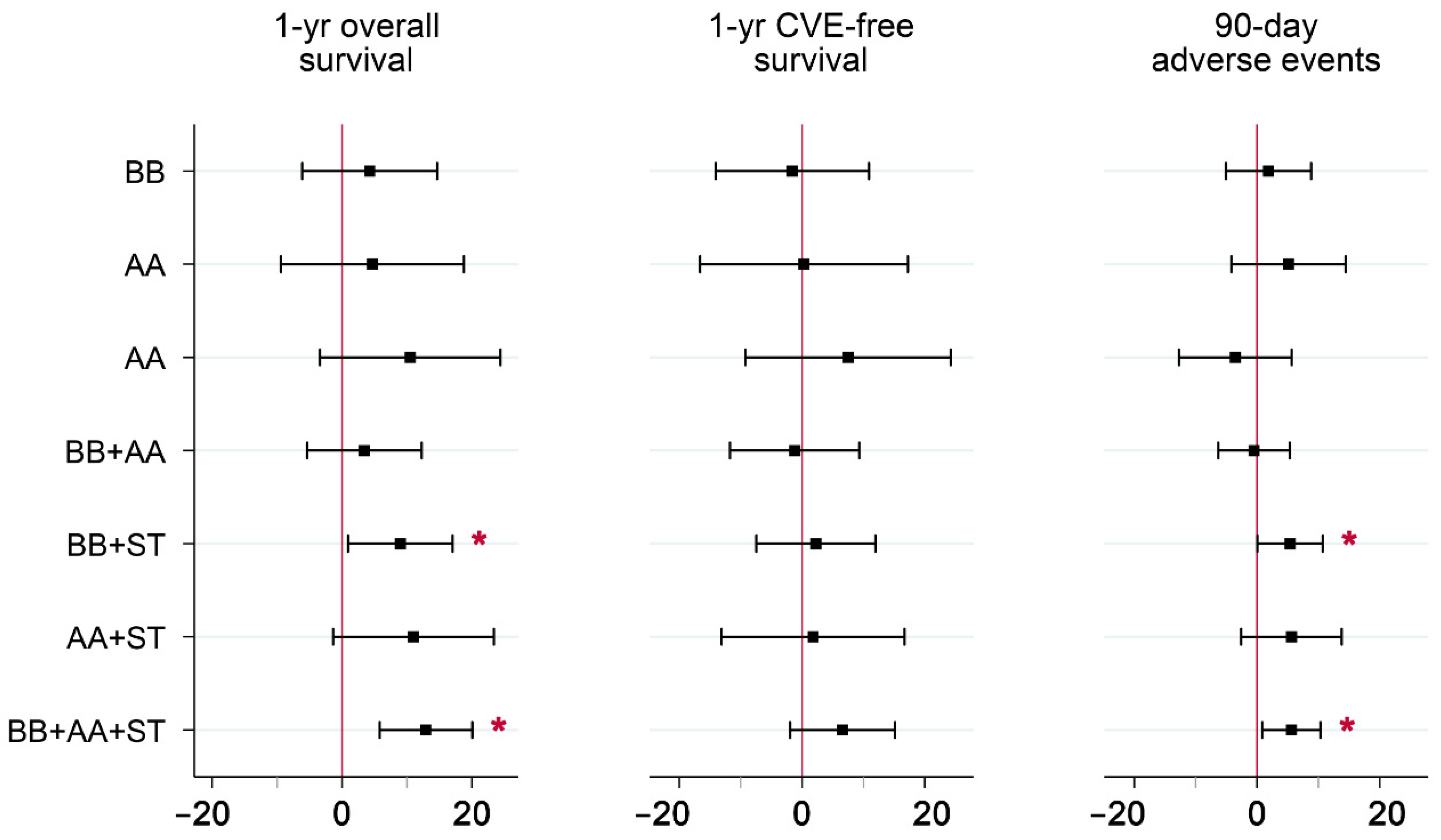
| One-Year Overall Survival | One-Year CVE-Free Survival | 90-Day Adverse Events | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Group | β Coef | SE | p-Value | β Coef | SE | p-Value | β Coef | SE | p-Value |
| None | −12.91 | 3.63 | <0.001 | −6.60 | 4.37 | 0.130 | −5.61 | 2.42 | 0.020 |
| BB | −8.66 | 3.90 | 0.026 | −8.18 | 4.68 | 0.080 | −3.74 | 2.62 | 0.153 |
| AA | −8.23 | 6.17 | 0.182 | −6.30 | 7.40 | 0.395 | −0.45 | 4.06 | 0.911 |
| ST | −2.41 | 5.99 | 0.687 | 0.89 | 7.21 | 0.901 | −9.15 | 3.97 | 0.021 |
| BB+AA | −9.46 | 3.41 | 0.006 | −7.81 | 4.09 | 0.056 | −6.10 | 2.28 | 0.007 |
| BB+ST | −3.93 | 3.20 | 0.220 | −4.33 | 3.86 | 0.261 | −0.22 | 2.15 | 0.919 |
| AA+ST | −1.91 | 5.52 | 0.729 | −4.81 | 6.63 | 0.469 | 0.01 | 3.66 | 0.999 |
| Full Sample | None | BB | AA | ST | BB+AA | BB+ST | AA+ST | BB+AA+ST | p-Value ** | |
|---|---|---|---|---|---|---|---|---|---|---|
| Severity of AMI | 7.55 | 6.77 | 7.03 | 6.76 | 7.96 | 7.21 | 8.01 | 8.17 | 7.89 | <0.001 |
| Disease burden | 3.37 | 2.99 | 3.16 | 2.99 | 3.83 | 3.13 | 3.49 | 3.60 | 3.53 | <0.001 |
| % w/potential contraindication | 46.30 | 54.84 | 52.03 | 57.04 | 52.14 | 48.97 | 43.72 | 43.29 | 34.00 | <0.001 |
| ADL | 0.43 | 0.91 | 0.66 | 0.56 | 0.48 | 0.40 | 0.27 | 0.27 | 0.28 | <0.001 |
| % w/diff in any ADL domain | 25.71 | 45.16 | 34.46 | 29.63 | 25.71 | 26.80 | 17.59 | 19.51 | 19.67 | <0.001 |
| % w/diff in 2+ ADL domains | 7.05 | 14.52 | 10.14 | 9.63 | 7.14 | 6.19 | 4.52 | 3.05 | 5.67 | <0.001 |
| ACE-27 score | 1.87 | 2.04 | 2.03 | 2.03 | 1.96 | 1.89 | 1.75 | 1.87 | 1.67 | <0.001 |
| % overweight (BMI > 25) | 66.95 | 51.61 | 61.49 | 62.22 | 70.00 | 66.49 | 66.33 | 73.17 | 74.00 | <0.001 |
| % underweight (BMI < 18.5) | 3.70 | 8.06 | 4.05 | 5.19 | 6.43 | 3.09 | 3.52 | 1.83 | 1.33 | <0.001 |
| % cath w/in 24 h | 39.32 | 16.94 | 27.70 | 23.70 | 38.57 | 30.41 | 52.76 | 45.12 | 55.33 | <0.001 |
| Full Sample | Areas in Quintile 1 (Lowest Use) | Areas in Quintile 2 | Areas in Quintile 3 | Areas in Quintile 4 | Areas in Quintile 5 (Highest Use) | p-Value ** | |
|---|---|---|---|---|---|---|---|
| Severity of AMI | 7.55 | 7.43 | 7.88 | 7.62 | 7.44 | 7.40 | 0.589 |
| Disease burden | 3.37 | 3.46 | 3.35 | 3.33 | 3.24 | 3.46 | 0.700 |
| % w/ potential contraindication | 46.30 | 44.26 | 44.52 | 47.39 | 47.4 | 48.19 | 0.255 |
| ADL | 0.43 | 0.48 | 0.37 | 0.46 | 0.42 | 0.43 | 0.840 |
| % w/diff in any ADL domain | 25.71 | 25.00 | 23.32 | 28.92 | 25.61 | 25.70 | 0.643 |
| % w/diff in 2+ ADL domains | 7.05 | 7.77 | 6.71 | 6.27 | 7.27 | 7.23 | 0.894 |
| ACE-27 score | 1.87 | 1.93 | 1.83 | 1.89 | 1.85 | 1.84 | 0.403 |
| % overweight (BMI > 25) | 66.95 | 66.22 | 68.55 | 66.2 | 67.82 | 65.86 | 0.901 |
| % underweight (BMI < 18.5) | 3.70 | 2.36 | 4.24 | 4.53 | 4.50 | 2.81 | 0.667 |
| % cath w/in 48 h | 39.32 | 41.28 | 38.79 | 39.86 | 36.65 | 40.00 | 0.610 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schroeder, M.C.; Chapman, C.G.; Chrischilles, E.A.; Wilwert, J.; Schneider, K.M.; Robinson, J.G.; Brooks, J.M. Generating Practice-Based Evidence in the Use of Guideline-Recommended Combination Therapy for Secondary Prevention of Acute Myocardial Infarction. Pharmacy 2022, 10, 147. https://doi.org/10.3390/pharmacy10060147
Schroeder MC, Chapman CG, Chrischilles EA, Wilwert J, Schneider KM, Robinson JG, Brooks JM. Generating Practice-Based Evidence in the Use of Guideline-Recommended Combination Therapy for Secondary Prevention of Acute Myocardial Infarction. Pharmacy. 2022; 10(6):147. https://doi.org/10.3390/pharmacy10060147
Chicago/Turabian StyleSchroeder, Mary C., Cole G. Chapman, Elizabeth A. Chrischilles, June Wilwert, Kathleen M. Schneider, Jennifer G. Robinson, and John M. Brooks. 2022. "Generating Practice-Based Evidence in the Use of Guideline-Recommended Combination Therapy for Secondary Prevention of Acute Myocardial Infarction" Pharmacy 10, no. 6: 147. https://doi.org/10.3390/pharmacy10060147
APA StyleSchroeder, M. C., Chapman, C. G., Chrischilles, E. A., Wilwert, J., Schneider, K. M., Robinson, J. G., & Brooks, J. M. (2022). Generating Practice-Based Evidence in the Use of Guideline-Recommended Combination Therapy for Secondary Prevention of Acute Myocardial Infarction. Pharmacy, 10(6), 147. https://doi.org/10.3390/pharmacy10060147






