Prilling and Coating of Ammonium Dinitramide (ADN) Solid Green Propellant in Toluene Mixture Using Ultrasound Sonication
Abstract
:1. Introduction
2. Materials and Methods
2.1. ADN Synthesis
2.2. Melt Prilling and Coating of Pristine ADN by Conventional Method
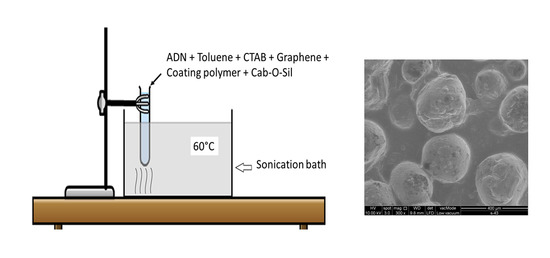
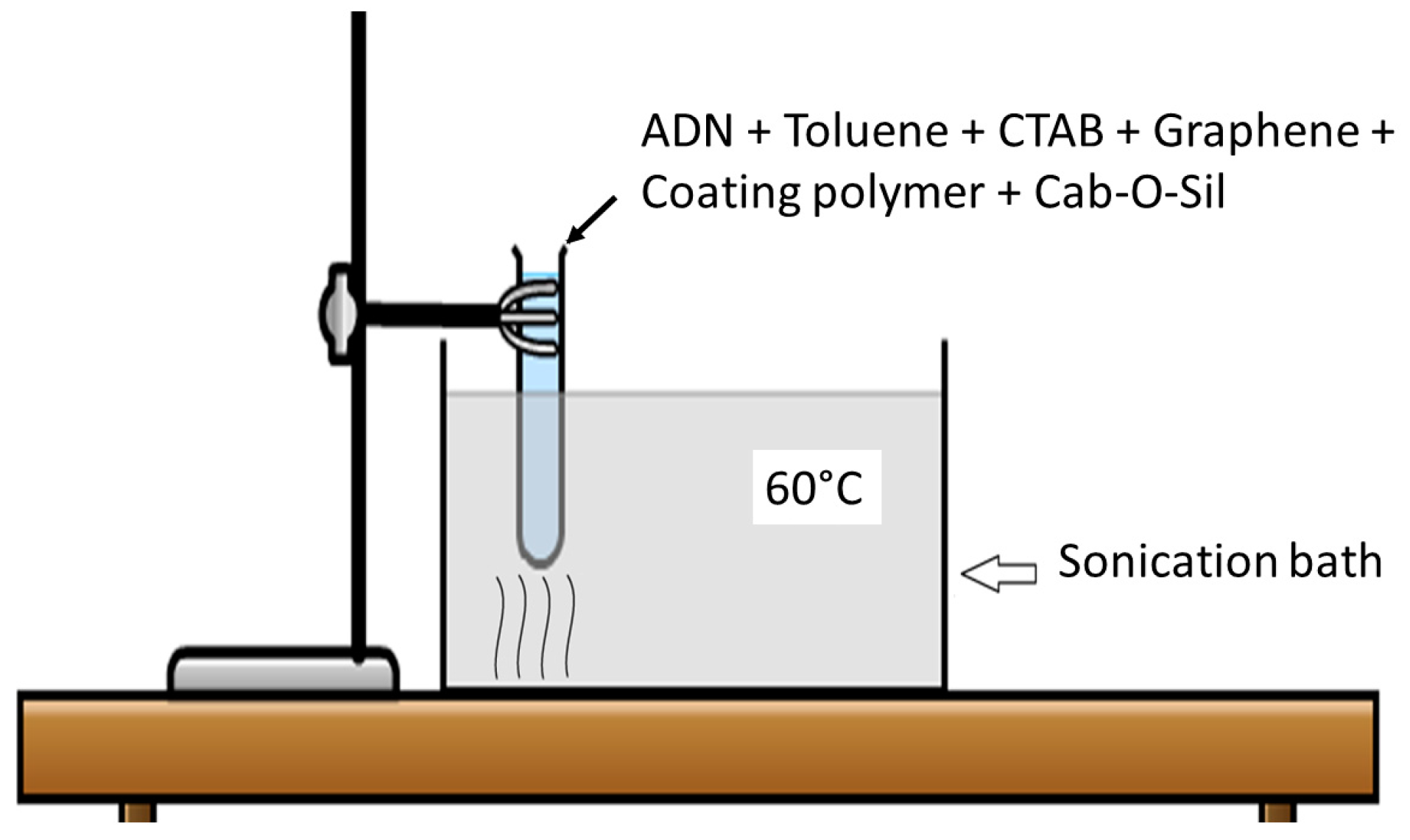
2.3. Prilling and PS Coating of Pristine ADN Particles by Ultrasound Sonication
2.4. Prilling and HTPB Coating of Pristine ADN Particles by Ultrasound Sonication
2.5. Morphology and Thermal Stability
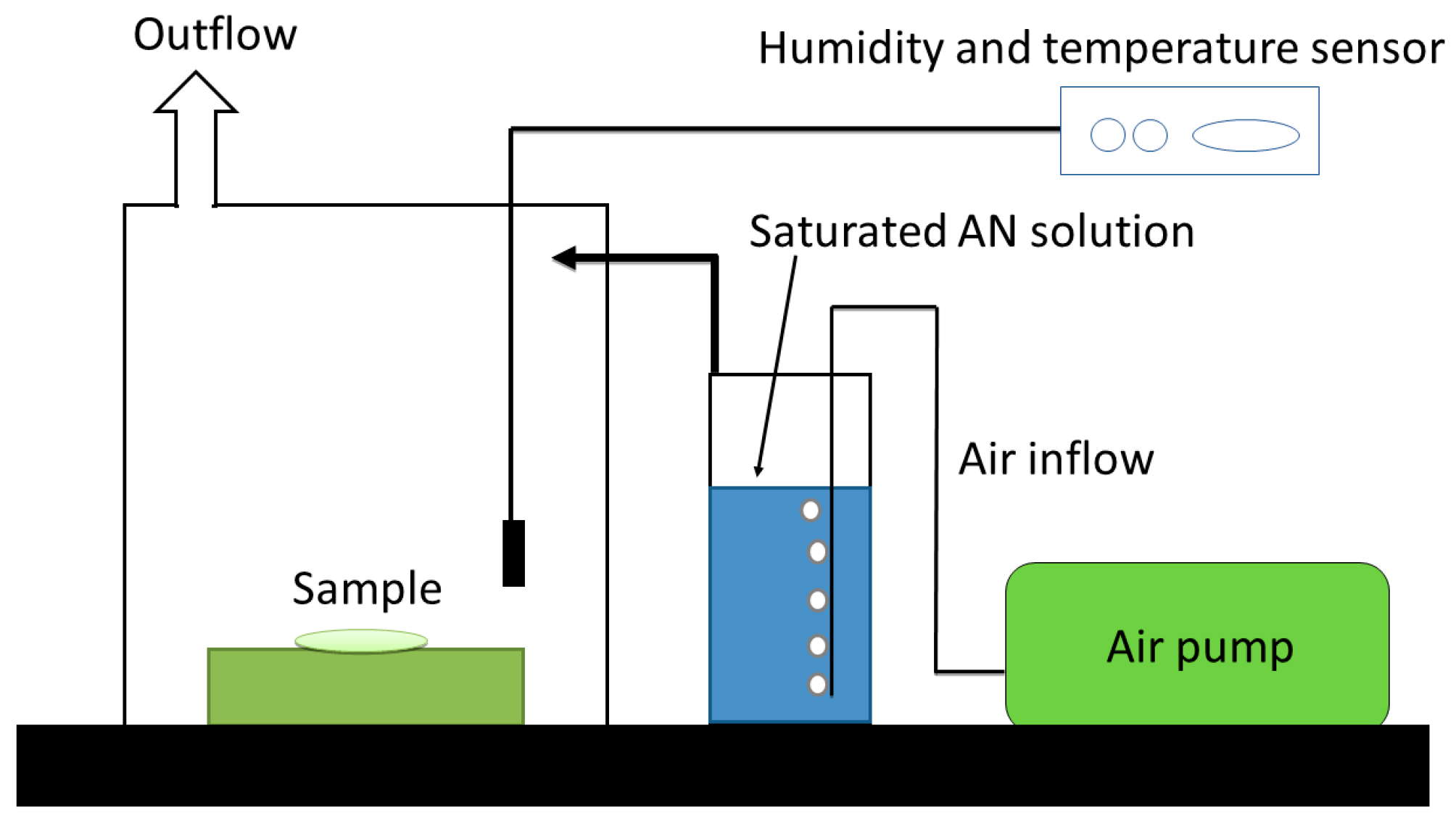
2.6. Water Absorption Test
3. Results and Discussion
3.1. Selection of Sonication Medium and Polymer
3.2. Crystal Morphology and Particle Size
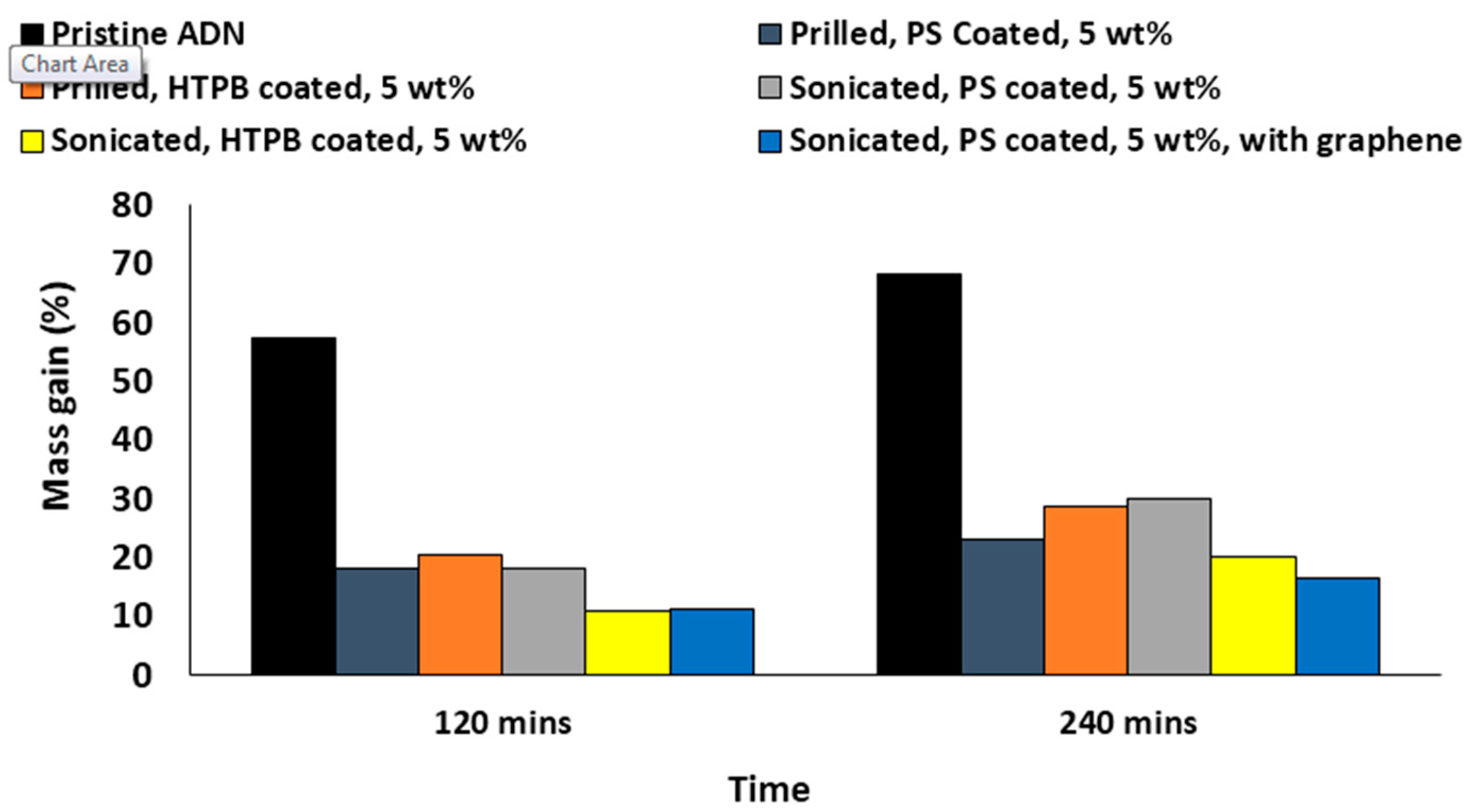
3.3. Water Absorption Testing
3.4. Effect of Additives
3.5. Thermal Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Luk’yanov, O.A.; Tartakovsky, V.A. Synthesis and Characterization of Dinitramidic Acid and Its Salts. In Solid Propellant Chemistry, Combustion, and Motor Interior Ballistics; Brill, T.B., Ren, W.-Z., Zarchan, P., Yang, V., Eds.; American Institute of Aeronautics and Astronautics: Washington, DC, USA, 2000; Volume 185, pp. 207–219, ISBN-13 978-1563474422. [Google Scholar]
- Nagamachi, M.Y.; de Oliveira, J.S.I.; Kawamoto, A.K.; de Dutra, R.L. AND—The new oxidizer around the corner for an environmentally friendly smokeless propellant. J. Aerosp. Technol. Manag. 2009, 1, 153–160. [Google Scholar] [CrossRef]
- Östmark, H.; Bemm, U.; Langlet, A.; Sanden, R.; Wingborg, N. The properties of ammonium dinitramide (ADN): Part 1, basic properties and spectroscopic data. J. Energ. Mater. 2000, 18, 123–138. [Google Scholar] [CrossRef]
- Cui, J.; Han, J.; Wang, J.; Huang, R. Study on the Crystal Structure and Hygroscopicity of Ammonium Dinitramide. J. Chem. Eng. Data 2010, 55, 3229–3234. [Google Scholar] [CrossRef]
- Ramaswamy, A. Energetic-material combustion experiments on propellant formulations containing prilled ammonium dinitramide. Combust. Explos. Shock Waves 2000, 36, 119–124. [Google Scholar] [CrossRef]
- Heintz, T.; Pontius, H.; Aniol, J.; Birke, C.; Leisinger, K.; Reinhard, W. Ammonium Dinitramide (ADN)—Prilling, Coating, and Characterization. Propellants Explos. Pyrotech. 2009, 34, 231–238. [Google Scholar] [CrossRef]
- Bayat, Y.; Zeynali, V. Preparation and Characterization of Nano-CL-20 Explosive. J. Energ. Mater. 2011, 4, 281–291. [Google Scholar] [CrossRef]
- Fuhr, F.I. Crystallization of the Energetic Oxidizer Salt Ammonium Dinitramide: Theoretical and Experimental Considerations. Ph.D. Thesis, Martin Luther University, Halle-Wittenberg, Germany, 2008. [Google Scholar]
- Teipel, U. Production Particles of Explosives. Propellants Explos. Pyrotech. 1999, 24, 134–139. [Google Scholar] [CrossRef]
- Hahma, A.; Edvinsson, H.; Östmark, H. The Properties of Ammonium Dinitramide(ADN): Part 2: Melt Casting. J. Energ. Mater. 2010, 28, 114–138. [Google Scholar] [CrossRef]
- Östmark, H.; Helte, A.; Karlsson, S.; Hahma, A.; Edvinsson, H. Detonation Properties and Reaction Rate Modeling of Melt Cast Ammonium Dinitramide (ADN). In Proceedings of the 12th International Detonation Symposium, San Diego, CA, USA, 11–16 August 2002; pp. 775–780. [Google Scholar]
- Larson, A.; Wingborg, N. Green Propellants Based on Ammonium Dinitramide (ADN). In Advances in Spacecraft Technologies; Hall, J., Ed.; Slavka Krautzeka: Rijeka, Croatia, 2011; ISBN 978-953-307-551-8. [Google Scholar]
- Kim, J.-W.; Shin, M.-S.; Kim, J.K.; Kim, H.S.; Koo, K.K. Evaporation Crystallization of RDX by Ultrasonic Spray. Ind. Eng. Chem. Res. 2011, 50, 12186–12193. [Google Scholar] [CrossRef]
- Kuppa, R.; Moholkar, V.S. Physical features of ultrasound-enhanced heterogeneous permanganate oxidation. Ultrason. Sonochem. 2010, 17, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Malani, R.S.; Moholkar, V.S.; Choudhury, H.A. Acid catalyzed biodiesel synthesis from Jatropha oil: Mechanistic aspectsof ultrasonic intensification. Chem. Eng. J. 2013, 31, 262–272. [Google Scholar] [CrossRef]
- Qadir, L.R.; Osburn-Atkinson, E.J.; Swider-Lyons, K.E.; Cepak, V.M.; Rolison, D.R. Sonochemically induced decomposition of energetic materials in aqueous media. Chemosphere 2003, 50, 1107–1114. [Google Scholar] [CrossRef]
- Wingborg, N. Ammonium Dinitramide-Water: Interaction and Properties. J. Chem. Eng. Data 2006, 51, 1582–1586. [Google Scholar] [CrossRef]
- Langlet, A.; Östmark, H.; Wingborg, N. Method of Preparing Dinitramidic Aicd and Salts Thereof. U.S. Patent 5,976,483, 8 August 1999. [Google Scholar]
- Kohga, M. From Cross-linking to Plasticization—Characterization of Glycerin/HTPB Blends. Propellants Explos. Pyrotech. 2009, 34, 436–443. [Google Scholar] [CrossRef]
- Wexler, A. Practical Laboratory Data. In CRC Handbook of Chemistry and Physics; Lide, D.R., Ed.; CRC Press: New York, NY, USA, 2003. [Google Scholar]
- Teipel, U.; Heintz, T. Surface Energy and Crystallization Phenomena of Ammonium Dinitramide. Propellants Explos. Pyrotech. 2005, 30, 404–411. [Google Scholar] [CrossRef]
- Agrawal, A. Surface Tension of Polymers. Available online: http://web.mit.edu/nnf/education/wettability/summerreading-2005short.pdf (accessed on 12 June 2017).
- Van Krevelen, D.W.; Nijenhuis, K.T. Properties of Polymers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780080548197. [Google Scholar]
- Aqra, F.; Ayyad, A. Surface energies of metals in both liquid and solid states. Appl. Surf. Sci. 2011, 257, 6372–6379. [Google Scholar] [CrossRef]
- ICI Americas Inc. The HLB System: A Time-Saving Guide to Emulsifier Selection. Available online: http://www.firp.ula.ve/archivos/historicos/76_Book_HLB_ICI.pdf (accessed on 12 June 2017).
- Wang, S.; Zhang, Y.; Abidi, N.; Cabrales, L. Wettabiity and Surface Free Energy of Graphene Films. Langmuir 2009, 25, 11078–11081. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Hsu, M.; Lu, H.; Lai, M.; Liu, P.; Hsu, C.; Ji, W.; Chuang, T.; Wei, Y.; Yeh, J.; et al. Room-temperature cured hydrophobic epoxy/graphene composites as corrosion inhibitorfor cold-rolled steel. Carbon 2014, 66, 144–153. [Google Scholar] [CrossRef]
- Luechinger, N.A.; Athanassiou, E.K.; Strak, W.J. Graphene-stabilized copper nanoparticles as an air-stable substitute for silver andgold in low-cost ink-jet printable electronics. Nanotechnology 2008, 19, 445201. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K.; Korgel, B.A. The importance of the CTAB surfactant on the colloidal seed-mediated synthesis of gold nanorods. Langmuir 2008, 24, 44–649. [Google Scholar] [CrossRef] [PubMed]
- Teipel, U.; Heintz, T.; Krause, H. Crystallization of Spherical Ammonium Dinitramide (ADN) particles. Propellants Explos. Pyrotech. 2000, 25, 81–85. [Google Scholar] [CrossRef]
- Sabourin, J.L.; Dabbs, D.M.; Yetter, R.A.; Dryer, F.L.; Aksay, I.A. Functionalized Graphene Sheet Colloids for Enhanced Fuel/Propellant Combustion. ACS Nano 2009, 3, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Thakre, P.; Yang, V. Thermal Decomposition and Combustion of Ammonium Dinitramide (review). Combust. Explos. Shock Waves 2005, 41, 657–679. [Google Scholar] [CrossRef]
- Matsunnaga, H.; Habu, H.; Atsumi, M. Thermal behavior of new oxidizer ammonium dinitramide. J. Therm. Anal. Calorim. 2012, 111, 1183–1188. [Google Scholar] [CrossRef]
- Jones, D.; Kwok, W.S.M.; Vachon, M.; Badeen, C.; Ridley, W. Characterization of ADN and ADN-Based Propellants. Propellants Explos. Pyrotech. 2005, 30, 140–147. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Wight, C.A. Ammonium Dinitramide: Kinetics and Mechanism of Thermal Decomposition. J. Phys. Chem. 1997, 101, 5653–5658. [Google Scholar] [CrossRef]
- Bottaro, J.C.; Penwell, E.P.; Schmitt, R.J. 1,1,3,4-Tetraoxo-1,2,3-triazapropene Anion, a New Oxy Anion of Nitrogen: The Dinitramide Anion and Its Salts. Am. Chem. Soc. 1997, 119, 9405–9410. [Google Scholar] [CrossRef]
- Tompa, A.S. Thermal analysis of ammonium dinitramide (ADN). Thermochim. Acta 2000, 357, 177–193. [Google Scholar] [CrossRef]





| Chemical Name | Function | Weight % |
|---|---|---|
| TDI | Crosslinker [19] | 10 |
| Glycerol | Catalyst | 10 |
| HTPB monomer | Monomer | 80 |
| Interface Components | Interface Energy |
|---|---|
| ADN-toluene | |
| ADN-PS | |
| ADN-graphene | |
| Toluene-PS | |
| Toluene-graphene | |
| Graphene-PS |
| Components | Work of Adhesion |
|---|---|
| ADN-PS | |
| ADN-graphene | |
| PS-graphene |
| Sample Type | Decomposition Onset Temperature (°C) | Enthalpy of Decomposition (kJ/g) | Maximum Peak Temperature (°C) |
|---|---|---|---|
| Pristine ADN | 177 | 1.86 | 193 |
| Prilled, PS, 5 wt % | 181 | 3.35 | 186 |
| Prilled, HTPB, 5 wt % | 170 | 2.19 | 190 |
| Sonicated, PS, 5 wt % | 171 | 1.81 | 192 |
| Sonicated, HTPB, 5 wt % | 178 | 1.78 | 181 |
| Sonicated, HTPB, 20 wt % | 196 | 2.62 | 198 |
| Pristine ADN [30] | 126 | 1.97 | 159 |
| Prilled ADN [37] | 168 | 2.01 | 195 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A.; Chin, J.; Cheah, K.H. Prilling and Coating of Ammonium Dinitramide (ADN) Solid Green Propellant in Toluene Mixture Using Ultrasound Sonication. Aerospace 2018, 5, 29. https://doi.org/10.3390/aerospace5010029
Rahman A, Chin J, Cheah KH. Prilling and Coating of Ammonium Dinitramide (ADN) Solid Green Propellant in Toluene Mixture Using Ultrasound Sonication. Aerospace. 2018; 5(1):29. https://doi.org/10.3390/aerospace5010029
Chicago/Turabian StyleRahman, Asad, Jitkai Chin, and Kean How Cheah. 2018. "Prilling and Coating of Ammonium Dinitramide (ADN) Solid Green Propellant in Toluene Mixture Using Ultrasound Sonication" Aerospace 5, no. 1: 29. https://doi.org/10.3390/aerospace5010029
APA StyleRahman, A., Chin, J., & Cheah, K. H. (2018). Prilling and Coating of Ammonium Dinitramide (ADN) Solid Green Propellant in Toluene Mixture Using Ultrasound Sonication. Aerospace, 5(1), 29. https://doi.org/10.3390/aerospace5010029