Study on Plasma-Chemical Mode of Pulsed Coaxial Dielectric Barrier Discharge Plasma Based on Mass Spectrometry
Abstract
1. Introduction
2. Experimental Device and Measurement Method
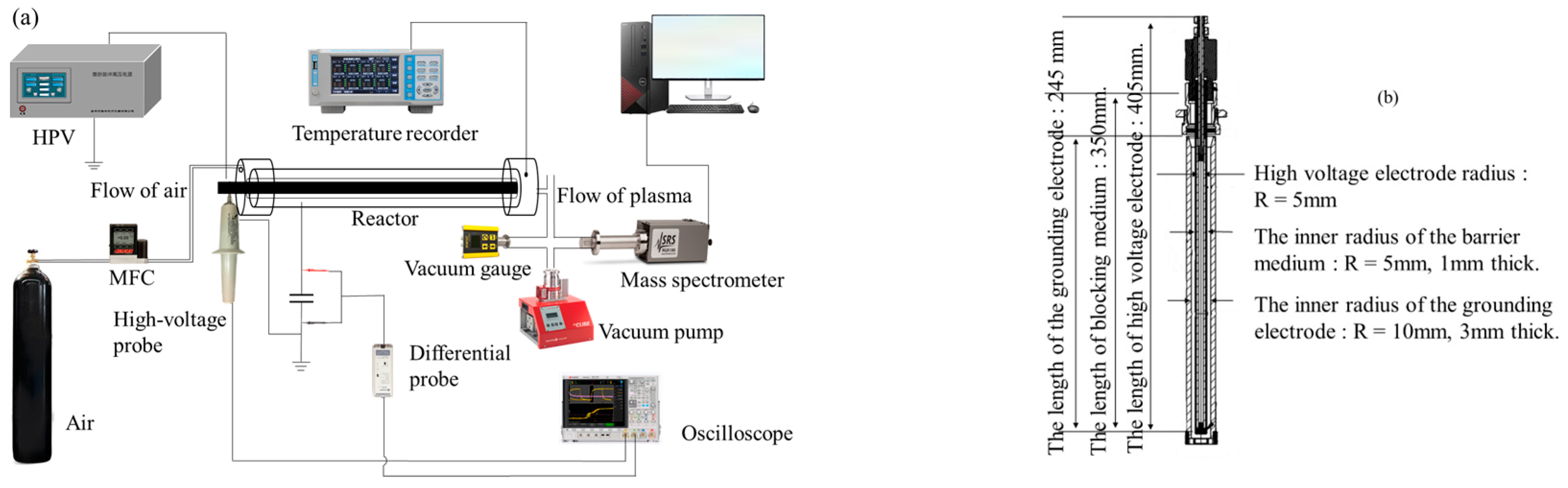
2.1. Coaxial DBD Device and Diagnostic System
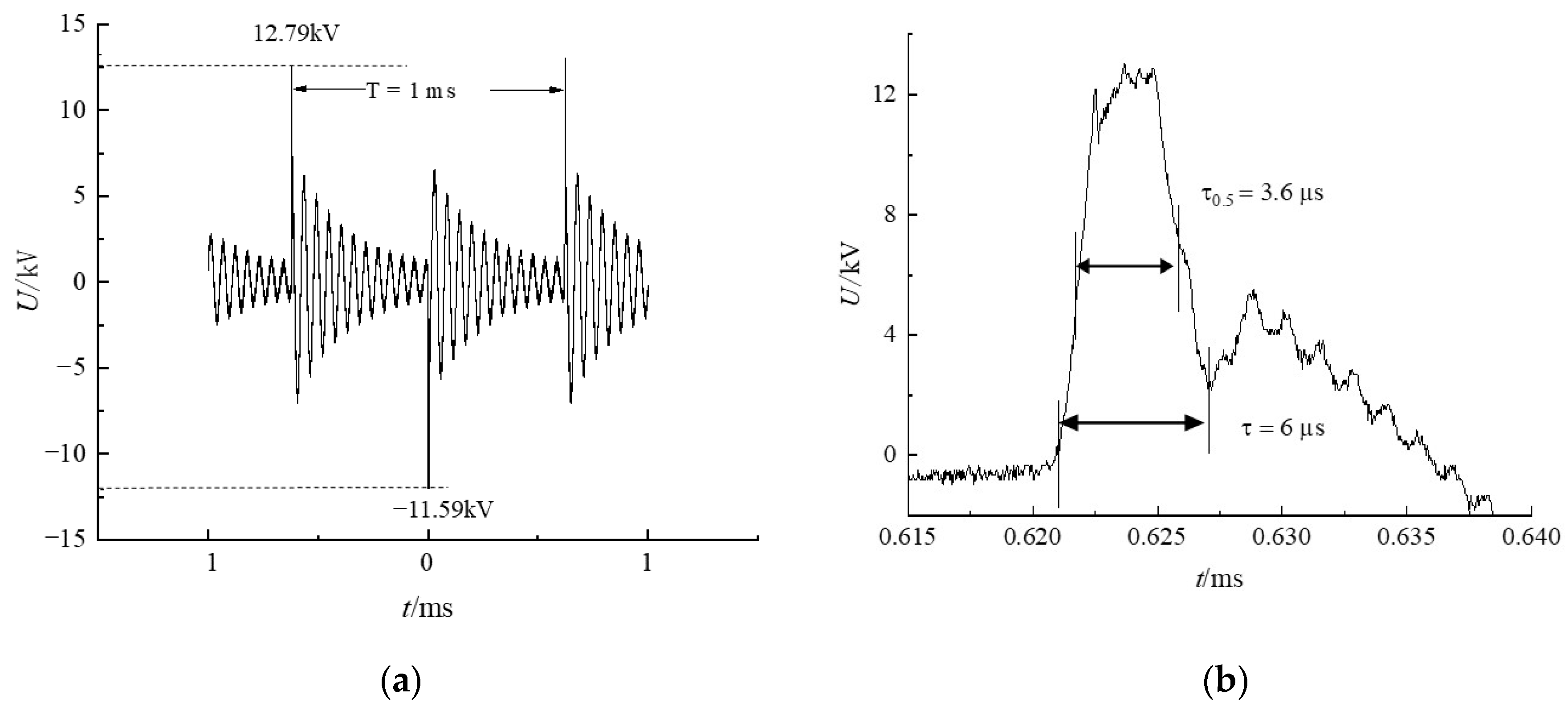
2.2. Measurements of Lissajous Figure and Active Substances
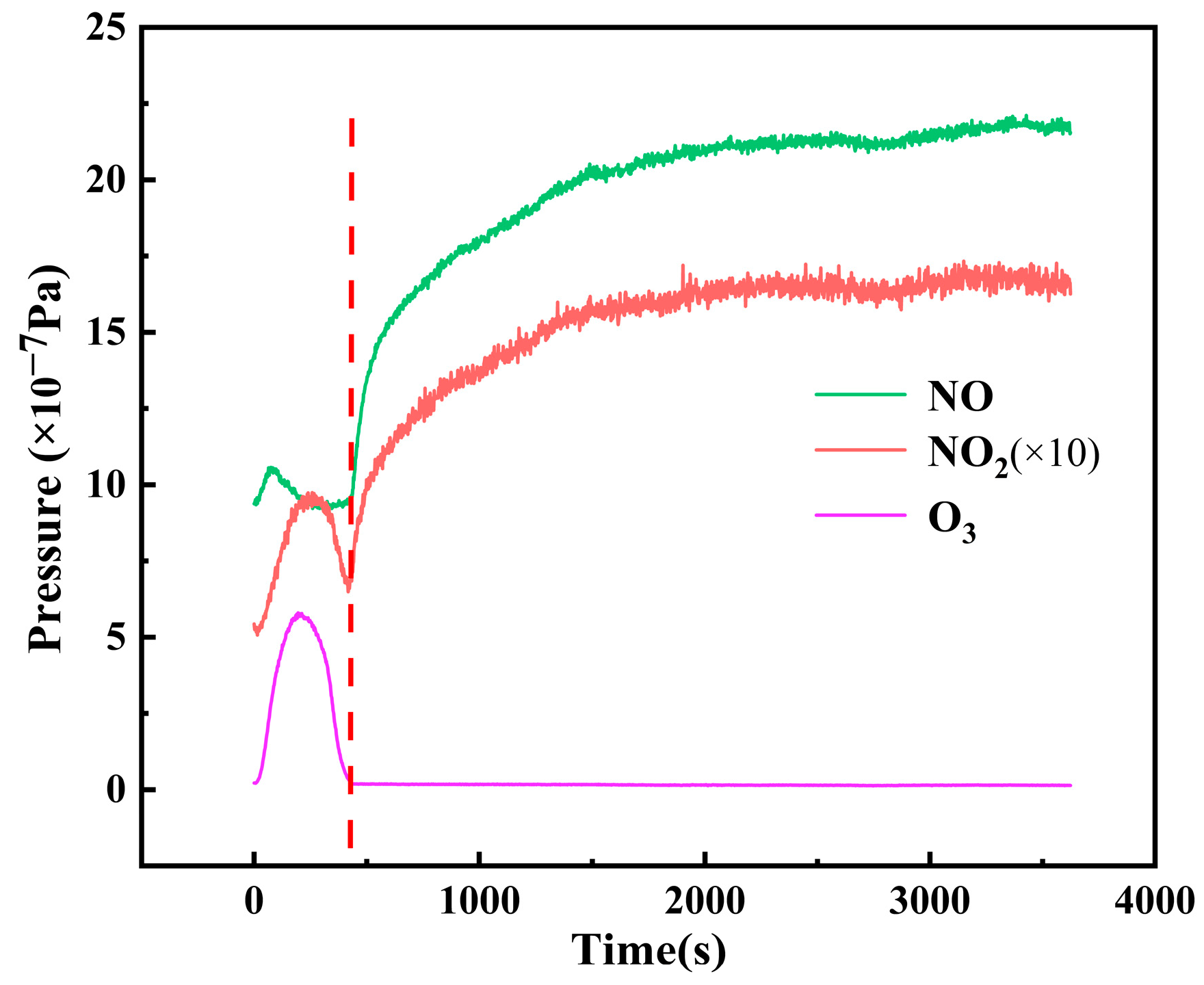
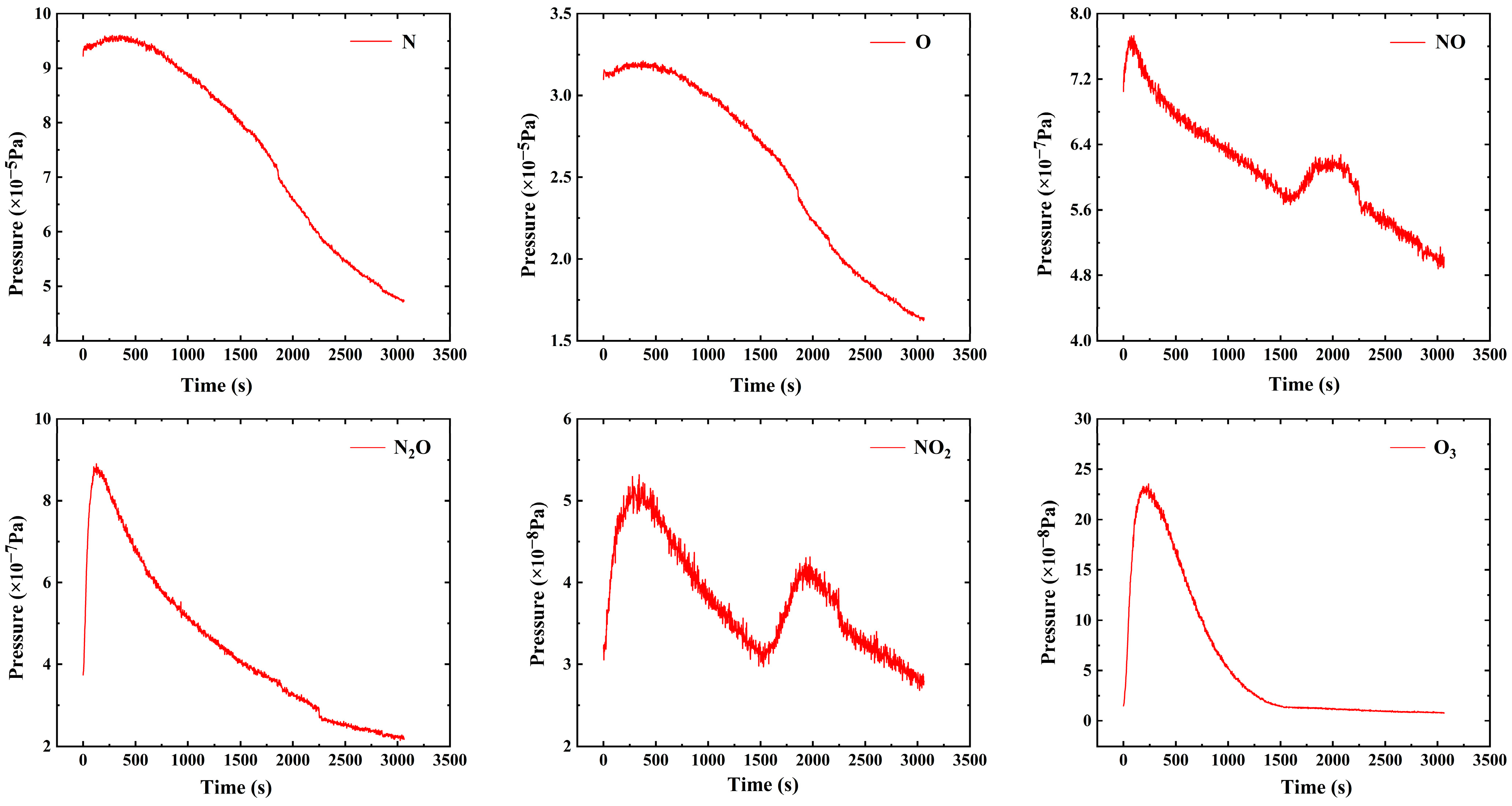
3. Experimental Results and Analysis
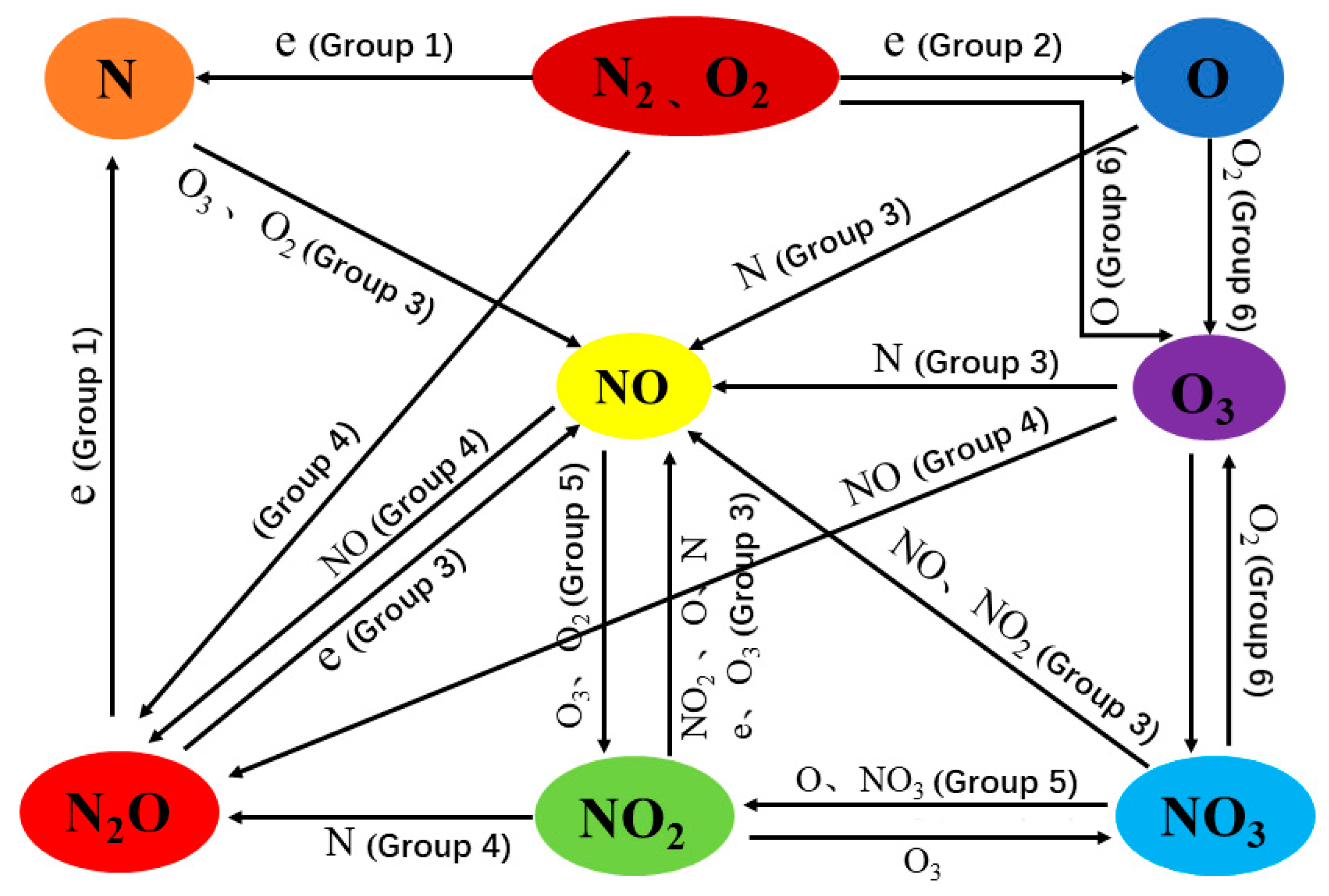
4. Investigation of the Chemical Reaction Mechanism in the Nitrogen-Oxygen System Using Mass Spectrometry
5. Conclusions
- (1)
- Gas flow rate and control voltage significantly influence the O3-NOx chemical equilibrium. Higher flow rates prolong the transition from O3-NOx coexistence to NOx-dominant mode, while increased control voltage accelerates this transition.
- (2)
- At fixed 140 V control voltage with flow rates ranging from 0.5 to 5.5 L/min, the corresponding energy densities (6037.2–552.1 J/L) yielded transition times from 408 s to 1210 s.
- (3)
- At constant 3.5 L/min flow rate, control voltages of 120–140 V (energy densities 656.6–867.8 J/L) produced transition times from 2367 s to 718 s, demonstrating greater sensitivity to voltage variation.
- (1)
- System scale-up while maintaining reaction characteristics
- (2)
- Practical implementation studies addressing environmental factors and parameter optimization for enhanced treatment efficiency
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Mu, X.G.; Wang, X.J.; Zhang, Y.; Liu, B.; Yang, J.J. Major products and their formation and transformation mechanism through degrading UDMH wastewater via DBD low temperature plasma. Environ. Technol. 2021, 42, 2709. [Google Scholar] [CrossRef] [PubMed]
- Kogelschatz, U. Fundamentals and Applications of Dielectric Barrier Discharges; ABB Corporate Research Ltd.: Baden, Switzerland, 2003. [Google Scholar]
- Eliasson, B.; Kogelschatz, U. Modeling and applications of silent discharge plasmas. IEEE Trans. Plasma Sci. 1991, 19, 309. [Google Scholar] [CrossRef]
- Du, Z.H.; Lin, X. Research Progress in Application of Low Temperature Plasma Technology for Wastewater Treatment. IOP Conf. Ser. Earth Environ. Sci. 2020, 512, 012031. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial application. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Nascimento, F.D.; Stancampiano, A. Plasma Electrode Dielectric Barrier Discharge: Development, Characterization and Preliminary Assessment for Large Surface Decontamination. Plasma Chem. Plasma Process. 2023, 46, 1791–1817. [Google Scholar] [CrossRef]
- Nascimento, F.D.; Graça Sampaio, A.D. A Low Cost, Flexible Atmospheric Pressure Plasma Jet Device with Good Anti-microbial Efficiency. IEEE Trans. Radiat. Plasma Med. Sci. 2023, 8, 307–322. [Google Scholar] [CrossRef]
- Sanghoo, P.; Wonho, C.; Cheorun, J. Interplay among ozone and nitrogen oxides in air plasmas: Rapid change in plasma chemistry. Chem. Eng. J. 2018, 352, 1014–1021. [Google Scholar] [CrossRef]
- Glaze, W.H. Drinking-water treatment with ozone. Environ. Sci. Technol. 1987, 21, 224. [Google Scholar] [CrossRef]
- Gott, R.P.; Moller, S.D.; Hodges, J.C.; Xu, K.G. Plasma-Based water purification for crewed space missions: Laboratory experimental comparisons for on-board applicability. Adv. Space Res. 2021, 68, 1591–1600. [Google Scholar] [CrossRef]
- Di, L.; Zhang, J.; Zhang, X. A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Process. Polym. 2018, 15, 1700234. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, Y.Y.; Kong, F.; Wang, R.X.; Ren, C.Y.; Shao, T. Surface charge decay of epoxy resin treated by AP-DBD deposition and direct fluorination. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 768–775. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, X.; Shao, T.; Zhang, C. Influence of oxygen content on argon/oxygen dielectric barrier discharge plasma treatment of polyethylene terephthalate film. IEEE Trans. Plasma Sci. 2017, 45, 310–317. [Google Scholar] [CrossRef]
- Pascual, A.; Llorca, I.; Canut, A. Use of ozone in food industries for reducing the environmental impact of cleaning and disinfection activities. Trends Food Sci. Technol. 2007, 18, 29–35. [Google Scholar] [CrossRef]
- Ivanov, M. Effects of high voltage electrical discharge plasma on olive mill wastewater treatment. Sustainability 2021, 13, 1552. [Google Scholar] [CrossRef]
- Lobna, M.; Chedly, T.; Sven-Uwe, G.; Latifa, B. A comparative study on ozone, hydrogen peroxide and UV based advanced oxidation processes for efficient removal of diethyl phthalate in water. J. Hazard. Mater. 2019, 363, 401–411. [Google Scholar] [CrossRef]
- Ashford, B.; Tu, X. Non-thermal plasma technology for the conversion of CO2. Curr. Opin. Green Sustain. Chem. 2017, 3, 45–49. [Google Scholar] [CrossRef]
- Pecchillo, C.T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOx Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef]
- Kevil, C.G.; Lefer, D.J. Review focus on inorganic nitrite and nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011, 89, 489. [Google Scholar] [CrossRef]
- Ratovitski, E.A.; Cheng, X.Q.; Yan, D.Y.; Sherman, J.H.; Canady, J.; Trink, B.; Michael, K. Anti-Cancer Therapies of 21st Century: Novel Approach to Treat Human Cancers Using Cold Atmospheric Plasma. Plasma Process. Polym. 2014, 11, 1128. [Google Scholar] [CrossRef]
- Tanaka, H.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Nakamura, K.; Utsumi, F.; Kajiyama, H.; Kano, H.; Okazaki, Y.; Toyokuni, S.; et al. Plasma Medical Science for Cancer Therapy: Toward Cancer Therapy Using Nonthermal Atmospheric Pressure Plasma. IEEE Trans. Plasma Sci. 2014, 42, 3760–3764. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; Van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11, 115012. [Google Scholar] [CrossRef]
- Bansemer, R.; Schmidt-Bleker, A.; Van Rienen, U.; Weltmann, K.D. Investigation and control of the O-3- to NO-transition in a novel sub-atmospheric pressure dielectric barrier discharge. Plasma Sources Sci. Technol. 2017, 26, 065005. [Google Scholar] [CrossRef]
- Yu, H.J.; Xia, W.W.; Wang, L.; Wang, Y.; Xu, S.Y.; Yao, L. Research of kinetics and anharmonicity for related reactions of NOx + H2O and cracking NOx. Chem. Phys. Lett. 2022, 798, 139580. [Google Scholar] [CrossRef]
- Abou Saoud, W.; Assadi, A.A.; Guiza, M.; Loganathan, S.; Bouzaza, A.; Aboussaoud, W.; Ouederni, A.; Rtimi, S.; Wolbert, D. Synergism between non-thermal plasma and photocatalysis: Implications in the post discharge of ozone at a pilot scale in a catalytic fixed-bed reactor. Appl. Catal. B Environ. 2019, 241, 227–235. [Google Scholar] [CrossRef]
- Catto, A.C.; Oliveira, M.C.; Ribeiro, R.A.P.; Avansi, W.; Longo, E. Hematite rhombuses for chemiresitive ozone sensors: Experimental and theoretical approaches. Appl. Surf. Sci. 2021, 563, 150209. [Google Scholar] [CrossRef]
- Gao, K.; Gong, D.D.; Liu, R.J.; Su, Z.H.; Jia, P.P.; Li, X.C. Measurement of ozone concentration in Atmospheric Pressure Air Barrier Discharge by Optical Absorption Spectroscopy. Spectrosc. Spectr. Anal. 2020, 40, 461–464. [Google Scholar] [CrossRef]
- Glascock, M.S.; Rovey, J.L.; Williams, S.; Thrasher, J. Plume Characterization of Electric Solid Propellant Pulsed Microthrusters. J. Propuls. Power 2017, 33, 870–880. [Google Scholar] [CrossRef]
- Große-Kreul, S.; Hübner, S.; Schneider, S.; Ellerweg, D.; Keudell, A.; Matejčík, S.; Benedikt, J. Mass spectrometry of atmospheric pressure plasmas. Plasma Sources Sci. Technol. 2015, 24, 044008. [Google Scholar] [CrossRef]
- Engeln, R.; Klarenaar, B.; Guaitella, O. Foundations of optical diagnostics in low-temperature plasmas. Plasma Sources Sci. Technol. 2020, 29, 063001. [Google Scholar] [CrossRef]
- Grill, A. Cold Plasma in Materials Fabrication: Form Fundamentals to Applications; IEEE Press: New York, NY, USA, 1994. [Google Scholar]
- Hippler, R. Low Temperature Plasma Physics: Fundamental Aspects and Applications; WILEY-VCH: Berlin, Germany, 2021. [Google Scholar]
- Pavlovich, M.J.; Clark, D.S.; Graves, D.B. Quantification of air plasma chemistry for surface disinfection. Plasma Sources Sci. Technol. 2014, 23, 065036. [Google Scholar] [CrossRef]
- Shimizu, T.; Sakiyama, Y.; Graves, D.B.; Zimmermann, J.L.; Morfill, G.E. The dynamics of ozone generation and mode transition in air surface micro-discharge plasma at atmospheric pressure. New J. Phys. 2012, 14, 103028. [Google Scholar] [CrossRef]
- Pierotti, G.; Piferi, C.; Popoli, A.; Cavedon, M.; Cristofolini, A.; Martines, E.; Riccardi, C. A novel two-stage kinetic model for surface DBD simulations in air. Plasma Sources Sci. Technol. 2023, 32, 064005. [Google Scholar] [CrossRef]
- Malik, M.A.; Jiang, C.Q.; Heller, R. Ozone-free nitric oxide production using an atmospheric pressure surface discharge—A way to minimize nitrogen dioxide co-production. Chem. Eng. J. 2016, 283, 631–638. [Google Scholar] [CrossRef]
- Liu, K.; Hu, Y.; Lei, J. The chemical product mode transition of the air DBD driven by AC power: A plausible evaluation parameter and the chemical behaviors. Phys. Plasma 2017, 24, 103513. [Google Scholar] [CrossRef]
- Simek, M.; Peka’rek, S.; Prukner, V. Ozone Production Using a Power Modulated Surface Dielectric Barrier Discharge in Dry Synthetic Air. Plasma Chem. Plasma Process. 2012, 32, 743–754. [Google Scholar] [CrossRef]
- Huang, L.C.; Ye, J.F.; Wang, D.K.; Zheng, Y.Z. Experimental Study on Degradation of Unsymmetrical Dimethylhydrazine Wastewater by Low Temperature Plasma. Chin. J. Energetic Mater. 2022, 30, 1013–1021. [Google Scholar] [CrossRef]
- Stanford Research Systems. Available online: http://www.thinksrs.com/downloads/pdfs/manuals/RGAm.pdf (accessed on 1 May 2024).
- Zhou, Z.Y.; Du, X.; Yang, J.; Wang, Y.; Li, C.; Wei, S.; Du, L.; Li, Y.; Qi, F.; Wang, Q. The vacuum ultraviolet beamline/endstations at NSRL dedicated to combustion research. J. Synchrotron Radiat. 2016, 23, 1035–1045. [Google Scholar] [CrossRef]
- Wang, C.Q.; Zhang, G.X. Effect of Measurement Elements on Discharge Characteristics of Dielectric Barrier Discharge. Phys. Procedia 2012, 32, 664–668. [Google Scholar] [CrossRef]
- Wang, R.X.; Yang, Y.; Chen, S.; Jiang, H.; Martin, P. Power Calculation of Pulse Power-Driven DBD Plasma. IEEE Trans. Plasma Sci. 2021, 49, 2210–2216. [Google Scholar] [CrossRef]
- Crounse, J.D.; Nielsen, L.B.; Jorgensen, S.; Kjaergaard, H.G.; Wennberg, P.O. Autoxidation of organic compounds in the atmosphere. J. Phys. Chem. Lett. 2013, 4, 3513–3520. [Google Scholar] [CrossRef]
- Available online: https://www.chembk.com/cn/chem/ (accessed on 1 May 2024).
- Robert, E.; Darny, T.; Dozias, S.; Iseni, S.; Pouvesle, J.M. New insights on the propagation of pulsed atmospheric plasma streams: From single jet to multi jet arrays. Phys. Plasmas 2015, 22, 122007. [Google Scholar] [CrossRef]
- Bourdon, A.; Darny, T.; Pechereau, F.; Pouvesle, J.-M.; Viegas, P.; Iséni, S.; Robert, E. Numerical and experimental study of the dynamics of a μs helium plasma gun discharge with various amounts of N2 admixture. Plasma Sources Sci. Technol. 2016, 25, 035002. [Google Scholar] [CrossRef]
- Obradovic, B.M.; Cvetanović, N.; Ivković, S.S.; Sretenović, G.B.; Kovačević, V.V.; Krstić, I.B.; Kuraica, M.M. Methods for spectroscopic measurement of electric field in atmospheric pressure helium discharges. Eur. Phys. J. Appl. Phys 2017, 77, 30802. [Google Scholar] [CrossRef]
- Alexandra, P.; Oliver, J.S. Dielectric barrier discharge in mass spectrometry—An overview over plasma investigations and ion sources applications. Trends Anal. Chem. 2024, 170, 117420. [Google Scholar] [CrossRef]
- Yagi, S.; Tanaka, M. Mechanism of Ozone Generation In Air-Fed Ozonizers. J. Phys. D-Appl. Phys. 1979, 12, 1509–1520. [Google Scholar] [CrossRef]
- Eliasson, B.; Kogelschatz, U.; Baessler, P. Dissociation of O2 in N2/O2 Mixtures. J. Phys. B At. Mol. Phys. 1984, 17, L797–L801. [Google Scholar] [CrossRef]
- Kossyi, I.A.; Kostinsky, A.Y.; Matveyev, A.A.; Silakov, V.P. Kinetic scheme of the non-equilibrium discharge in nitrogen-oxygen mixtures. Plasma Sources Sci. Technol. 1992, 1, 207–220. [Google Scholar] [CrossRef]
- Stefanovic, I.; Bibinov, N.K.; Deryugin, A.A.; Vinogradov, I.P.; Napartovich, A.P.; Wiesemann, K. Kinetics of ozone and nitric oxides in dielectric barrier discharges in O2/NOx and N2/O2/NOx mixtures. Plasma Sources Sci. Technol. 2001, 10, 406–416. [Google Scholar] [CrossRef]
- Van Gaens, W.; Bogaerts, A. Kinetic modelling for an atmospheric pressure argon plasma jet in humid air. J. Phys. D Appl. Phys. 2013, 46, 275201. [Google Scholar] [CrossRef]
- Shkurenkov, I.; Burnette, D.; Lempert, W.R. Kinetics of excited states and radicals in a nanosecond pulse discharge and afterglow in nitrogen and air. Plasma Sources Sci. Technol. 2014, 23, 065003. [Google Scholar] [CrossRef]

| Control Voltage/V | 120 | 130 | 140 |
|---|---|---|---|
| Actual positive pulse peak/kV | 11.90 | 12.79 | 14.33 |
| Actual negative pulse peak/kV | −11.13 | −11.57 | −12.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zheng, Y.; Du, B.; Han, J.; Wen, M.; Zhang, T. Study on Plasma-Chemical Mode of Pulsed Coaxial Dielectric Barrier Discharge Plasma Based on Mass Spectrometry. Aerospace 2025, 12, 433. https://doi.org/10.3390/aerospace12050433
Wang D, Zheng Y, Du B, Han J, Wen M, Zhang T. Study on Plasma-Chemical Mode of Pulsed Coaxial Dielectric Barrier Discharge Plasma Based on Mass Spectrometry. Aerospace. 2025; 12(5):433. https://doi.org/10.3390/aerospace12050433
Chicago/Turabian StyleWang, Diankai, Yongzan Zheng, Baosheng Du, Jianhui Han, Ming Wen, and Tengfei Zhang. 2025. "Study on Plasma-Chemical Mode of Pulsed Coaxial Dielectric Barrier Discharge Plasma Based on Mass Spectrometry" Aerospace 12, no. 5: 433. https://doi.org/10.3390/aerospace12050433
APA StyleWang, D., Zheng, Y., Du, B., Han, J., Wen, M., & Zhang, T. (2025). Study on Plasma-Chemical Mode of Pulsed Coaxial Dielectric Barrier Discharge Plasma Based on Mass Spectrometry. Aerospace, 12(5), 433. https://doi.org/10.3390/aerospace12050433






