Research on Ablation Device Suitable for Thermal Protection System of Solid Rocket Ramjet
Abstract
1. Introduction
2. Experimental Setup, Methods and Materials
2.1. Experimental Setup
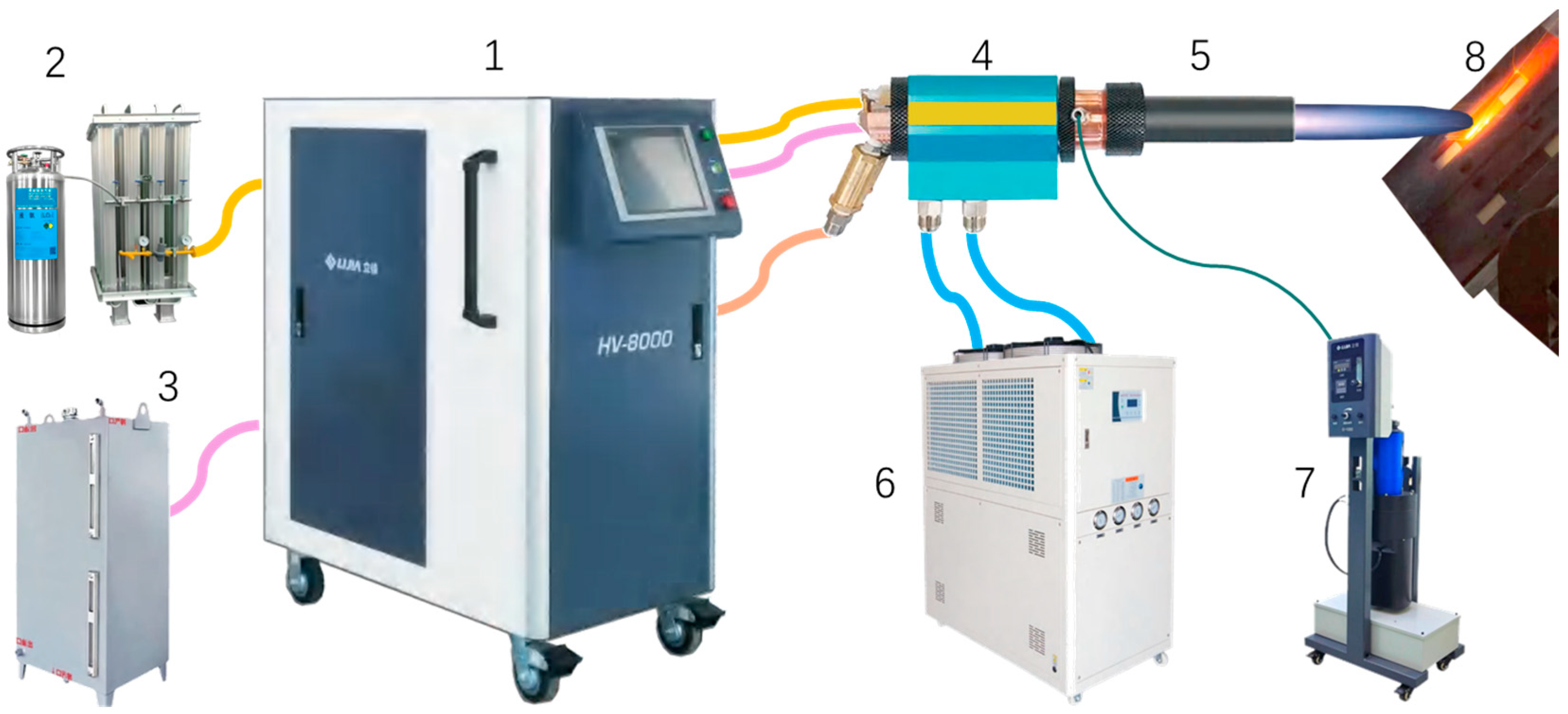
2.1.1. Design of Ablation Experimental Device to Simulate Solid Rocket Ramjet
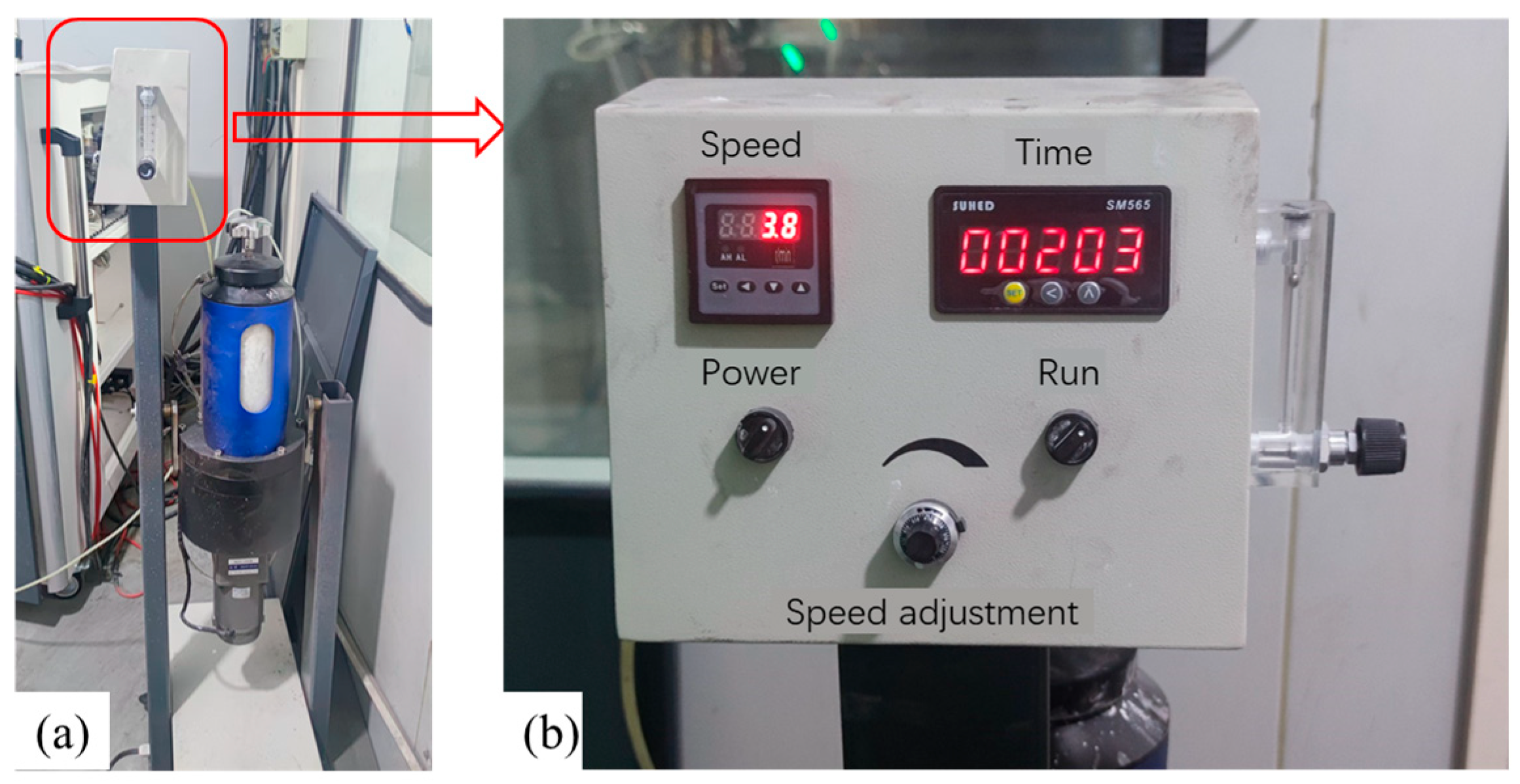
2.1.2. Specimen Fixture and Back Wall Temperature Measurement System Design
2.2. Experimental Methods
2.2.1. Oxygen Concentration Simulation Methods
2.2.2. Temperature Simulation Methods
2.2.3. Two-Phase Flow Simulation Methods
2.2.4. Pressure Environment Simulation Methods
2.3. Materials
3. Results and Discussion
3.1. Determination of Oxygen-to-Fuel Ratio
3.2. Temperature Calibration
3.3. Repeatability and Discriminability of the Experimental System
3.4. Pressure Environment and Two-Phase Flow
3.4.1. Pressure Environment
3.4.2. Two-Phase Flow Environment
4. Conclusions
- (1)
- When the oxygen-to-fuel ratio exceeds the theoretical value and continues to increase, combustion gas parameters (including combustion temperature, gas constant, and specific heat ratio) exhibit a decreasing trend, whereas oxygen concentration increases gradually. Selecting an oxygen-to-fuel ratio of 3.5 results in conditions that approximate the actual operational scenarios of a typical ramjet.
- (2)
- Ablation experimental data indicate that for identical TPS materials, the maximum relative error in ablation rate is only 5.67%. This fully demonstrates that the ablation test system is reasonably and reliably designed, meeting the requirements for ablation experiments on thermal insulation materials.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full name |
| TPS | Thermal Protection System |
| C/C | Carbon/Carbon Fiber |
| SiC | Silicon Carbide |
| CEA | Chemical Equilibrium Application |
| EPDM | Ethylene Propylene Diene Monomer |
| PIC | Phenolic Impregnated Carbon |
| PR | Phenolic Resin |
References
- Shi, D.L.; Sun, L.; Cheng, J.M.; Bao, F.; Feng, X. Study on mixing and combustion characteristics of solid rocket ramjet with a large angle of attack. Aerosp. Sci. Technol. 2023, 141, 108534. [Google Scholar] [CrossRef]
- Guo, M.F.; Li, J.; Li, K. Carbon nanotube reinforced ablative material for thermal protection system with superior resistance to high-temperature dense particle erosion. Aerosp. Sci. Technol. 2020, 106, 106234. [Google Scholar] [CrossRef]
- Mandal, S.; Hashim, S.A.; Roy, A.; Karmakar, S. A short review of challenges and prospects of boron-laden solid fuels for ramjet applications. FirePhysChem 2023, 3, 179–200. [Google Scholar] [CrossRef]
- Leuchtmann, C.; Gallegos, D.F.; Young, G.; Schoenitz, M.; Dreizin, E.L. Combustion of solid fuels containing boron, borides, and boron based composites in a solid fuel ramjet. Fuel 2024, 371, 132101. [Google Scholar] [CrossRef]
- Guo, M.F.; Du, J.F.; Zhang, Y.C. ZrO2-reinforced polymer-matrix composites used for thermal protection systems of ultra-high temperature aerospace propulsion. Aerosp. Sci. Technol. 2024, 145, 108906. [Google Scholar] [CrossRef]
- Meng, X.Y.; Gao, J.F.; Tian, H.; Niu, X.; Chen, R.; Cai, G. Study on the dynamic numerical simulation of flow and combustion in hybrid rocket engines based on a discrete phase model. Acta Astronaut. 2024, 215, 156–167. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; He, Z.P.; Xu, Q.; Cheng, S. Study on the collision characteristics between high-temperature alumina droplets and char layer. Acta Astronaut. 2024, 225, 870–880. [Google Scholar] [CrossRef]
- Guo, M.F.; Yu, K.X.; Yang, J.P.; Zhang, P.; Zhang, Y.; Zhu, D. B2O3-reinforced ablative materials with superior and comprehensive ablation resistance used in aerospace propulsion thermal protection systems. Polym. Degrad. Stab. 2024, 223, 110740. [Google Scholar] [CrossRef]
- Guo, M.F.; Zhang, P.; Yu, K.X.; Yang, J.; Wang, H.; Zhang, Y.; Du, J.; Zhu, D. Hollow microsphere-reinforced ablative materials for thermal protection systems of solid rocket engines. Acta Astronaut. 2024, 221, 309–317. [Google Scholar] [CrossRef]
- Shojaie-bahaabad, M.; Bozorg, M.; Najafizadeh, M.; Cavaliere, P. Ultra high temperature ceramic coatings in thermal protection systems (TPS). Ceram. Int. 2024, 50, 9937–9951. [Google Scholar] [CrossRef]
- Al Saad, A.; Martinez, C.; Trice, R.W. Ablation performance of rare earth oxide (REO)-stabilized tetragonal and cubic zirconia coatings as a thermal protection system (TPS) for carbon/carbon composites. J. Eur. Ceram. Soc. 2023, 43, 6449–6460. [Google Scholar] [CrossRef]
- Xu, W.J.; Song, W.D.; Jia, X.F.; Ma, C.; Wang, J.; Qiao, W.; Ling, L. Nano-silica modified lightweight and high-toughness carbon fiber/phenolic ablator with excellent thermal insulation and ablation performance. Def. Technol. 2024, 31, 192–199. [Google Scholar] [CrossRef]
- Wang, R.N.; Cheng, C.; Yang, X.H.; Bai, L.; Zhang, J.; Fei, J.; Fu, Q. Ablation behavior of C/C-ZrC-SiC composites under oxyacetylene, plasma, and plasma-solid particle environments: A comparative investigation. Mater. Charact. 2025, 219, 114649. [Google Scholar] [CrossRef]
- Yan, C.L.; Luo, P.; Zhang, J.J.; Zhao, Z.; Liu, R. Ablation behavior of Cf/ZrC–SiC ultra–high temperature ceramic composites in oxyacetylene torch and plasma wind tunnel. Mater. Today Commun. 2024, 41, 110477. [Google Scholar] [CrossRef]
- Baker, B.; Venkatachalam, V.; Zoli, L.; Vinci, A.; Failla, S.; Sciti, D.; Binner, J. Ablation behaviour of carbon fibre ultra-high temperature composites at oblique angles of attack. Mater. Des. 2021, 212, 110199. [Google Scholar] [CrossRef]
- Liu, L.; Ma, J.T.; Feng, W.; Han, Q.; Wang, X.; Wang, Y.; Wang, P.; Yang, Z.; Guo, Y. Dynamic ablation of C/C-SiC-AlSi and C/C-SiC-ZrB2-AlSi at changing impacted angle. Vacuum 2024, 230, 113640. [Google Scholar] [CrossRef]
- Kumari, K.A.; Pandey, K.M.; Ray, M.; Sharma, K.K. The numerical investigation of combustion performance of scramjet combustor with variation in angle of attack. Results Eng. 2022, 15, 100507. [Google Scholar] [CrossRef]
- Wang, Z.W.; Bai, X.H.; Liu, H.Y.; Fu, S.; Liu, C. Study on flow structure and heat transfer characteristics of a novel double layer heat shield in an afterburner. Therm. Sci. Eng. Prog. 2024, 48, 102353. [Google Scholar] [CrossRef]
- Li, W.; Tan, X.M.; Leng, Y.H.; Wang, C.; Shan, Y.; Zhang, J. Study on the cooling performance of flame stabilizer in integrated afterburner with different cooling configurations. Aerosp. Sci. Technol. 2025, 157, 109867. [Google Scholar] [CrossRef]
- Li, J.; Zhu, G.; Hu, B.Z.; Lv, X.; Gao, B.; Liu, S. Simulated experiment on case overheating failure of solid rocket engine under flight overload condition. Appl. Therm. Eng. 2020, 172, 115135. [Google Scholar] [CrossRef]
- Hou, Y.N.; Yee, C.; Li, W.; Koo, J.H.; Li, L.; Rech, B.; Fahy, W.; Wu, H.; Buffy, J.J. A novel ablative material for thermal protection system, Carbon fiber/polysiloxane composites. Aerosp. Sci. Technol. 2022, 129, 107822. [Google Scholar] [CrossRef]
- Su, X.J.; Hu, B.T.; Quan, Y.; Qin, Y.; Feng, Q.; Hu, C. Ablation behavior and mechanism of bulk MoAlB ceramic at ∼1670–2550 °C in air plasma flame. J. Eur. Ceram. Soc. 2021, 41, 5474–5483. [Google Scholar] [CrossRef]
- Geng, L.; Cheng, S.; Fu, Q.G.; Li, H. Laser ablation behavior and mechanism of carbon/carbon composites. Acta Mater. Compos. Sin. 2022, 39, 4337–4343. [Google Scholar]
- Momozawa, A.; Yokote, N.; Terutsuki, D.; Komurasaki, K. Dynamic oxidation of SiC with arc-heated plasma wind tunnel and laser heating. Vacuum 2021, 185, 109899. [Google Scholar] [CrossRef]
- Cohen, L.S.; Couch, H.T.; Murrin, T.A. Performance of Ablative Materials in Ramjet Environments. In Proceedings of the East Hartford, Thermophysics and Heat Transfer Conference, Boston, MA, USA, 15–17 July 1974. [Google Scholar]
- Liu, Y.; Guan, Y.W.; Li, J.; Liu, P.; Zhang, X. Insulator ablation modes in different impact conditions of alumina droplets onto wall surfaces. Acta Astronaut. 2018, 153, 138–145. [Google Scholar] [CrossRef]
- Li, J.; Xi, K.; Lv, X.; Li, Q.; Wang, S.-X. Characteristics and formation mechanism of compact/porous structures in char layers of EPDM insulation materials. Carbon 2018, 127, 498–509. [Google Scholar] [CrossRef]
- Li, J.; Guo, M.F.; Liu, Y.; He, G.-Q. Review on ablation research of insulation materials for solid rocket engines. J. Astronaut. 2019, 40, 1146–1156. [Google Scholar]
- Lou, Y.C.; Yu, X.J.; He, G.Q.; Li, J.; Chen, J. A simulation experimental method for insulation ablation under oxygen-rich condition. J. Solid Rocket Technol. 2006, 3, 229–231. [Google Scholar]
- Shi, Y.A.; Zha, B.L.; Su, Q.D.; Jia, X.-D. Effects of oxygen content on the ablation behavior of silicone rubber-based insulation material. Int. J. Aerosp. Eng. 2019, 3, 6592972. [Google Scholar] [CrossRef]
- Gao, Y.; Zha, B.L.; Wang, J.J.; Sun, Z.-S.; Zhang, Z.-F.; Shi, Y.-A. Ablation mechanism of C/C–SiC and C/C–SiC–ZrC composites in hypersonic oxygen-enriched environment. Ceram. Int. 2022, 48, 22985–22993. [Google Scholar] [CrossRef]
- Li, J.; Hu, B.W.; Hui, K.; Li, K.; Wang, L. Erosion resistance of ethylene propylene diene monomer insulations reinforced with precoated multi-walled carbon nanotubes. Acta Astronaut. 2022, 198, 251–257. [Google Scholar] [CrossRef]
- Chen, Z.C.; Wang, L.M.; Liu, S.Y.; Wang, J.; Xia, Z.; Hu, S. Numerical study on the effect of multi-hole injector on three-dimensional flow and heat transfer properties of a novel combined solid rocket engine. Int. J. Therm. Sci. 2024, 200, 108990. [Google Scholar] [CrossRef]
- Meng, X.Y.; Tian, H.; Yu, R.P.; Lu, Y.; Gu, X.; Tan, G.; Cai, G. Three-dimensional numerical simulation of hybrid rocket engine based on dynamic mesh technology. Aerosp. Sci. Technol. 2023, 141, 108573. [Google Scholar] [CrossRef]
- Meng, X.Y.; Tian, H.; He, L.F.; Gao, J.; Niu, X.; Cai, G. Numerical and experimental research on axial injection end-burning hybrid rocket engines with polyethylene fuel. Chin. J. Aeronaut. 2024, 37, 91–105. [Google Scholar] [CrossRef]
- Shoyama, T.; Wada, Y.; Shimagaki, M.; Hashimoto, T.; Takada, S. Liquid propellant rocket engine cycles partially using electric turbopump. Acta Astronaut. 2024, 224, 138–147. [Google Scholar] [CrossRef]
- Guo, M.F.; Yu, K.X.; Yang, J.P.; Zhang, P.; Zhang, Y.; Kan, X.; Guo, Y. Polymer–matrix composite design for extreme environments in aerospace propulsion. Acta Astronaut. 2025, 236, 1130–1140. [Google Scholar] [CrossRef]
- Choi, J.-Y. A Quasi Global Mechanism of Kerosene Combustion for Propulsion Applications. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Diego, CA, USA, 31 July–3 August 2011. [Google Scholar] [CrossRef]
- Available online: https://www.nasa.gov/glenn/research/chemical-equilibrium-with-applications/ (accessed on 15 January 2024).
- Kavga, A.; Thomopoulos, V.; Pischinas, E.; Tsipianitis, D.; Nikolakopoulos, P. Design and simulation of a greenhouse in a computational environment (ANSYS/FLUENT) and an automatic control system in a LABVIEW environment. Simul. Modell. Pract. Theory 2023, 129, 102837. [Google Scholar] [CrossRef]
- Jovanović, R.; Swiatkowski, B.; Kakietek, S.; Škobalj, P.; Lazović, I.; Cvetinović, D. Mathematical modelling of swirl oxy-fuel burner flame characteristics. Energy Convers. Manag. 2019, 191, 193–207. [Google Scholar] [CrossRef]
- Cao, H.Y.; Liu, P.; Cheng, J.M.; Yang, Y.; Yang, B. Thermometry of Ablation Testing of Thermal Insulation Material Based on Multispectral Imaging. J. Thermophys. Heat Transf. 2025. [Google Scholar] [CrossRef]
- Hu, H.W.; Li, J.; Xu, J.L.; Bai, H.; Wang, Y. Excellent ablation resistance of silicone insulations reinforced with three carbon-based nano-fillers under oxygen-enriched environment. Acta Astronaut. 2025, 229, 55–64. [Google Scholar] [CrossRef]
- Wang, Y.W.; Li, J.; Wan, L.Q.; Wang, L.; Li, K. A lightweight rubber foaming insulation reinforced by carbon nanotubes and carbon fibers for solid rocket engines. Acta Astronaut. 2023, 208, 270–280. [Google Scholar] [CrossRef]











| Parameter | Value |
|---|---|
| Pressure (kPa) | 900 |
| Temperature (K) | 3206.57 |
| Density (kg/m3) | 0.987 |
| Specific heat ratio | 1.123 |
| Molar fraction of O2 | 0.36645 |
| Molar fraction of H2 | 0.00893 |
| Molar fraction of OH | 0.07930 |
| Molar fraction of H2O | 0.18900 |
| Molar fraction of CO | 0.08151 |
| Molar fraction of CO2 | 0.22125 |
| Molar fraction of O | 0.04432 |
| Molar fraction of HO2 | 0.00022 |
| Molar fraction of H2O2 | 0.00001 |
| Molar fraction of H | 0.00902 |
| Test Piece | Quality Before Experiment (g) | Quality After Experiment (g) | Mass Ablation Rate (g/s) | Average Value | Relative Error | |
|---|---|---|---|---|---|---|
| Silicone rubber 1 | #1 | 53.84 | 40.54 | 0.0222 | ||
| #2 | 53.71 | 39.71 | 0.0233 | 0.02275 | 2.42% | |
| Silicone rubber 2 | #1 | 55.45 | 46.48 | 0.0149 | ||
| #2 | 55.79 | 47.83 | 0.0133 | 0.0141 | 5.67% | |
| Phenolic resin | #1 | 63.34 | 30.15 | 0.0553 | ||
| #2 | 63.88 | 32.57 | 0.0522 | 0.05375 | 2.88% | |
| Title of the Sample | 0 s | 100 s | 200 s | 300 s | 400 s | 500 s | 600 s | |
|---|---|---|---|---|---|---|---|---|
| Silicone rubber 1 | #1 | 311.55 | 347.98 | 421.09 | 503.25 | 574.42 | 633.06 | 679.82 |
| #2 | 315.61 | 352.32 | 426.34 | 504.27 | 576.72 | 636.98 | 686.19 | |
| Average value | 313.58 | 350.15 | 423.72 | 503.76 | 575.57 | 635.02 | 683.01 | |
| Relative error | 0.65% | 0.62% | 0.62% | 0.10% | 0.20% | 0.31% | 0.47% | |
| Silicone rubber 2 | #1 | 306.97 | 329.19 | 386.23 | 524.60 | 685.68 | 849.05 | 1075.43 |
| #2 | 308.58 | 335.12 | 389.85 | 480.14 | 631.73 | 803.18 | 984.57 | |
| Average value | 307.78 | 332.16 | 388.04 | 502.37 | 658.71 | 826.12 | 1030 | |
| Relative error | 0.26% | 0.90% | 0.47% | 4.4% | 4.1% | 2.8% | 4.4% | |
| Phenolic resin | #1 | 309.42 | 375.12 | 502.44 | 650.53 | 932.71 | 1046.5 | 1080.8 |
| #2 | 311.09 | 348.58 | 393.57 | 480.19 | 600.88 | 1066.9 | 1104.7 | |
| Average value | 310.26 | 361.85 | 448.01 | 565.36 | 766.80 | 1056.7 | 1092.8 | |
| Relative error | 0.27% | 3.7% | 12.2% | 15.1% | 21.6% | 1.0% | 1.1% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Yan, H.; Feng, X.; Zhu, G.; Liu, J.; Qi, X. Research on Ablation Device Suitable for Thermal Protection System of Solid Rocket Ramjet. Aerospace 2025, 12, 772. https://doi.org/10.3390/aerospace12090772
Cheng J, Yan H, Feng X, Zhu G, Liu J, Qi X. Research on Ablation Device Suitable for Thermal Protection System of Solid Rocket Ramjet. Aerospace. 2025; 12(9):772. https://doi.org/10.3390/aerospace12090772
Chicago/Turabian StyleCheng, Jiming, Hang Yan, Xiping Feng, Guoqiang Zhu, Jie Liu, and Xintong Qi. 2025. "Research on Ablation Device Suitable for Thermal Protection System of Solid Rocket Ramjet" Aerospace 12, no. 9: 772. https://doi.org/10.3390/aerospace12090772
APA StyleCheng, J., Yan, H., Feng, X., Zhu, G., Liu, J., & Qi, X. (2025). Research on Ablation Device Suitable for Thermal Protection System of Solid Rocket Ramjet. Aerospace, 12(9), 772. https://doi.org/10.3390/aerospace12090772






