Abstract
This study explored how a sample of Australian pharmacists would convey information about the side effects of a medicine, if they were to counsel a patient. A qualitative method was selected and written responses to a case-based scenario were analysed using inductive thematic analysis. The grounded theory approach elicited a fluid and dynamic model for side effect counselling. The study identified strategies for counselling, such as encouraging adherence through emphasising the benefits of the medication, referral to the prescriber, and providing empathy and reassurance to ease anxiety and address concerns. Pharmacists acknowledged the potential for risk, although only a minority used numerical descriptors. The final themes or outcomes were that pharmacists aim to allay fears, minimise harm and promote medication use when counselling about side effects. Professional empathy, the acknowledgment of patient concerns, and the importance of providing tailored information to promote medication adherence, emerged as features of the quality use of medicines. This study contributes to existing literature by identifying the role of allaying patients’ fears when conveying side effect information. It also describes a process to convey tailored information. Implications for practice include the importance of effective use of communication strategies to encourage adherence, as the appropriate use of medication can lead to positive health outcomes.
1. Introduction
All medicines have the potential to cause side effects, and patients wish to be informed of possible side effects no matter how rare [1]. However, patients are often unsatisfied with the side effect information provided by health care professionals [2,3,4], and report frustration at receiving inadequate information [4,5]. Without sufficient knowledge of drugs and the risks associated with side effects, patients will continually adjust how they take their medicines [6,7]. It has also been suggested that patients feel more capable of making informed treatment decisions when side effects are discussed [4]. Knowledge of side effects correlates with better adherence [8] and patients who are satisfied with medication information are more likely to be adherent [2].
Despite patients’ preference for side-effect information, and the established financial and health consequences of non-adherence, studies have shown that health providers are reluctant to discuss side effects with patients [4,9,10]. A large primary health care study in the United Kingdom reported that there had been no mention of side effects, risks, or precautions between General Practitioners (GPs) and their patients in more than two-thirds (67.5%) of consultations involving new drugs [11], and similar findings were reported from the United States [5]. The reasons for GPs not discussing side effects are varied, and include time constraints [4,5,9], other topics that are perceived to be more important [12,13], fears that information may reduce adherence, and reliance on pharmacists to supplement or provide information [5,9]. It is therefore imperative that pharmacists have the skills to communicate risk effectively, and adequately address side-effect-related concerns when discussing medicines with patients.
In their need to “make sense” of medication-taking, patients will actively seek information, with studies showing that pharmacists are considered as reliable sources of drug information [6]. For example, among users of antidepressants, 92% of patients chose pharmacists as their first or second preference for verbal information about their medicines [14], while among those taking antiepileptic medicines, 76% wanted their pharmacist to be more involved in discussing side effects [15].
Pharmacists worldwide are expected to provide advice on medicines [16], and in Australia, they are required to “assist consumer understanding and adherence”; this is an element of the Competency Standard “Dispense Prescribed Medicines” [17]. Excellent prescribing practice is of questionable benefit if patients feel that the risk of side effects outweighs the benefits of the medication [18]. Patient education, also referred to as patient counselling, remains poorly defined [19] with a lack of research into the process [20]. “Patient counselling” is a term used by the pharmacy profession and includes an advisory role about the use of medicines and it should be differentiated from the psychotherapeutic activity commonly associated with the term counselling [19].
Dyck et al. explored how pharmacists counsel on medication side effects by qualitatively examining how pharmacists framed information about side effects, with the authors concluding that safety was the pharmacists’ major concern [20]. They also noted that pharmacists preferred to use qualitative descriptors of side effect risk, such as “may experience” and “might occur”. These authors recommended that further studies should investigate how to convey information without causing undue alarm. This formed the basis for the current exploratory study to investigate strategies pharmacists would use to communicate information about side effects to patients. In order to better understand how pharmacists would counsel on side effects, we selected a qualitative approach to explore how pharmacists would counsel a (hypothetical) patient about two side effects of a commonly prescribed drug, and the descriptors they would use to convey side effect risk.
2. Experimental Section
2.1. Methodology
Exploratory methodology was selected as more studies using qualitative methods to investigate pharmacist communication are necessary [21]. The inductive nature of analysis allows researchers to gain insights and generate explanatory theoretical frameworks [22].
A case-based scenario was designed, and pharmacists described in writing how they would describe side effects to a hypothetical patient. Providing written responses was expected to encourage participation and hence the generation of data as pharmacists could respond at a convenient time.
The fictitious scenario (see Appendix) described a concerned patient asking the pharmacist about diarrhoea and headache, two side effects that are often associated with atorvastatin. The drug is frequently prescribed in Australia [23] and the two side effects are attributable to a range of medicines, thereby expanding the context beyond that of a single drug [24,25]. The numerical probability of each side effect was also included in the scenario. It was piloted to ensure face and content validity, and the final version was distributed to a sample of community and hospital pharmacists.
2.2. Data Collection and Participants
Data collection occurred in two stages among community and hospital pharmacists. In Australia, community pharmacists play a pivotal role in the supply of medicines, dispensing prescription and pharmacist-only medicines, recommending Over-The-Counter (OTC) and Complementary and Alternative Medicines (CAMs), and providing professional services [26]. Hospital pharmacists provide a range of dispensing, clinical and patient education services [27], while an Accredited Pharmacist provides medication review services and may be employed in either sector [28].
From a database developed in Brisbane, Australia, and containing contact details of approximately 900 pharmacists who were preceptors for undergraduate pharmacy students, a random sample of community pharmacists (n = 200) was invited to participate. Letters of invitation were mailed between June and August 2010. The second stage occurred early in 2011 among hospital pharmacists and the scenario was distributed via email to members of the Society of Hospital Pharmacists of Australia (n = 2,865). Demographic details of participants included gender, age, and years since graduation as a pharmacist. Ethical approval for both stages was obtained from the Human Research Ethics Committee of the School of Pharmacy at the University of Queensland.
2.3. Data Analysis
Raw data were analysed using inductive thematic analysis and a constructivist grounded theory approach facilitated the emergence of the core category [29]. Written responses from community pharmacists were copied word for word into a Microsoft Word© document and examined. This microanalysis (open coding) identified codes which were arranged into categories during the subsequent process of axial coding. Preliminary analysis was undertaken by two researchers (Thanh Huynh and Damir Krehula and secondary coding by Therése Kairuz. Sensitising questions (as described by Corbin and Strauss) were used by Damir Krehula to allow increased insight into the data [29]; for example: What is the problem being addressed? Why has the pharmacist chosen to say this? What is the pharmacist attempting to convey? When no new codes could be discovered during subsequent analysis, the categories and themes were considered to be appropriately dense.
The sensitising questions assisted in the conceptualisation of more abstract concepts during axial coding. Codes were continuously sorted in terms of their properties and those sharing mutual properties were organised into categories. This process facilitated an inquiry process and concepts were compared in terms of what and why something was said. Extensive memos and field notes assisted with the refinement of ideas. Axial coding was reviewed by Therése Kairuz and differences that emerged were discussed and revised until consensus was reached.
Data from hospital pharmacists were analysed in a similar manner and revealed no new properties or codes; it was established that data saturation had been achieved [30]. Relationships between concepts were continually proposed, ideas were diagrammatically represented, and a theoretical framework began to develop. To ensure its validity and consistency, the developing framework was reviewed for quality through consideration of its fit, logic, relevance, and depth [21]. Hypothesised relationships were verified by Kim Bellamy, and theoretical comparisons were used to ensure theory cohesion. During this process the underlying goal, or core category, emerged.
3. Results and Discussion
3.1. Respondents
There were 35 participating pharmacists, 24 from community pharmacies and 11 from the hospital sector. Participant demographics reflected the general trend of gender among pharmacists in Australia (female > male) and age, as about two-thirds of registered pharmacists are less than 45 years old [31]. There were no discernible trends about conveying side effect information when related to pharmacist age, gender or experience.
There was a total of 63 different responses, 35 regarding diarrhoea and 28 regarding headache; 7 responses were identical and were only analysed once. The majority of respondents were female (n = 22), and there were slightly more young respondents (n = 18), aged between 25 and 44 years. Experience as a pharmacist since graduation ranged from 3 to 41 years, and the majority of pharmacists (13/35) had 5 to 14 years experience (Table 1).

Table 1.
Participant demographics (n = 35).
| Participants | (n) | |
|---|---|---|
| Gender | Female | 22 |
| Male | 11 | |
| Missing data | 2 | |
| Age group (years) | 25–34 | 12 |
| 35–44 | 6 | |
| 45–54 | 8 | |
| 55–64 | 6 | |
| Missing data | 3 | |
| Experience (years) | 5–14 | 13 |
| 15–24 | 8 | |
| 25–34 | 4 | |
| 35–44 | 7 | |
| Missing data | 3 |
3.2. Themes
Preliminary analysis identified three codes related to conveying side effect information for both diarrhoea and headache: probability (or likelihood), duration, and severity. The other eight codes included advice about medication, benefits of medication, referral to a General Practitioner (GP), and empathy and reassurance. During secondary analysis, these 11 codes were condensed to five categories: discussion of side effects, management of side effects, GP referral, easing anxiety and encouraging medication use. Codes and categories were considered to be strategies that pharmacists would use to convey side effect information. From the data, three themes emerged: allay fears, minimize harm and promote medication use (Table 2). The themes reflect the outcomes of effectively conveying side effect information.

Table 2.
The coding process of the data.
| Raw data: Examples of quotes | Code | Category | Theme |
|---|---|---|---|
| “Approximately 1 in 100 people who take this medicine will experience diarrhoea. Usually it is not debilitating nor does it last very long.” | Side Effect Probability Side Effect Severity Side Effect Duration | Discussion of Side Effect(s) | Allay fears |
| “CMIs* can scare the patients at times but…” | Empathy | Easing Anxiety | |
| “Hopefully this adverse reaction would not be a problem for you.” | Reassurance | ||
| “Speak with your doctor to see if a different lipid lowering drug would suit you.” | Referral, to encourage use of medication | GP Referral | Minimise harm |
| “If the headache continues for more than a few days, you need to see your GP.” | Referral, to ensure well-being | ||
| “If you get a headache, you can take Panadol.” | Side effect advice | Side Effect Management | |
| “If you experience diarrhoea, ensure you maintain your fluid intake.” | Lifestyle Advice | ||
| “Good cholesterol control reduces your risk of having a heart attack or stroke.” | Medication Benefits | Encourage Use of Medication | Promote medication use |
| “I would suggest starting the medication, giving it a 2 week trial and then…” | Medication Advice |
* CMI is Consumer Medicines Information, which is available to consumers for medicines registered in Australia.
3.3. Descriptors and Strategies
Descriptors of risk were used by approximately one-third of respondents. The frequency of side effects was described in simple terms as “transient” and “doesn’t last very long”. Similarly, descriptors of probability were also vague, such as “uncommon” and “unlikely”. A minority of responses included precise descriptors such as “1 in 100”. In the following quote, a precise description of probability is followed by reassurance: “Approximately 1 in 100 people who take this medicine will experience diarrhoea. Usually it is not debilitating nor does it last very long” (Hospital 5).
There were a variety of strategies to promote medication use. One strategy was referral to a doctor: “If you experience side effects when you take this medication, speak with your doctor to see if a different lipid-lowering drug would suit you” (Community 24). In some instances, medication use was explicitly encouraged through personal recommendations: “I would suggest starting the medication, giving it a 2 week trial and then…” (Community 22). Strategies to allay anxiety included providing reassurance and demonstrating empathy; this usually occurred early in the process: “CMIs can scare the patients at times… sometimes the list can be quite overwhelming”(Community 4.) A few pharmacists (n = 8) included the benefits of treatment: “….shown to reduce the risk of strokes and heart attacks” (Community 22) and “Good cholesterol control reduces your risk of having a heart attack or stroke” (Hospital 8).
The majority of respondents (94%) incorporated strategies to reduce medication-related anxiety, using reassuring phrases and drawing on experience to address concerns: “I haven’t heard of people having a problem with it…” (Community 7). Most responses included a combination of strategies, such as allaying fears whilst minimising harm. When comparing responses for the two side effects, there was variation in how the strategies were used or combined (Table 3).
3.4. Emergence of a Theoretical Model for Counselling about Side Effects
Pharmacists would generally initiate patient counselling by allaying fears, followed by promoting medication use or by discussing ways to minimise harm. Each of these themes contributed to a “core category” which was identified as the quality use of medicines. The Quality Use of Medicines (QUM) is a term used by the Australian government and includes the safe, judicious, appropriate and efficacious use of medicines [32]. Regardless of the sequence of themes, the process of side effect counselling appeared to be fluid and dynamic (Figure 1).

Table 3.
Strategies and strategy combinations for side effect counselling used by respondents for each side effect.
| Strategy | Strategy Combinations | Diarrhoea N = 34 | Headache N = 32 |
|---|---|---|---|
| Allay fears | ( single process) | 4 | 3 |
| Allay fears AND minimise harm | 11 | 15 | |
| Allay fears AND promote medication use | 3 | 2 | |
| Allay fears AND minimise harm AND promote medication use | 14 | 8 | |
| Minimise harm | (single process) | 1 | 1 |
| Promote medication use | ( single process) | 0 | 0 |
| Promote medication use AND minimise harm | 1 | 3 |
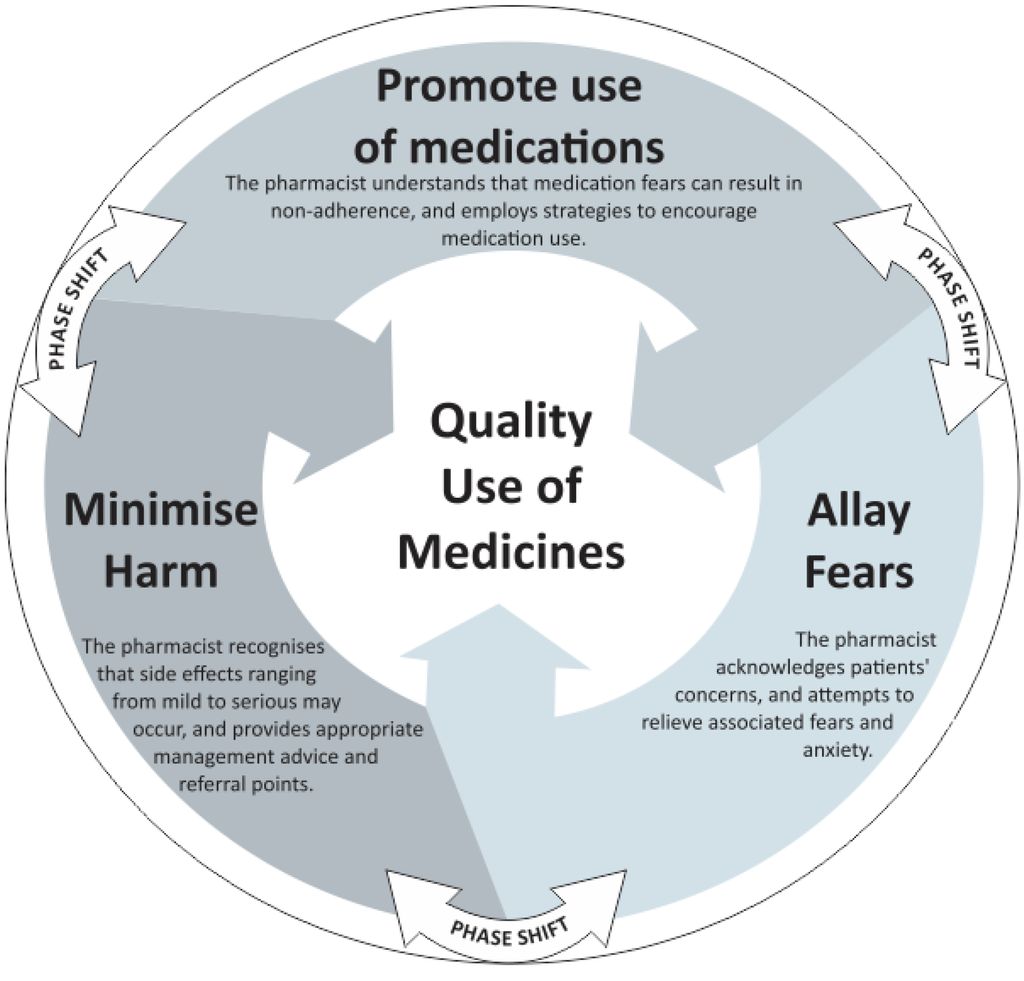
Figure 1.
Model of counselling about side effects.
3.5. Discussion of Findings
The systematic, reflective and comparative data analysis underpinned descriptive findings between hospital and community pharmacists. This added to the richness of the data and assisted with the densification and conceptualisation of categories and themes. Maximising differences between comparative groups permitted the researchers to maximise differences without focusing on them per se, creating a more powerful means for generating theory [30]. The main finding of this study is that the participants–hospital and community pharmacists–aim to allay patient fears in addition to minimising harm and promoting medication use, when conveying information about side effects, which formed an integral part of promoting the quality use of medicines.
Patient education and counselling about medicines is a core function of pharmacists, and the skills to identify patient concerns and address them appropriately are fundamental to professional practice. Skills include the provision of information and advice [21,33,34], emotional capacity [33,34,35] and the structuring of information to achieve a goal [36], especially when patients are unfamiliar with aspects of taking a medicine [19]. Qualitative research is interpretive and does not attempt to generalize; instead, it can generate a potential hypothesis. In this study, the fluid and dynamic process for conveying information to educate and counsel patients about the side effects of medicines is reflected in our model.
3.5.1. Allaying Fears
The core theme of “promoting the quality use of medicines” was grounded in allaying fears, promoting the use of medicines, and minimising harm, which underpin the safe and appropriate use of medicines. The core theme integrated with the process of conveying side effect information.
A fear of side effects contributes to medication non-adherence should patients intentionally decide against taking their medicines [37]. Our findings suggest that pharmacists perceived that medicines affect patients in different ways and show an appreciation of the multi-faceted factors affecting adherence. Side effect counselling would extend beyond the provision of information or advice; in the data, frequent use of the word “you” personalised the written responses and it was apparent that the well-being of the (hypothetical) patient in the scenario was pivotal for many pharmacists. Allaying fears is a unique finding in this study and it may reflect that respondents were influenced by a core objective of the National Medicines Policy in Australia, the Quality Use of Medicines (QUM), which is the safe, effective, judicious and efficacious use of medication [32]. It is suggested that the acceptance and implementation of national policies by health professions may have a beneficial and constructive effect on professional practice.
The term QUM may not be fully understood in countries where the term “rational use of medicines” is more commonly used. Potential misunderstandings may arise and it is recommended that terms related to pharmacy practice should be harmonised to facilitate the dissemination and implementation of findings and improve practice worldwide.
3.5.2. Descriptors of Side Effects
Our findings are similar to those of other studies regarding how information is conveyed about the probability, severity and/or duration associated with side effects. Descriptors were often vague [19] or described as “unlikely,” “mild” or “transient” [38,39]. This can be problematic as patients often overestimate risk when qualitative descriptors are used [40] which may adversely affect their willingness to take a medication [38]. The use of vague verbal descriptors could also be an avoidance of responsibility and may explain why some pharmacists in this study would refer the patient to a GP if a side effect was “bothersome” or caused patient “concern”. Hypothetical referrals of this nature were more frequent among community than hospital pharmacists, and may reflect a fear of professional liability. It is also possible that it is reflective of a more isolated professional environment, as community pharmacists often work alone compared to hospital pharmacists who work within teams. Pharmacists may deliberately use vague descriptors to address possible issues related to patient understanding, and may indicate a subconscious effort to address patient health literacy [40]. It has been reported that patients prefer numerical descriptors such as “0.001%” or “1 in 100” [41], which may allow for more accurate prediction of risk; however, an advantage of vague descriptors is that they are simple to use and to understand. We suggest that simple yet accurate descriptors of risks should be balanced with discussion about the benefits of the medication. Further research is needed to identify whether pharmacists require training in numeracy and how to convey numeric information to patients.
3.5.3. Strategies for Side Effect Counselling
The codes and categories identified during data analysis reflected strategies that could be used during side effect counselling, such as referral to a GP or describing the benefits of medication. The variation in the number and sequence of strategies in this study is reflected in the fluid nature of our model. Although the sequence and use of strategies varied, allaying fears was the strategy used by most pharmacists. It was pleasing that most respondents would minimise harm whilst allaying fears, demonstrating the professionalism expected of a pharmacist.
When empathy and reassurance were incorporated in the responses, they were often as platforms to provide information. Demonstrating empathy has been identified as a “pay-off” in the Four Habits Model of the medical interviewing process, and can enhance engagement with patients during consultations [42]. In addition to the use of strategies during the process of conveying side effect information, the inclusion of a simple question, such as “Do you feel that this medication may do you more harm than good?” may pave the way to discussing and allaying fears. The question is based on a statement from the Merck Adherence Estimator demonstrating the link between pharmacist communication and medication adherence [43].
Surprisingly, explanations were seldom included in responses. This may be reflective of the nature of data collection, or an authoritarian approach to providing information. It may also indicate a reluctance to engage with the patient or to take responsibility for patient care. The lack of explanations may also be the result of the non-interactive nature of data collection in this study. Although the written responses to a hypothetical patient may be considered theoretical, our approach avoided external influences which may affect pharmacist behaviour, such as those that affect participants during observed health professional and patient interactions (the Hawthorne effect).
3.5.4. Limitations
The use of a single drug and two common side effects and the small number of respondents may be considered limiting. However, although the data were small, they were rich, and line-by-line analysis provided deep insight. It is highly probable that an external factor affected our response rate; in early 2011 the state of Queensland experienced the worst floods in decades [44] and affected thousands of people; none-the-less, data saturation was reached. Our findings may have been influenced by, and be limited to, the Australian context in which the participating pharmacists practiced. The respondents who volunteered to participate may be considered “motivated” pharmacists and therefore our counselling model may not reflect the practice of all pharmacists.
4. Conclusions
This study contributes to existing literature on patient education by describing a process to convey side effect information and it identifies the pharmacist’s role in allaying patients’ fears. The core theme, the quality use of medicines, is a unique finding and may reflect the influence of a national policy on practice. Our counselling model reflects a process to convey side effect information that is dynamic yet fluid. The model incorporates a variety of strategies to allay fears, minimise harm and promote medication use. Recognition of the role of professional empathy, the acknowledgment of patient concerns, and the importance of providing tailored information to allay fears and promote medication adherence emerged as integral features of the quality use of medicines. Implications for practice include the importance of effective communication strategies to convey side effect information and encourage adherence; ultimately, the appropriate use of medication can lead to positive health outcomes.
Acknowledgments
The authors thank Dr Neil Cottrell for input and expertise in the early stages of this study, the participants for providing responses, the Society of Hospital Pharmacists of Australia for distributing the survey, and the University of Queensland for providing resources.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ziegler, D.; Mosier, M.; Buenaver, M.; Okuyemi, L. How Much Information About Adverse Effects of Medication Do Patients Want From Physicians? Arch. Intern. Med. 2001, 161, 706–713. [Google Scholar] [CrossRef]
- Bowskill, R.; Clatworthy, J.; Parham, R.; Rank, T.; Horne, R. Patients’ perceptions of information received about medication prescribed for bipolar disorder: implications for informed choice. J. Affect. Disord. 2007, 100, 253–257. [Google Scholar] [CrossRef]
- Enlund, H.; Vainio, K.; Wallenius, S.; Poston, J.W. Adverse drug effects and the need for drug information. Med. Care 1991, 29, 558–564. [Google Scholar] [CrossRef]
- Nair, K.; Dolovich, L.; Cassels, A.; McCormack, J.; Levine, M.; Gray, J.; Mann, K.; Burns, S. What patients want to know about their medications. Focus group study of patient and clinician perspectives. Can. Fam. Physician. 2002, 48, 104–110. [Google Scholar]
- Tarn, D.M.; Paterniti, D.A.; Williams, B.R.; Cipri, C.S.; Wenger, N.S. Which providers should communicate which critical information about a new medication? Patient, pharmacist, and physician perspectives. J. Am. Geriatr. Soc. 2009, 57, 462–469. [Google Scholar] [CrossRef]
- Bajcar, J. Task analysis of patients' medication-taking practice and the role of making sense: A grounded theory study. Res. Soc. Admin. Pharm. 2006, 2, 59–82. [Google Scholar] [CrossRef]
- Horne, R.; Weinman, J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J. Psychosom. Res. 1999, 47, 555–567. [Google Scholar] [CrossRef]
- Okuyan, B.; Sancar, M.; Izzettin, F.V. Assessment of medication knowledge and adherence among patients under oral chronic medication treatment in community pharmacy settings. Pharmacoepidem. Dr. S. 2013, 22, 209–214. [Google Scholar] [CrossRef]
- McGrath, J.M. Physicians’ perspectives on communicating prescription drug information. Qual. Health Res. 1999, 9, 731–745. [Google Scholar] [CrossRef]
- Schommer, J.; Wiederholt, J. Pharmacists’ views of patient counselling. Am. Pharm. 1994, NS34, 46–53. [Google Scholar]
- Makoul, G.; Arntson, P.; Schofield, T. Health promotion in primary care: physician-patient communication and decision making about precription medications. Soc. Sci. Med. 1995, 41, 1241–1254. [Google Scholar] [CrossRef]
- Berry, D.; Michas, I.; Gillie, T.; Forster, M. What do patients want to know about their medicines, and what do doctors want to tell them?: A comparative study. Psych. Health. 1997, 12, 467–480. [Google Scholar] [CrossRef]
- Mottram, D.R.; Reed, C. Comparative evaluation of patient information leaflets by pharmacists, doctors and the general public. J. Clin. Pharm. Ther. 1997, 22, 127–134. [Google Scholar] [CrossRef]
- Sleath, B.; Wurst, K. Patient receipt of, and preferences for receiving, antidepressant information. Int. J. Pharm. Pract. 2002, 10, 235–241. [Google Scholar] [CrossRef]
- McAuley, J.W.; Miller, M.A.; Klatte, E.; Shneker, B.F. Patients with epilepsy’s perception on community pharmacist’s current and potential role in their care. Epilepsy Behav. 2009, 14, 141–145. [Google Scholar] [CrossRef]
- Wiedenmayer, K.; Summers, R.; Mackie, C.; Gous, A.; Everard, M. Developing pharmacy practice: A focus on patient care. Available online: http://www.fip.org/files/fip/publications/DevelopingPharmacyPractice/DevelopingPharmacyPracticeEN.pdf. (accessed on 10 September 2013).
- Professional Practice Standards version 4: Standard 3: Counselling. Available online: http://www.psa.org.au/supporting-practice/professional-practice-standards/version-4 (accessed on 10 September 2013).
- Barnett, N. Developing your consultation skills to support medicines adherence. Clin. Pharm. 2012, 4, 266–268. [Google Scholar]
- Pilnick, A. “Patient counseling” by pharmacists: Four approaches to the delivery of counselling sequences and their interactional reception. Soc. Sci. Med. 2003, 56, 835–849. [Google Scholar] [CrossRef]
- Dyck, A.; Deschamps, M.; Taylor, J. Pharmacists’ discussions of medication side effects: A descriptive study. Patient Educ. Couns. 2005, 56, 21–27. [Google Scholar] [CrossRef]
- Shah, B.; Chewning, B. Conceptualising and measuring pharmacist-patient communications: A review of published studies. Res. Soc. Admin. Pharm. 2006, 2, 153–185. [Google Scholar] [CrossRef]
- Charmaz, K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis; Thousand Oaks: California, CA, USA, 2006. [Google Scholar]
- Australian Government Department of Health. PBS Expenditure and Prescriptions: Expenditure and Prescriptions twelve months to 30 June 2012. Available online: http://www.pbs.gov.au/info/statistics/expenditure-and-prescriptions-30-06-2012 (accessed on 21 November 2013).
- Ferrari, A. Headache: One of the most common and troublesome adverse reactions to drugs. Curr. Drug. Saf. 2006, 1, 43–58. [Google Scholar] [CrossRef]
- Triantafyllou, K.; Vlachogiannakos, J.; Ladas, S.D. Gastrointestinal and liver side effects of drugs in elderly patients. Best. Pract. Res. Cl. Ga. 2010, 24, 203–215. [Google Scholar] [CrossRef]
- Pharmacy Board of Australia. Available online: http://www.pharmacyboard.gov.au/Codes-Guidelines.aspx (accessed on 30 October 2013).
- The Society of Hospital Pharmacists of Australia. Available online: http://www.shpa.org.au/About (accessed on 30 October 2013).
- Australian Association of Consultant Pharmacy. Available online: http://www.aacp.com.au/ (accessed on 30 October 2013).
- Corbin, J. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory; Thousand Oaks: California, CA, USA, 2008. [Google Scholar]
- Glaser, B.G.; Strauss, A.L. The Discovery of Grounded Theory; Aldine Publishing Company: Chicago, IL, USA, 1967. [Google Scholar]
- Australian Health Practitioner Registration Authority. Pharmacy Board of Australia: Data tables. June 2012. Available online: http://www.ahpra.gov.au (accessed on 10 September 2013).
- Australian Government Deaprtment of Health. Quality Use of Medicines (QUM). Available online: http://www.health.gov.au/internet/main/publishing.nsf/Content/nmp-quality.htm (accessed on 30 October 2013).
- Hargie, O.; Morrow, N.; Woodman, C. Pharmacists’ evaluation of key communication skills in practice. Patient Educ. Couns. 2000, 39, 61–70. [Google Scholar] [CrossRef]
- Kimberlin, C.L. Communicating with patients: Skills assessment in US colleges of pharmacy. Am. J. Pharm. Educ. 2006, 70, 67. [Google Scholar] [CrossRef]
- Blom, L.; Wolters, M.; Hoor-Suykerbuyk, M.T.; van Paassen, J.; van Oyen, A. Pharmaceutical education in patient counselling: 20 h spread over 6 years? Patient Educ. Couns. 2011, 83, 465–471. [Google Scholar] [CrossRef]
- Kokkinn, B.; Stupans, I. Improving pharmacy counseling skills: an interdisciplinary model of support for students with English as an additional language. Int. J. Pharm. Pract. 2011, 19, 435–437. [Google Scholar] [CrossRef]
- Krueger, K.P.; Berger, B.A.; Felkey, B. Medication adherence and persistence: A comprehensive review. Adv. Ther. 2005, 22, 313–356. [Google Scholar] [CrossRef]
- Berry, D.C.; Raynor, D.K.; Knapp, P.; Bersellini, E. Patients’ understanding of risk associated with medication use: Impact of European Commission Guidelines and other risk scales. Drug Safety 2003, 26, 1–11. [Google Scholar] [CrossRef]
- Knapp, P.; Raynor, D.K.; Berry, D.C. Comparison of two methods of presenting risk information to patients about the side effects of medicines. Qual. Saf. Health Care 2004, 13, 176–180. [Google Scholar] [CrossRef]
- Berkman, N.D.; Sheridan, S.L.; Donahue, K.E.; Halpern, D.J.; Crotty, K. Low health literacy and health outcomes: An updated systematic review. Ann. Intern. Med. 2011, 155, 97–107. [Google Scholar] [CrossRef]
- Lipkus, I.M. Numeric, verbal, and visual formats of conveying health risks: Suggested best practices and future recommendations. Med. Decis. Making. 2007, 27, 696–713. [Google Scholar] [CrossRef]
- Frankel, R.M.; Stein, T. Getting the most out of the clinic encounter: The Four Habits Model. Perm. J. 1999, 3, 79–88. [Google Scholar]
- McHorney, C.A. The Adherence Estimator: A brief, proximal screener for patient propensity to adhere to prescription medications for chronic disease. Curr. Med. Res. Opin. 2009, 25, 215–238. [Google Scholar] [CrossRef]
- ABC News. Brisbane floods: before and after. Available online: http://www.abc.net.au/news/specials/qld-floods/ (accessed on 30 October 2013).
Appendix
Please read the information below about a customer coming to see you and asking questions regarding side effects. There is no right or wrong answer; it is the words that you would use to discuss and/or describe the side effects with the customer that is important.
You are a pharmacist currently working at a community pharmacy. A customer was recently prescribed “Atorvastatin 40mg” for her high cholesterol and received a Consumer Medicines Information (CMI) Leaflet. The medication was dispensed two days ago. She has read the CMI and is concerned about experiencing diarrhoea or headache because these are described as “common” side effects. She hands you the CMI which reads:
- Tell your doctor if you notice any of the following and if they worry you:
- Constipation, diarrhea
- Unusual tiredness or weakness
- Stomach or belly pain, nausea
- Headache
- Trouble sleeping
You know that diarrhoea occurs in >1% (AMH) of people who take atorvastatin (reported as 3.8% in eMIMS) and headache occurs in >1% (AMH) of people who take atorvastatin (reported as 2.5% in eMIMS). What are the words you would use to describe and/or discuss the side effects of (i) diarrhoea and (ii) headache with the customer?
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).