Recent Advances in Sickle-Cell Disease Therapies: A Review of Voxelotor, Crizanlizumab, and L-glutamine
Abstract
1. Introduction
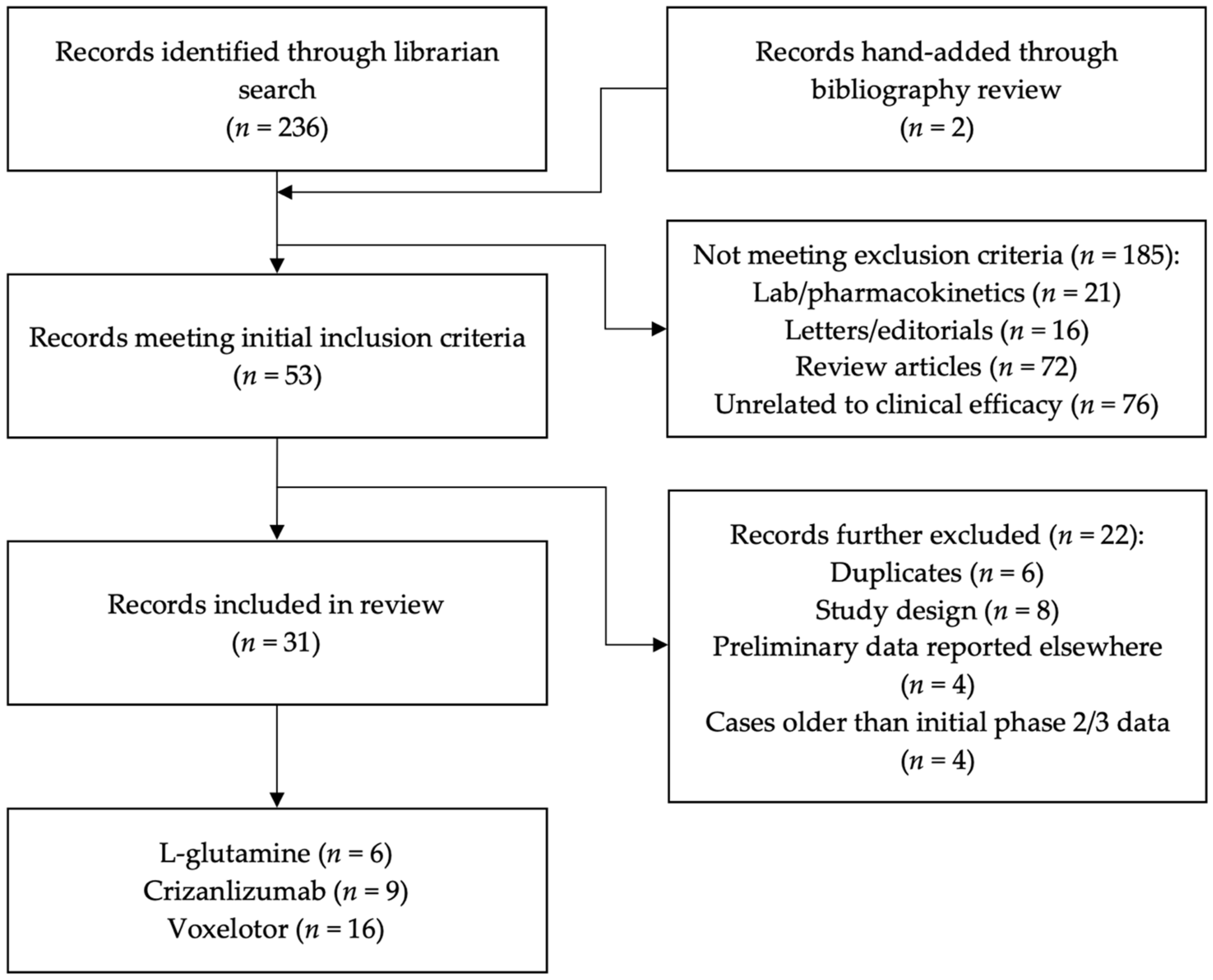
2. Literature Search and Articles Selection
2.1. Inclusion and Exclusion Criteria
2.2. Data Extraction
3. Results
3.1. Description of Included Studies
3.1.1. L-glutamine
3.1.2. Crizanlizumab
3.1.3. Voxelotor
3.2. Efficacy
3.2.1. L-glutamine
3.2.2. Crizanlizumab
3.2.3. Voxelotor
3.3. Quality of Life and Patient-Reported Outcomes Data
3.3.1. L-glutamine
3.3.2. Crizanlizumab
3.3.3. Voxelotor
3.4. Side Effects and Prescribing Data
3.4.1. L-glutamine
3.4.2. Crizanlizumab
3.4.3. Voxelotor
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle cell disease. N. Engl. J. Med. 2017, 376, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Hassell, K.L. Population estimates of sickle cell disease in the US. Am. J. Prev. Med. 2010, 38, S512–S521. [Google Scholar] [CrossRef] [PubMed]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of sickle cell disease. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Tsaras, G.; Owusu-Ansah, A.; Boateng, F.O.; Amoateng-Adjepong, Y. Complications associated with sickle cell trait: A brief narrative review. Am. J. Med. 2009, 122, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.M.; Thompson, A.A.; Lai, J.S.; Penedo, F.J.; Rychlik, K.; Liem, R.I. Health-related quality of life and adherence to hydroxyurea in adolescents and young adults with sickle cell disease. Pediatr. Blood Cancer 2017, 64, e26369. [Google Scholar] [CrossRef] [PubMed]
- Panepinto, J.A.; Bonner, M. Health-related quality of life in sickle cell disease: Past, present, and future. Pediatr. Blood Cancer 2012, 59, 377–385. [Google Scholar] [CrossRef]
- Lubeck, D.; Agodoa, I.; Bhakta, N.; Danese, M.; Pappu, K.; Howard, R.; Gleeson, M.; Halperin, M.; Lanzkron, S. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw. Open 2019, 2, e1915374. [Google Scholar] [CrossRef]
- Platt, O.S. Hydroxyurea for the treatment of sickle cell anemia. N. Engl. J. Med. 2008, 358, 1362–1369. [Google Scholar] [CrossRef]
- Charache, S.; Terrin, M.L.; Moore, R.D.; Dover, G.J.; Barton, F.B.; Eckert, S.V.; McMahon, R.P.; Bonds, D.R.; the Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N. Engl. J. Med. 1995, 332, 1317–1322. [Google Scholar] [CrossRef]
- Thornburg, C.D.; Calatroni, A.; Telen, M.; Kemper, A.R. Adherence to hydroxyurea therapy in children with sickle cell anemia. J. Pediatr. 2010, 156, 415–419. [Google Scholar] [CrossRef]
- Cox, S.E.; Hart, E.; Kirkham, F.J.; Stotesbury, H. L-Glutamine in sickle cell disease. Drugs Today 2020, 56, 257–268. [Google Scholar] [CrossRef]
- Niihara, Y.; Miller, S.T.; Kanter, J.; Lanzkron, S.; Smith, W.R.; Hsu, L.L.; Gordeuk, V.R.; Viswanathan, K.; Sarnaik, S.; Osunkwo, I.; et al. A Phase 3 Trial of l-Glutamine in Sickle Cell Disease. N. Engl. J. Med. 2018, 379, 226–235. [Google Scholar] [CrossRef]
- Blair, H.A. Crizanlizumab: First approval. Drugs 2020, 80, 79–84. [Google Scholar] [CrossRef]
- Ataga, K.I.; Kutlar, A.; Kanter, J.; Liles, D.; Cancado, R.; Friedrisch, J.; Guthrie, T.H.; Knight-Madden, J.; Alvarez, O.A.; Gordeuk, V.R.; et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 429–439. [Google Scholar] [CrossRef]
- Blair, H.A. Voxelotor: First approval. Drugs 2020, 80, 209–215. [Google Scholar] [CrossRef]
- Vichinsky, E.; Hoppe, C.C.; Ataga, K.I.; Ware, R.E.; Nduba, V.; El-Beshlawy, A.; Hassab, H.; Achebe, M.M.; Alkindi, S.; Brown, R.C.; et al. A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease. N. Engl. J. Med. 2019, 381, 509–519. [Google Scholar] [CrossRef]
- Lam, H.; Becerra, J.M.; Stark, C.W.; Niihara, Y.; Callaghan, M. The Evaluation of Transfusion Data from the Phase 3 Clinical Study of L-Glutamine in Sickle Cell Disease. Blood 2021, 138, 3116. [Google Scholar] [CrossRef]
- Niihara, Y.; Stark, C.W.; Razon, R.; Tran, L.T.; Becerra, J.M.; Panosyan, E.H.; Lasky, J.L. Consistent benefit of L-glutamine observed across patients with low, medium, and high number of crises reported in the year prior to screening-analysis from the phase 3 study of L-glutamine in sickle cell anemia. Blood 2018, 132, 1065. [Google Scholar] [CrossRef]
- Wilson, S.; Wright, F.; Carden, M.A. L-Glutamine Decreases Opioid Use in Individuals with Sickle Cell Disease and Chronic Pain: A Case Series. Blood 2019, 134, 4849. [Google Scholar] [CrossRef]
- Gotesman, M.; Elgar, G.; Pak, Y.; Hernandez Santiago, L.; Alvarez, A.; Lin, H.; Lasky, J.L.; Panosyan, E.H. Pediatric Patients with Sickle Cell Disease at Harbor-UCLA & Early Experience of L-Glutamine Therapy. Blood 2020, 136, 6. [Google Scholar] [CrossRef]
- Ogu, U.O.; Thomas, M.; Chan, F.; Sebastian, G.; Minniti, C.P. Barriers to the Use of Endari™ in an Urban Adult Sickle Cell Center. Blood 2019, 134, 2287. [Google Scholar] [CrossRef]
- Ataga, K.I.; Kutlar, A.; DeBonnett, L.; Lincy, J.; Kanter, J. Crizanlizumab Treatment Is Associated with Clinically Significant Reductions in Hospitalization in Patients with Sickle Cell Disease: Results from the Sustain Study. Blood 2019, 134, 2289. [Google Scholar] [CrossRef]
- Kutlar, A.; Kanter, J.; Liles, D.K.; Alvarez, O.A.; Cançado, R.D.; Friedrisch, J.R.; Knight-Madden, J.M.; Bruederle, A.; Shi, M.; Zhu, Z.; et al. Effect of crizanlizumab on pain crises in subgroups of patients with sickle cell disease: A SUSTAIN study analysis. Am. J. Hematol. 2019, 94, 55–61. [Google Scholar] [CrossRef]
- Smith, W.R.; Ataga, K.I.; Saraf, S.L.; Adisa, O.A.; Bailey, M.; Ramscar, N.; Bonner, A.; Brown, S.; Pastor, L. The Effect of Crizanlizumab on the Number of Days Requiring Opioid Use for Management of Pain Associated with Vaso-Occlusive Crises in Patients with Sickle Cell Disease: Results from the Sustain Trial. Blood 2020, 136, 32–33. [Google Scholar] [CrossRef]
- Shah, N.; Boccia, R.; Kraft, W.K.; Hardesty, B.M.; Paulose, J.; Lainé, D.; Purkayastha, D.; Bhor, M.; Achebe, M.; Cataldo, V.; et al. Pro3 successor study: Treatment and health care resource utilization by sickle cell patients who participated in the sustain study in the united states. Value Health 2019, 22, S335. [Google Scholar] [CrossRef]
- Kanter, J.; Liles, D.K.; Smith-Whitley, K.; Brown, C.; Kutlar, A.; Elliott, B.; Shah, A.; Lincy, J.; Poggio, S.; Ataga, K.I. Crizanlizumab 5.0 Mg/Kg Exhibits a Favorable Safety Profile in Patients with Sickle Cell Disease: Pooled Data from Two Phase II Studies. Blood 2019, 134, 991. [Google Scholar] [CrossRef]
- Li, V.J.; Adesina, O.O.; Fertrin, K.Y. Crizanlizumab-Associated Painful Febrile Reaction in Sickle Cell Disease Patients. Blood 2021, 138, 4186. [Google Scholar] [CrossRef]
- Kanter, J.; Hellemann, G.; Cohen, A.J.; Manwani, D.; Idowu, M.; Guarino, S.; Saif Ur Rehman, S.; Treadwell, M.; Clay, E.L.J.; Owusu-Ansah, A.; et al. Early Evaluation of the Use of Crizanlizumab in Sickle Cell Disease: A National Alliance of Sickle Cell Centers Study. Blood 2021, 138, 3113. [Google Scholar] [CrossRef]
- Kanter, J.; Shah, A.; Joshi, V.; Mehta, H.; Levine, M.; Arunagiri, U.; Paulose, J.; Donohue, B.; Scalera, A.; Manwani, D. Rare Cases of Infusion-Related Reactions (IRRs) Presenting As Pain Events during or after Crizanlizumab Infusion in Patients (Pts) with Sickle Cell Disease (SCD): A Systematic Evaluation of Post-Marketing (PM) Reports. Blood 2021, 138, 3112. [Google Scholar] [CrossRef]
- Howard, J.; Vichinsky, E.; Knight-Madden, J.; Tonda, M.; Washington, C.; Tong, B.; Lehrer-Graiwer, J.; Gordeuk, V.R. Correlation of Voxelotor Exposure with Hemoglobin Response and Measures of Hemolysis in Patients from the HOPE Study. Blood 2019, 134, 1020. [Google Scholar] [CrossRef]
- Howard, J.; Ataga, K.I.; Brown, R.C.; Achebe, M.; Nduba, V.; El-Beshlawy, A.; Hassab, H.; Agodoa, I.; Tonda, M.; Gray, S.; et al. Voxelotor in adolescents and adults with sickle cell disease (HOPE): Long-term follow-up results of an international, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Haematol. 2021, 8, e323–e333. [Google Scholar] [CrossRef]
- Minniti, C.P.; Knight-Madden, J.; Tonda, M.; Gray, S.; Lehrer-Graiwer, J.; Biemond, B.J. The impact of voxelotor treatment on leg ulcers in patients with sickle cell disease. Am. J. Hematol. 2021, 96, E126–E128. [Google Scholar] [CrossRef]
- Vichinsky, E.; Gordeuk, V.R.; Telfer, P.; Inati, A.; Tonda, M.; Gray, S.; Agodoa, I.; Ataga, K.I. Higher Hemoglobin Levels Achieved with Voxelotor Are Associated with Lower Vaso-occlusive Crisis Incidence: 72-Week Analysis from the HOPE Study. Blood 2020, 136, 31–32. [Google Scholar] [CrossRef]
- Achebe, M.; Hassab, H.; Alkindi, S.; Brown, R.C.C.; Telfer, P.; Biemond, B.J.; Gordeuk, V.R.; Lipato, T.; Tonda, M.; Gray, S. Long-Term Safety and Efficacy of Voxelotor for Patients with Sickle Cell Disease: Results from an Open-Label Extension of the Phase 3 HOPE Trial. Blood 2021, 138, 3114. [Google Scholar] [CrossRef]
- Estepp, J.; Kalpatthi, R.; Woods, G.; Trompeter, S.; Liem, R.; Sims, K.; Inati, A.; Inusa, B.; Campbell, A.; Piccone, C.; et al. Safety and efficacy of voxelotor in pediatric patients with sickle cell disease aged 4–11 years. HemaSphere 2021, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; Hoppe, C.; Inati, A.; Wang, W.; Hsu, L.L.; Gordeuk, V.R.; Liem, R.; Woods, G.; Piccone, C.; Fong, E.; et al. Results from a phase 2a study (GBT440-007) evaluating adolescents with sickle cell disease treated with multiple doses of voxelotor (GBT440), a hbs polymerization inhibitor. HemaSphere 2018, 2, 304–305. [Google Scholar] [CrossRef]
- Brown, C.; Hoppe, C.; Inati, A.; Abboud, M.; Wang, W.; Liem, R.; Woods, G.; Hsu, L.; Gordeuk, V.; Piccone, C.; et al. Phase 2A study (GBT440-007) of voxelotor in adolescents with sickle cell disease. Pediatr. Blood Cancer 2019, 66, S241–S242. [Google Scholar] [CrossRef]
- Muschick, K.; Fuqua, T.; Stoker-Postier, C.; Anderson, A. Real-world experience of pediatric patients with sickle cell disease treated with oxbryta®. Pediatr. Blood Cancer 2021, 68, S121–S122. [Google Scholar] [CrossRef]
- Phan, V.; Caldarera, L.; Cortez, A.L.; Wheeler, K.; Larkin, S.K.; Park, S.N.; Dulman, R.Y.; Briere, N.; Lewis, A.; Kuypers, F.A.; et al. The Effect of Voxelotor on Exercise Capacity of Youths with Sickle Cell Anemia. Blood 2021, 138, 2045. [Google Scholar] [CrossRef]
- Zaidi, A.U.; Lipato, T.; Alvarez, O.A.; Lonshteyn, A.; Weycker, D.; Pham, N.; Delea, T.E.; Agodoa, I.; Cong, Z.; Shah, N. Real-World Effectiveness of Voxelotor for Treating Sickle Cell Disease in the US. Blood 2020, 136, 25. [Google Scholar] [CrossRef]
- Ware, R.; Brown, C.; De Montalembert, M.; Tonda, M.; Tong, B.; Hoppe, C.; Lehrer-Graiwer, J.; Abboud, M.R. Concomitant hydroxyurea and voxelotor: Results from the hope study. HemaSphere 2020, 4, 711. [Google Scholar] [CrossRef]
- Betancourt, J.L.; You, S.; Campbell, S.; Pink, S.; Thomas, M.; Curtis, S.A.; Minniti, C.P. Real World Clinical Experience with Oxbryta Therapy in Individuals with Sickle Cell Disease. Blood 2020, 136, 15–16. [Google Scholar] [CrossRef]
- Shah, N.; Zaidi, A.U.; Callaghan, M.U.; Liles, D.; Johnson, C.E.; De Castro, L.M. Real World Evidence of Prescription Patterns and Effect of Oxbryta (voxelotor) for Patients with Sickle Cell Disease. Blood 2020, 136, 31–32. [Google Scholar] [CrossRef]
- Andemariam, B.; Achebe, M.; Clay, E.L.J.; Drachtman, R.A.; Sharma, A.; Nero, A.C.; Osunkwo, I.; Sarnaik, S.; Idowu, M.; Shah, N. Real-World Experience of Patients with Sickle Cell Disease Treated with Voxelotor: A Multicenter, Retrospective Study. Blood 2021, 138, 3100. [Google Scholar] [CrossRef]
- Onasanya, O. Cost-effectiveness of Lglutamine (Endari) and off-label generic hydroxyurea comedication compared to hydroxyurea (Siklos) for averting pediatric sickle cell crisis-related hospital admissions. Pharmacoepidemiol. Drug Saf. 2020, 29, 647. [Google Scholar] [CrossRef]
- Alashgar, A.; Holdford, D.; Pontinha, V. PRO51 Cost Effectiveness of Crizanlizumab Compared to Hydroxyurea for Minimizing the Frequency of Vaso-Occlusive (VOC) PAIN Episodes in Patients with Sickle CELL Disease SCD from a Payer Perspective. Value Health 2020, 23, S699. [Google Scholar] [CrossRef]
- Ataga, K.I.; Saraf, S.L.; Derebail, V.K.; Sharpe, C.C.; Inati, A.; Lebensburger, J.D.; DeBonnett, L.; Zhang, S.; Bartolucci, P. The Effect of Crizanlizumab Plus Standard of Care (SoC) Versus Soc Alone on Renal Function in Patients with Sickle Cell Disease and Chronic Kidney Disease: A Randomized, Multicenter, Open-Label, Phase II Study (STEADFAST). Blood 2019, 134, 1018. [Google Scholar] [CrossRef]
- Abboud, M.R.; Howard, J.; Cançado, R.; Smith, W.R.; Güvenç, B.; Espurz, N.; Weill, M.; de Montalembert, M. Crizanlizumab Versus Placebo, with or without Hydroxyurea/Hydroxycarbamide, in Adolescent and Adult Patients with Sickle Cell Disease and Vaso-Occlusive Crises: A Randomized, Double-Blind, Phase III Study (STAND). Blood 2019, 134, 998. [Google Scholar] [CrossRef]
- Heeney, M.; Rees, D.; De Montalembert, M.; Odame, I.; Merino, R.; Elliott, B.; Bodla, S.; Kanter, J. Crizanlizumab dose confirmation in pediatric patients with sickle cell disease: Solace-kids design. Pediatr. Blood Cancer 2019, 66, S240–S241. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Study to Evaluate the Effect of GBT440 in Pediatrics With Sickle Cell Disease (HOPE Kids). Available online: https://clinicaltrials.gov/ct2/show/NCT02850406 (accessed on 15 August 2022).
- Clinicaltrials.gov. Study to Evaluate the Effect of GBT440 on TCD in Pediatrics With Sickle Cell Disease (HOPE Kids 2). Available online: https://clinicaltrials.gov/ct2/show/NCT04218084 (accessed on 15 August 2022).
| Source (Country) | Outcome Measured | Study Design | Age, Years | Group, n | Summary of Findings |
|---|---|---|---|---|---|
| Clinical Efficacy | |||||
| Niihara 2018, USA | Primary: number of pain crises; secondary: hospitalizations, ED visits, changes in hematologic measures | RCT | 5–58, median 19 in treatment group, 17 in placebo | 230 |
|
| Niihara 2018, USA | Number of crises | Subgroup analysis of Niihara et al. | 5–58, median 19 in treatment group, 17 in placebo | 230 |
|
| Lam 2021, USA | Number of transfusions | Post hoc of Niihara et al. | 5–58, median 19 in treatment group, 17 in placebo | 230 |
|
| Wilson 2019, USA | Opioid use | Case series | Range 9–24 | 4 |
|
| Side Effects and Prescribing Data | |||||
| Gotesman 2020, USA | Prescribing data, compliance | Retrospective review | Mean 9.1 | 50 |
|
| Ogu 2019, USA | Compliance, side effects | Retrospective review | Mean 36, range 21–70 | 111 |
|
| Source (Country) | Outcome Measured | Study Design | Age, Years | Group, n | Summary of Findings |
|---|---|---|---|---|---|
| Clinical Efficacy | |||||
| Ataga 2017, USA | Primary: rate of sickle-cell pain crises Secondary: hospitalizations, time to first and second hospitalizations, annual rates of uncomplicated crises, ACS, patient-reported outcomes | RCT (SUSTAIN trial) | Median 29, range 16–63 | 198 |
|
| Ataga 2019, USA | Annual rate of hospitalization, time to first hospitalization | Post hoc of SUSTAIN data | Median 29, range 16–63 | 198 |
|
| Kutlar 2019, USA | Secondary endpoints of SUSTAIN | Post hoc of SUSTAIN data | Median 29, range 16–63 | 198 |
|
| Smith 2020, USA | Days of opioid use | Post hoc of SUSTAIN data | Median 29, range 16–63 | 198 |
|
| Shah 2019, USA | Pain crises events, treatment patterns, health care resources | Retrospective cohort review of SUSTAIN 1 year following study | ≥18 years old, median 37 | 6 |
|
| Side Effects and Prescribing Data | |||||
| Kanter 2019, USA | Safety, side effects | Pooled Phase 2 trial data (SUSTAIN + SOLACE-adults) SUSTAIN: RCT SOLACE-adult: PK/PD | Median 29, range 16–65 | 111 (SUSTAIN n = 66, SOLACE n = 45) |
|
| Kanter 2021, USA | Insurance approval, adherence | Retrospective multi-center review | ≥16 | 297 prescribed, 238 received infusion |
|
| Kanter 2021, USA | Infusion-related reaction | Retrospective review of safety database | Median 23 (range 16–38) | 28 |
|
| Li 2021, USA | Side effects | Case report | 20 and 48 | 2 |
|
| Source (Country) | Outcome Measured | Study Design | Age, Years | Group, n | Summary of Findings |
|---|---|---|---|---|---|
| Clinical Efficacy | |||||
| Vichinsky 2019, international | Primary: hemoglobin response | Phase 3 RCT (HOPE trial) | 12–64, median 24 | 274 |
|
| Howard 2019, international | Measurements of hemolysis | Post hoc of HOPE trial | 12–64, median 24 | 274 |
|
| Howard 2021, international | Changes from baseline hemoglobin, safety | 72-week follow-up of HOPE | 12–64, median 24 | 274 |
|
| Minniti 2021, international | Leg ulcers | Post hoc of HOPE | 12–64, median 24 | 274 |
|
| Vichinsky 2020, international | Hemoglobin response and VOE incidence | 72-week follow-up of HOPE | 12–64, median 24 | 274 |
|
| Achebe 2021, international | Hemoglobin response, markers of hemolysis | Long-term open-label extension of HOPE, 1500 mg | Median 25 | 178 |
|
| Estepp 2021, USA | Increase in hemoglobin, side effects | Open-label, multicenter, multiple-dose trial (HOPE KIDS 1) | 4–11, median 7 | 45 |
|
| Brown 2018, USA | Increase in hemoglobin | Phase 2a study of 900 mg/day in adolescents | 12–17, median 14 | 25 |
|
| Brown 2019, USA | Increase in hemoglobin | Phase 2a study of 1500 mg/day in adolescents | 12–17, median 14 | 15 |
|
| Muschick 2021, USA | Increase in hemoglobin | Single-center retrospective review | 12–21 | 17 |
|
| Phan 2021, USA | Exercise capacity | Pilot study | 12–20 | 9 |
|
| Zaidi 2020, USA | Effect on anemia | Retrospective review in Symphony Health system | Mean 35.7 | 1275 |
|
| Side Effects and Prescribing Data | |||||
| Ware 2020, international | Concomitant use of HU | Post hoc of HOPE | 12–64, median 24 | 274 |
|
| Betancourt 2020, USA | Real-world experience, barriers to use, health outcomes | Single-center retrospective review | 21–70, mean 41 | 54 |
|
| Shah 2020, USA | Prescribing, side effects, clinical efficacy | Multicenter retrospective review | Mean 33 | 60 |
|
| Andemariam 2021, USA | Prescribing, side effects, clinical efficacy | Multicenter retrospective post-marketing review | Mean 34.3 | 300, 49 at time of data analysis |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migotsky, M.; Beestrum, M.; Badawy, S.M. Recent Advances in Sickle-Cell Disease Therapies: A Review of Voxelotor, Crizanlizumab, and L-glutamine. Pharmacy 2022, 10, 123. https://doi.org/10.3390/pharmacy10050123
Migotsky M, Beestrum M, Badawy SM. Recent Advances in Sickle-Cell Disease Therapies: A Review of Voxelotor, Crizanlizumab, and L-glutamine. Pharmacy. 2022; 10(5):123. https://doi.org/10.3390/pharmacy10050123
Chicago/Turabian StyleMigotsky, Michael, Molly Beestrum, and Sherif M. Badawy. 2022. "Recent Advances in Sickle-Cell Disease Therapies: A Review of Voxelotor, Crizanlizumab, and L-glutamine" Pharmacy 10, no. 5: 123. https://doi.org/10.3390/pharmacy10050123
APA StyleMigotsky, M., Beestrum, M., & Badawy, S. M. (2022). Recent Advances in Sickle-Cell Disease Therapies: A Review of Voxelotor, Crizanlizumab, and L-glutamine. Pharmacy, 10(5), 123. https://doi.org/10.3390/pharmacy10050123







