Experience, Knowledge, and Perceptions of Pharmacogenomics among Pharmacists and Nurse Practitioners in Alberta Hospitals
Abstract
1. Introduction
2. Methods
2.1. Survey Design
2.2. Participants
2.3. Statistical Analysis
3. Results
3.1. Participants
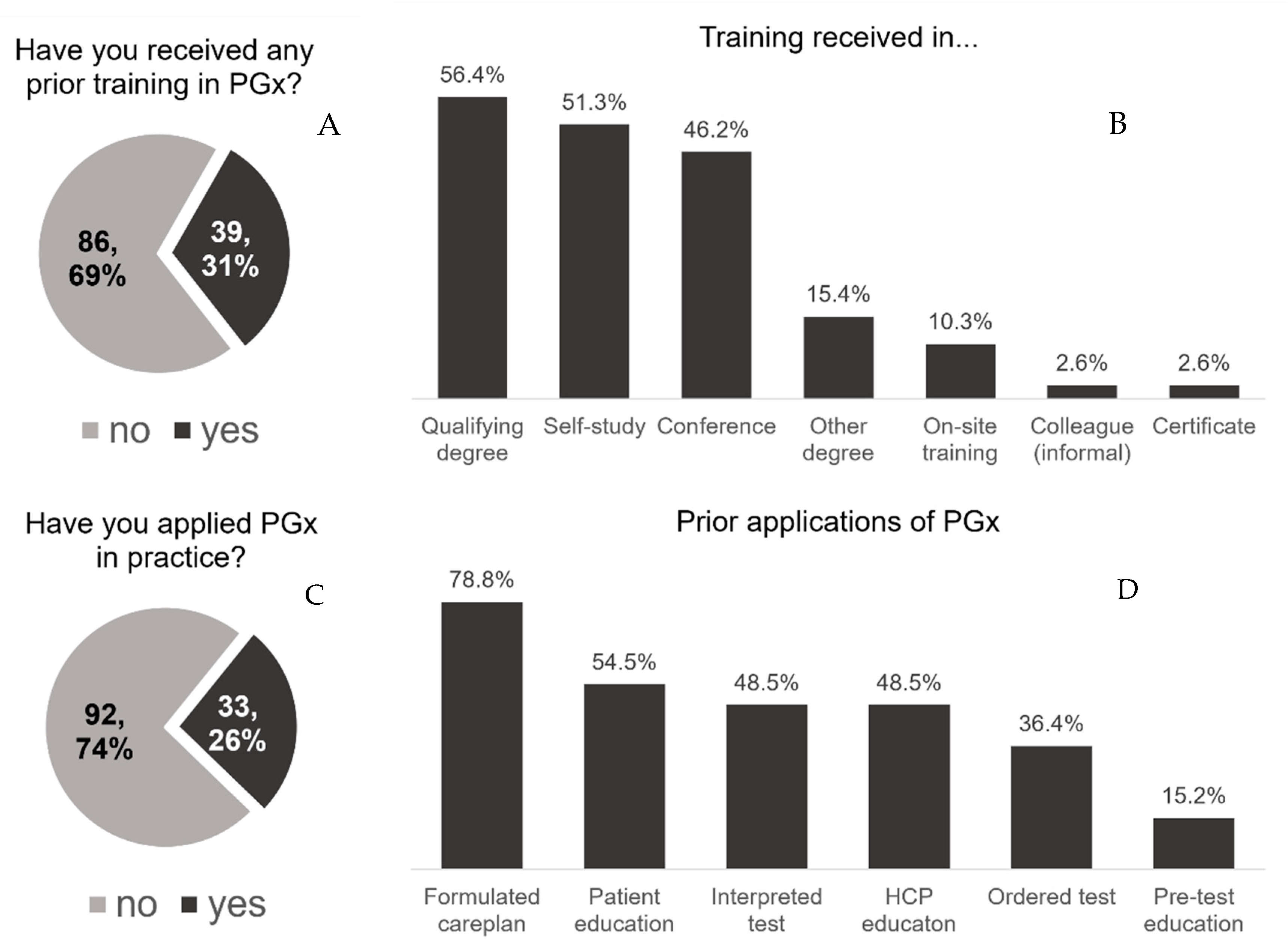
3.2. Pharmacogenomics Training and Experience
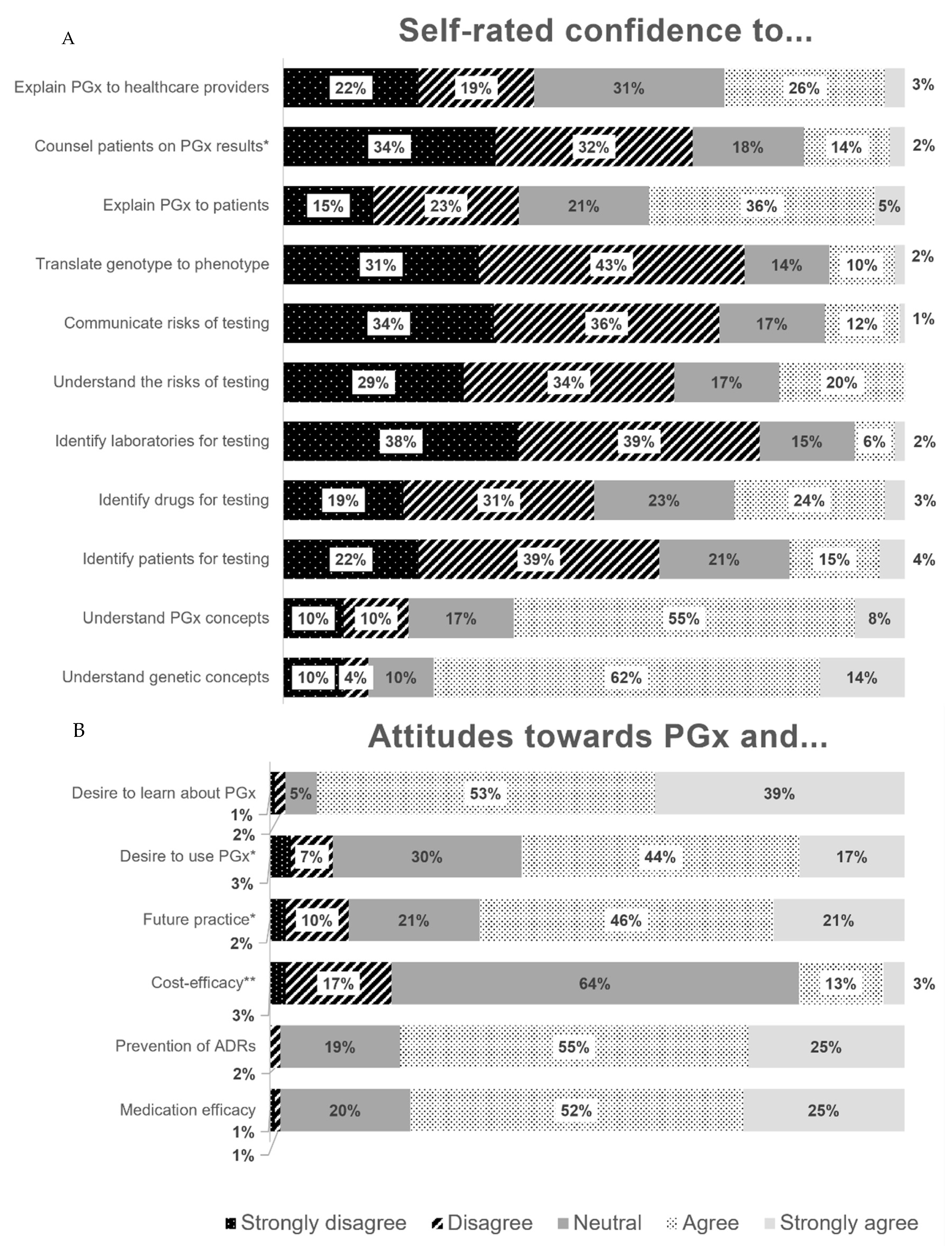
3.3. Knowledge of Pharmacogenomics
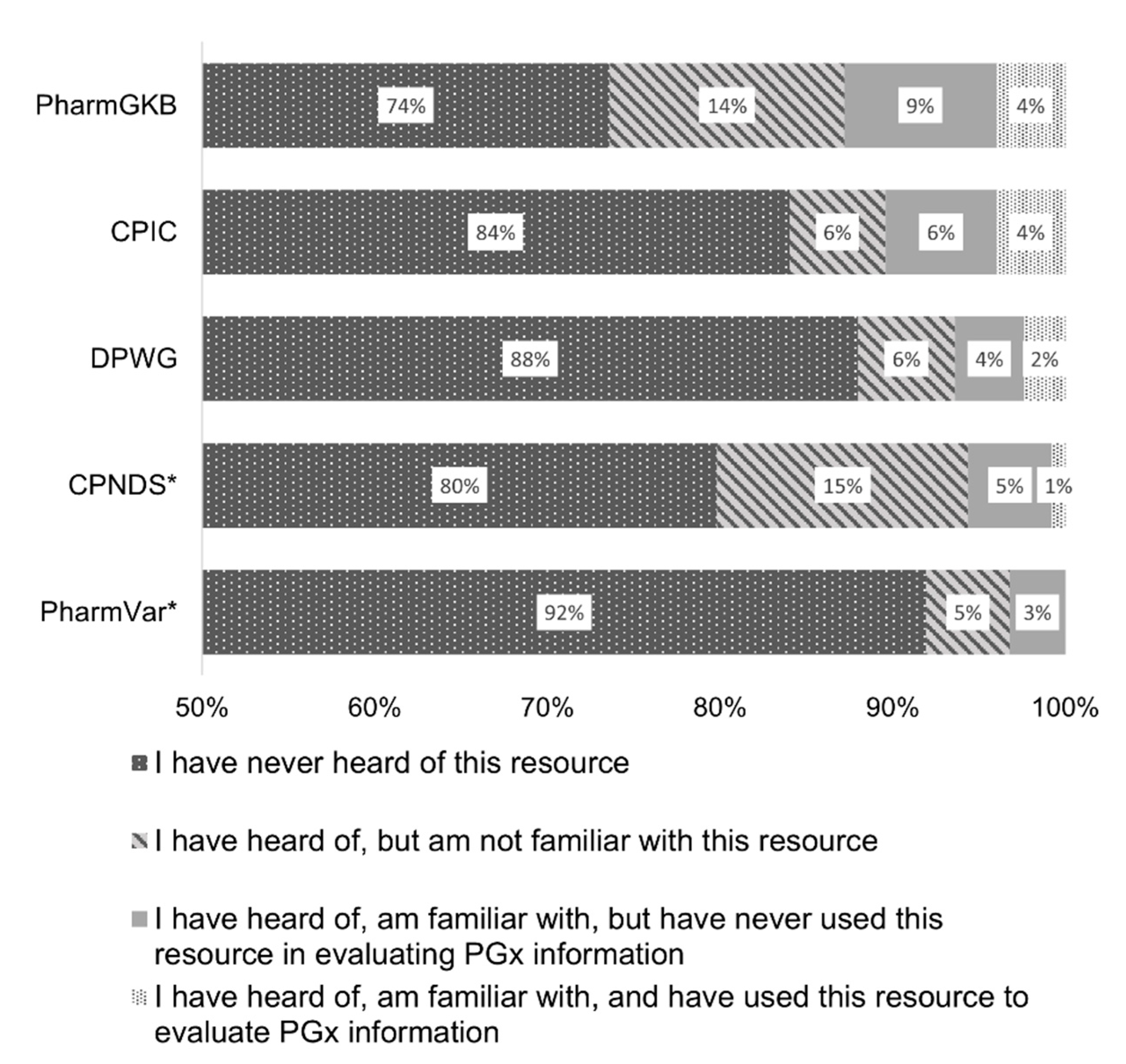
3.3.1. Resource Knowledge
3.3.2. Applying Knowledge
3.3.3. Obtaining Knowledge
3.4. Perceptions of Pharmacogenomics
Clinical Utility
3.5. Barriers to Implementation
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, W.E.; McLeod, H.L. Pharmacogenomics—Drug Disposition, Drug Targets, and Side Effects. N. Engl. J. Med. 2003, 348, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Relling, M.V.; Klein, T.E.; Gammal, R.S.; Whirl-Carrillo, M.; Hoffman, J.M.; Caudle, K.E. The Clinical Pharmacogenetics Implementation Consortium: 10 Years Later. Clin. Pharmacol. Ther. 2020, 107, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Swen, J.J.; Nijenhuis, M.; de Boer, A.; Grandia, L.; Maitland-van der Zee, A.H.; Mulder, H.; Rongen, G.A.P.J.M.; Van Schaik, R.H.N.; Schalekamp, T.; Touw, D.J.; et al. Pharmacogenetics: From Bench to Byte—An Update of Guidelines. Clin. Pharmacol. Ther. 2011, 89, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Table of Pharmacogenomic Biomarkers in Drug Labeling: The United States Food and Drug Administration 2020. Available online: https://www.fda.gov/drugs/science-and-research-drugs/table-pharmacogenomic-biomarkers-drug-labeling (accessed on 11 March 2021).
- Clinical Pharmacogenetics Implementation Consortium (CPIC). Implementation. 2022. Available online: https://cpicpgx.org/implementation/ (accessed on 2 August 2022).
- Hayashi, M.; Hamdy, D.A.; Mahmoud, S.H. Applications for pharmacogenomics in pharmacy practice: A scoping review. Res. Soc. Adm. Pharm. 2021, 18, 3094–3118. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Nagy, M.; Lynch, M.; Kamal, S.; Mohamed, S.; Hadad, A.; Abouelnaga, S.; Aquilante, C.L. Assessment of healthcare professionals’ knowledge, attitudes, and perceived challenges of clinical pharmacogenetic testing in Egypt. Pers. Med. 2020, 17, 251–260. [Google Scholar] [CrossRef]
- Edris, A.; Vanoverschelde, A.; Bushaj, P.; Van Nieuwerburgh, F.; Lahousse, L. Pharmacogenetics in clinical practice: Current level of knowledge among Flemish physicians and pharmacists. Pharmacogenom. J. 2021, 21, 78–84. [Google Scholar] [CrossRef]
- Amara, N.; Blouin-Bougie, J.; Bouthillier, D.; Simard, J. On the readiness of physicians for pharmacogenomics testing: An empirical assessment. Pharmacogenom. J. 2018, 18, 308–318. [Google Scholar] [CrossRef]
- Hayashi, M.; Mahmoud, S.H.; Hamdy, D.A. The Efficacy of a Didactic and Case-Based Pharmacogenomics Education Program on Improving the Knowledge and Confidence of Alberta Pharmacists. Pharmgenomics Pers Med. 2022, 15, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Nowlan, S.-E.; MacKinnon, N.J.; Hincapie, A.; Tachuk, M. A survey of Alberta pharmacists’ attitudes, comfort and perceived barriers to a community-based naloxone program. Can. Pharm. J. 2021, 154, 262–270. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, T.; Kirk, J.; Duff, E.; Golonka, R. A survey of nurse practitioner controlled drugs and substances prescribing in three Canadian provinces. J. Clin. Nurs. 2019, 28, 4342–4356. [Google Scholar] [CrossRef] [PubMed]
- Luzum, J.; Pakyz, R.E.; Elsey, A.R.; Haidar, C.E.; Peterson, J.F.; Whirl-Carrillo, M.; Handelman, S.; Palmer, K.; Pulley, J.M.; Beller, M.; et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: Outcomes and Metrics of Pharmacogenetic Implementations Across Diverse Healthcare Systems. Clin. Pharmacol. Ther. 2017, 102, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Blagec, K.; Swen, J.J.; Koopmann, R.; Cheung, K.-C.; van Rhenen, M.C.; Holsappel, I.; Konta, L.; Ott, S.; Steinberger, D.; Xu, H.; et al. Pharmacogenomics decision support in the U-PGx project: Results and advice from clinical implementation across seven European countries. PLoS ONE 2022, 17, e0268534. [Google Scholar] [CrossRef] [PubMed]
- Rosswurm, M.A.; Larrabee, J.H. A Model for Change to Evidence-Based Practice. Image J. Nurs. Scholarsh. 1999, 31, 317–322. [Google Scholar] [CrossRef]
- Volpi, S.; Bult, C.J.; Chisholm, R.L.; Deverka, P.A.; Ginsburg, G.S.; Jacob, H.J.; Kasapi, M.; McLeod, H.L.; Roden, D.M.; Williams, M.S.; et al. Research Directions in the Clinical Implementation of Pharmacogenomics: An Overview of US Programs and Projects. Clin. Pharmacol. Ther. 2018, 103, 778–786. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Kim, H.-S.K.; Oh, M.; Shin, J.-G. Survey of physicians’ views on the clinical implementation of pharmacogenomics-based personalized therapy. Transl. Clin. Pharmacol. 2020, 28, 34. [Google Scholar] [CrossRef]
- Meloche, M.; Kwon, H.J.; Letarte, N.; Bussières, J.-F.; Vadnais, B.; Hurlimann, T.; Lavoie, A.; Beauchesne, M.-F.; de Denus, S. Opinion, experience and educational preferences concerning pharmacogenomics: An exploratory study of Quebec pharmacists. Pharmacogenomics 2020, 21, 235–245. [Google Scholar] [CrossRef]
- Johansen Taber, K.; Dickinson, B. Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties. Pharmacogenom. Pers. Med. 2014, 145, 145–162. [Google Scholar] [CrossRef]
- Chang, J.S.; Pham, D.-A.; Dang, M.T.; Lu, Y.; VanOsdol, S.; Shin, J. Evaluation of popular drug information resources on clinically useful and actionable pharmacogenomic information. J. Med. Libr. Assoc. 2016, 104, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Vitek, C.R.R.; Abul-Husn, N.S.; Connolly, J.J.; Hartzler, A.L.; Kitchner, T.; Peterson, J.F.; Rasmussen, L.V.; E Smith, M.; Stallings, S.; Williams, M.S.; et al. Healthcare provider education to support integration of pharmacogenomics in practice: The eMERGE Network experience. Pharmacogenomics 2017, 18, 1013–1025. [Google Scholar] [CrossRef]
- Caraballo, P.J.; Hodge, L.S.; Bielinski, S.J.; Stewart, A.K.; Farrugia, G.; Schultz, C.G.; Rohrer-Vitek, C.R.; Olson, J.E.; Sauver, J.L.S.; Roger, V.L.; et al. Multidisciplinary model to implement pharmacogenomics at the point of care. Genet. Med. 2017, 19, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Jessel, C.D.; Al Maruf, A.; Oomen, A.; Arnold, P.D.; Bousman, C.A. Pharmacogenetic Testing Knowledge and Attitudes among Pediatric Psychiatrists and Pediatricians in Alberta, Canada. J. Can. Acad. Child Adolesc. Psychiatry 2022, 31, 18–27. [Google Scholar] [PubMed]
| Characteristic | n | (%) | |
|---|---|---|---|
| Profession | |||
| Pharmacist | 91 | (67.9) | |
| Nurse Practitioner | 37 | (27.6) | |
| Physician | 6 | (4.5) | |
| Years in Practice * | |||
| Less than 2 years | 8 | (6.0) | |
| 2–5 years | 20 | (15.0) | |
| 6–10 years | 26 | (19.6) | |
| 11–15 years | 18 | (13.5) | |
| 16–20 years | 19 | (14.3) | |
| More than 20 years | 42 | (31.6) | |
| Location of Practice (Number of inhabitants) † | |||
| Rural (0–50,000) or Locum | 13 | (9.8) | |
| Suburban (50,001–250,000) | 25 | (18.9) | |
| Urban (greater than 250,000) | 94 | (71.2) | |
| Specialty † | |||
| None/general medicine | 25 | (18.9) | |
| Pediatric/neonatal medicine | 17 | (12.9) | |
| Oncology | 17 | (12.9) | |
| Adult intensive care or emergency medicine | 17 | (12.9) | |
| Psychiatry | 10 | (7.5) | |
| Cardiology and stroke | 10 | (7.6) | |
| Geriatrics | 9 | (6.8) | |
| Infectious diseases | 7 | (5.3) | |
| Pain and palliative care | 7 | (5.3) | |
| Other ‡ | 14 | (9.8) | |
| Therapeutic | % Selected * (n = 114) | % Selected as Top Therapeutic Area (n = 116) |
|---|---|---|
| Oncology | 62.3 | 35.3 |
| Cardiovascular/stroke/vascular surgery | 59.7 | 7.8 |
| Psychiatry | 56.1 | 19.0 |
| Endocrinology/diabetes | 46.5 | 4.3 |
| Infectious diseases | 34.2 | 0 |
| Geriatrics | 34.2 | 2.6 |
| No specialty/general practice | 33.3 | 19.5 |
| Pediatrics | 29.0 | 4.3 |
| Nephrology | 28.1 | 0 |
| Gastroenterology | 27.2 | 0 |
| Critical care | 27.2 | 0.9 |
| Respiratory medicine | 25.4 | 0 |
| Pain management and palliative care | 19.3 | 0 |
| Emergency medicine | 9.7 | 0 |
| Other ** | 4.4 | n/a |
| Outpatient medicine | 0 | 0.9 |
| Unsure/unable to say | n/a | 5.2 |
| Profession | Component of Patient Care with PGx n (%) | ||
|---|---|---|---|
| Pre-Test Education | Test Interpretation | Post-Test Education and Follow-Up | |
| Physician | 17 (14.4) | 25 (21.6) | 21 (18.0) |
| Pharmacist | 44 (37.3) | 49 (42.2) | 48 (41.0) |
| Nurse practitioner | 8 (6.8) | 5 (4.3) | 8 (6.8) |
| Registered nurse | 2 (1.7) | 0 (0) | 3 (2.6) |
| Genetic counsellor | 33 (28.0) | 27 (23.3) | 23 (19.7) |
| More than one profession selected / those with knowledge and training regardless of profession | 10 (8.5) | 7 (6.0) | 9 (7.7) |
| Unsure / it depends | 3 (2.5) | 2 (1.7) | 4 (3.4) |
| None of the above | 1 (0.9) | 1 (0.9) | 1 (0.9) |
| Total responses | 118 | 116 | 117 |
| Barrier | % Selected * | % Selected as Top Barrier |
|---|---|---|
| Lack of health care providers knowledge | 87.2 | 41.9 |
| Cost / funding / reimbursement | 81.2 | 29.1 |
| Lack of guidelines / quality evidence | 70.1 | 11.1 |
| Lack of testing equipment | 58.1 | 2.6 |
| Lack of time to provide service | 46.2 | 3.4 |
| Delay in test results | 40.2 | 3.4 |
| Lack of electronic medical record integration | 38.5 | 2.6 |
| Ethical concerns | 29.1 | 2.6 |
| Legal concerns | 28.2 | 0.9 |
| Lack of patient acceptance | 15.4 | 0.9 |
| Social concerns | 12.8 | 0 |
| Unsure | 1.7 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, M.; Bousman, C.A. Experience, Knowledge, and Perceptions of Pharmacogenomics among Pharmacists and Nurse Practitioners in Alberta Hospitals. Pharmacy 2022, 10, 139. https://doi.org/10.3390/pharmacy10060139
Hayashi M, Bousman CA. Experience, Knowledge, and Perceptions of Pharmacogenomics among Pharmacists and Nurse Practitioners in Alberta Hospitals. Pharmacy. 2022; 10(6):139. https://doi.org/10.3390/pharmacy10060139
Chicago/Turabian StyleHayashi, Meagan, and Chad A. Bousman. 2022. "Experience, Knowledge, and Perceptions of Pharmacogenomics among Pharmacists and Nurse Practitioners in Alberta Hospitals" Pharmacy 10, no. 6: 139. https://doi.org/10.3390/pharmacy10060139
APA StyleHayashi, M., & Bousman, C. A. (2022). Experience, Knowledge, and Perceptions of Pharmacogenomics among Pharmacists and Nurse Practitioners in Alberta Hospitals. Pharmacy, 10(6), 139. https://doi.org/10.3390/pharmacy10060139






