Abstract
Propellants play a crucial role in the propulsion systems of aerospace vehicles, and their combustion characteristics are susceptible to external environmental conditions. This study systematically investigated the impact of various initial conditions on the combustion process of ADN-based propellant, including combustion products, equilibrium pressure, adiabatic temperature, and ignition delay time. The results indicate that the primary combustion products of ADN-based propellant include N2O, N2, CO2, OH, and others. ADN-based propellant exhibits a distinct two-stage combustion process under low pressure and temperature conditions (P0 = 2 atm, T0 = 586 K). Conversely, under high pressure and temperature conditions (P0 = 10 atm, T0 = 2930 K), the two stages of combustion occur almost simultaneously, making them difficult to distinguish. Furthermore, as the initial temperature increases, the ignition delay time decreases significantly, and the combustion rate accelerates. When the initial temperature rises from 400 K to 2800 K at a pressure of P0 =10 atm, the ignition delay time decreases from 3.5 ms to 0.6 μs. Interestingly, changes in initial pressure have a relatively minor impact on the ignition delay time compared to changes in temperature. Therefore, temperature has a more crucial influence on the combustion characteristics of ADN-based propellant than pressure. This study holds promise for providing new combustion optimization strategies for the aerospace industry and promoting the development of aircraft designs towards higher performance and sustainability.
1. Introduction
With the rapid development of aerospace technology, small satellites, microsatellites, and planetary probes, among other space exploration technologies, are thriving. Propellants, serving as the core power source for various carrier rockets and spacecraft, directly impact the performance of thrusters and are crucial for advancing new-generation carrier rockets and spacecraft. Ammonium dinitramide (ADN)-based propellants [1,2,3,4,5], with their significant advantages of high energy, high density, low toxicity, positive oxygen balance, and high specific impulse, have gradually emerged as a strong candidate for the new generation of hydrazine-based propellants. ADN, with the chemical formula NH4N(NO2)2, is a white crystal composed of the anion N(NO2)− and the cation NH4+ [6,7,8]. Since its first synthesis by the Zelinsky Institute of the former Soviet Union in 1971, the successful on-orbit application of ADN-based propellants has attracted widespread attention in the industry [9,10]. The combination of ADN as an oxidizer with methanol (CH3OH) dissolved in water has been recognized as a formulation strategy for ADN-based propellants, which are regarded as a promising substitute for conventional hydrazine-based monopropellants [11,12,13,14]. Yao et al. [15] from the Beijing Institute of Control Engineering used Tunable Diode Laser Absorption Spectroscopy (TDLAS) technology to accurately measure the CO concentration and gas temperature generated during the decomposition and combustion processes of ADN-based propellants. Zeng et al. [16] combined TDLAS with monochromatic radiation temperature measurement technology to deeply investigate the combustion characteristics of ADN-based thrusters under different operating modes and successfully measured intermediate products and gas temperatures. Wang et al. [17] from the Institute of Mechanics, Chinese Academy of Sciences systematically studied the influence of different laser energies and delay times on the plasma characteristics of ADN-based propellants using laser-induced emission spectroscopy technology, revealing important findings such as an initial electron temperature of 68,000 K and an electron density of 1.6 × 10−19. Yu et al. [18,19,20] from Beijing Jiaotong University focused on the influence laws of different microwave powers, gas flow rates, and propellant flow rates on microwave plasma ignition and the combustion of ADN-based liquid propellants. Experimental results showed that increasing microwave power significantly promotes the combustion of ADN-based liquid propellants. Within our research group, Jiang et al. [21] investigated the performance of ADN-based liquid propellant in a pulse laser ablation micro-propulsion system based on laser-induced breakdown spectroscopy. The results demonstrated that when the incident laser energy was added to 100 mJ and 21 wt. % AMIMDCA was used as fuel, a strong and stable plasma signal was observed. Moreover, Du and colleagues [22] documented the application of high-energy pulsed laser ablation to ADN-based propellant droplets positioned within micron-scale pits, yielding an impulse of 9.8 µN·s and a specific impulse of 234.9 s, thereby proving the feasibility of generating micro-thrust via the pulsed laser ablation of microdroplets.
Simultaneously, significant progress and fresh breakthroughs have been achieved in related theoretical modeling endeavors. Gustavo F. Velardez et al. [23] performed molecular dynamics calculations on the melting point and liquid properties of ADN. Zhang et al. [24] conducted comprehensive simulations to analyze the atomization characteristics, decomposition and combustion, and thruster structural parameters of ADN-based propellants, revealing the temperature and pressure distribution patterns inside the thruster. Jing et al. [25,26] at Tsinghua University proposed a detailed reaction mechanism for the combustion process of ADN-based propellants, encompassing 48 species and 242 chemical reactions. After that, the chemical mechanism of ADN-based propellants consisting of 18 species and 39 reactions was successfully simplified to simulate the reaction process of gaseous ADN and methanol, revealing the asynchrony between ADN decomposition and methanol oxidation within the reaction chamber. Liu et.al [27] at the Beijing Institute of Control Engineering employed three-dimensional numerical simulations to investigate the flow and phase change characteristics of the propellant at the microscale of capillaries within the ADN-based micro-thruster.
In summary, previous research on ADN has primarily focused on its physicochemical properties and dynamic behaviors. However, the thermal decomposition and detailed combustion mechanisms of ADN-based propellants remain unclear. To address these gaps, we investigated the combustion characteristics of ADN-based propellants under varied initial conditions. Our research systematically analyzed the influence of different temperatures and pressures on core combustion parameters, including combustion products (N2O, N2, CO2, NO, and OH), the equilibrium pressure (PE), adiabatic combustion temperature (Tad), and ignition delay time (τ). Our findings are expected to provide guidance for enhancing thruster propulsion performance and promoting the development of aerospace towards high performance and sustainability.
2. Theoretical Methods
OpenFOAM V6 is a well-established software program for computational fluid dynamics (CFD) calculations. Being an open-source platform, it effectively handles the computation of combustion reactions [28,29,30]. In this study, we utilize the reacting Foam solver within OpenFOAM to perform numerical simulations of ADN-based propellant. The solver introduces adaptive mesh refinement technology to enhance computational speed. The pertinent equations governing mass conservation, momentum conservation, species conservation, and energy conservation are presented in Equation (1), as detailed in [31]. The solution to the control equation is obtained through the integrated finite volume method solver, while the pressure implicit operator splitting (PISO) algorithm, sourced from [32], is employed to adjust the velocity field based on the pressure equation’s output.
where is the viscous stress tensor; is the i-component of the diffusion velocity for the kth component; is the mass fraction of the kth component; is the reaction rate of the kth component; is the heat release rate of the combustion process; is the heat flux, including the thermal diffusion term caused by the temperature gradient and the thermal diffusion term caused by the diffusion of components with different specific enthalpies; and is the deviatoric stress tensor [31].
The ADN-based propellant employed in the present investigation is designed as a mixture consisting of three components: 63% ADN, 11% methanol (CH3OH), and 26% water (H2O). The percentage mentioned here refers to the mass fraction. The ADN-based propellant composition (63% ADN, 11% methanol, and 26% water) was determined based on the experimental measurements by Jing et al. from Tsinghua University [26]. A simplified mechanism of ADN-based propellant with 18 species and 39 elementary reactions was used in our investigation [4]. The solution to the chemical reaction process employs the seulex ode solver, which utilizes the explicit Euler method for time advancement. Furthermore, to ensure the reliability of numerical simulation results obtained using the simplified mechanism, a comparative analysis was conducted for typical scenarios. As illustrated in Figure 1, we calculated the zero-dimensional homogeneous ignition process of ADN-based propellant at various temperatures (370 K, 400 K, 450 K, and 500 K) under atmospheric pressure conditions and compared the time-varying curves of mixture temperature obtained from both the simplified and detailed chemical reaction mechanisms. The results indicate that the simplified mechanism can accurately predict the ignition process of the mixture under different initial temperature conditions. Moreover, the diffusion problems (Figure S1) and shock tube problems (Figure S2) serve as validation cases. The research findings indicate a high degree of concordance between the data produced by our solver and the predictive results of the ASURF program, confirming the trustworthiness of our model. Therefore, all numerical simulation results in this work were obtained using the simplified mechanism. Moreover, the simulated operating conditions with different initial pressures and temperatures are shown in Table 1.
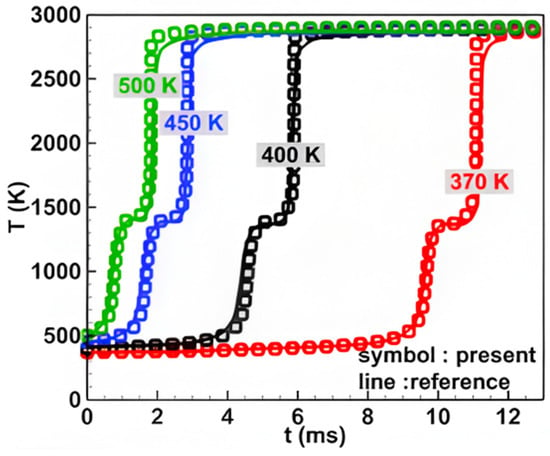
Figure 1.
Typical verification examples of zero-dimensional homogeneous ignition process of ADN-based propellant at various temperatures.

Table 1.
The simulated operating conditions with different initial pressures and temperatures.
3. Results and Discussion
3.1. Time-Evolution Temperature and Pressure Analysis of ADN-Based Propellant
As we all know, temperature and pressure are two key factors that affect the combustion reaction rate and product distribution. Figure 2 illustrates the evolution of temperature and pressure over time during the combustion of ADN-based propellants under different initial conditions. Under the initial conditions of pressure P0 = 2 atm and temperature T0 = 586 K (hereinafter referred to as the “low-temperature, low-pressure scenario”), the trends in temperature and pressure caused by the combustion of ADN-based propellants exhibit overall consistency. As shown in Figure 2a, the temperature and pressure generated by the combustion of ADN-based propellants display a clear two-stage increase at t = 0.3 ms and t = 0.75 ms. For instance, at t = 0.3 ms, the temperature rapidly rises from the initial 586 K to approximately 1200 K. Here, we define the period t < 0.3 ms as the first-stage ignition process and t > 0.3 ms as the second-stage ignition process, using 0.3 ms as the dividing line. In contrast to the low-temperature, low-pressure scenario, under the initial conditions of pressure P0 = 10 atm and temperature T0 = 2930 K (hereinafter referred to as the “high-temperature, high-pressure scenario”), as shown in Figure 2b, no two-stage characteristics are observed in the temperature and pressure of ADN-based propellant combustion. This is primarily due to the higher initial temperature, which accelerates the combustion reaction process, with the second-stage combustion occurring simultaneously with the first-stage combustion. Additionally, it is noteworthy that during the initial ignition stage (at t = 0.1 μs), there is a decline in temperature (approximately 40 K), which differs from the continuous temperature increase observed in the low-temperature, low-pressure scenario. This phenomenon may be attributed to the different initial conditions affecting the chemical combustion reaction of ADN-based propellants. By comparing the simulation results of the low-temperature, low-pressure scenario and the high-temperature, high-pressure scenario, it is found that the ignition time of ADN-based propellants is shortened from 0.8 ms in the low-temperature, low-pressure scenario to 4 μs in the high-temperature, high-pressure scenario, indicating that the high-temperature, high-pressure initial conditions effectively accelerate the combustion reaction rate of ADN-based propellants.
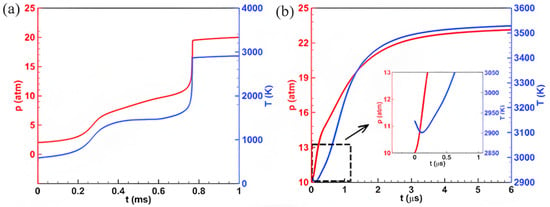
Figure 2.
Variation curves of pressure and temperature for ADN-based propellants under (a) P0 = 2 atm, T0 = 586 K; (b) P0 = 10 atm, T0 = 2930 K. Red represents pressure; blue represents temperature; the black box represents the enlarged image.
3.2. Combustion Products Analysis of ADN-Based Propellant
The evolution of primary combustion components and its mass fractions of ADN-based propellant were performed and are shown in Figure 3. The main combustion components involved in the combustion process of ADN-based propellant include N2O, N2, CO2, OH, CH3OH, and NH4N(NO2)2. As shown in Figure 3a, under low-temperature, low-pressure conditions, when t = 0.3 ms, the mass fraction of the reactant NH4N(NO2)2 rapidly decreases to zero, while the mass fraction of N2O increases from zero to 0.20, indicating that NH4N(NO2)2 is completely decomposed within the first stage, producing the intermediate product N2O. Concurrently, the mass fraction of CH3OH decreases from 0.16 to 0.10 during this stage, suggesting that CH3OH has begun to decompose. In the second-stage ignition process (t > 0.3 ms), as time progresses, CH3OH is completely consumed and converted into the product CO2, while the mass fraction of the intermediate product N2O decreases and results in the formation of the final product N2; complete ignition occurs in the mixture, and the temperature reaches the adiabatic combustion temperature. For high-temperature, high-pressure conditions (Figure 3b), due to the higher initial temperature, intermediate species such as N2O generated from the combustion reaction of NH4N(NO2)2 in the first stage are quickly consumed, leading to simultaneous production and consumption of N2O that cannot be clearly distinguished. Therefore, the second-stage ignition occurs concurrently with the first stage, which is consistent with previous analysis of temperature and pressure changes. Additionally, the consumption rate of CH3OH accelerates under high-temperature, high-pressure conditions and its mass fraction has already decreased to zero when NH4N(NO2)2 begins to be consumed. This contrasts with the pattern observed under low-temperature, low-pressure conditions, where NH4N(NO2)2 reacts with CH3OH first, potentially due to the rapid decomposition of CH3OH at high temperatures and pressures leading to a temperature drop during the initial ignition stage. Different initial conditions result in different combustion reaction mechanisms of ADN. Therefore, varying initial conditions can effectively regulate the combustion reaction of ADN-based propellants.
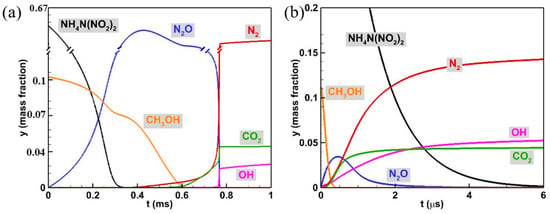
Figure 3.
Variation curves of the mass fraction over time for ADN-based propellants and the main combustion components. (a) P0 = 2 atm, T0 = 586 K; (b) P0 = 10 atm, T0 = 2930 K.
3.3. Equilibrium Pressure and Adiabatic Temperature Analysis of ADN-Based Propellant
The influence of initial temperature and pressure on the equilibrium pressure (PE) and adiabatic combustion temperature (Tad) of ADN-based propellants was further investigated. As shown in Figure 4a, when the initial pressure P0 remains constant, gradually increasing the initial temperature T0 results in an overall decreasing trend in PE after the combustion of ADN-based propellants. Additionally, there is a positive correlation between the initial pressure P0 and PE; as P0 increases, PE also increases. Unlike the trend observed for equilibrium pressure, when the initial pressure P0 is constant, the adiabatic combustion temperature Tad of ADN-based propellants after combustion increases with an increase in initial temperature, as illustrated in Figure 4b. Notably, the increase in Tad is much smaller than the change in T0. Taking the condition with P0 = 15 atm as an example, when the initial temperature varies from 400 K to 2800 K, the adiabatic combustion temperature only rises from 2962 K to 3789 K. This is because the heat release during the combustion process raises the temperature, inducing a shift in the reaction equilibrium towards the reverse direction. Simultaneously, the specific heat capacity of the substances increases with temperature, leading to the adiabatic combustion temperature increasing by a smaller amount than the initial temperature. In summary, the choice of initial temperature has a crucial impact on the combustion reaction. There exists an appropriate range for selecting the initial temperature; it is not necessarily better to choose a higher one.
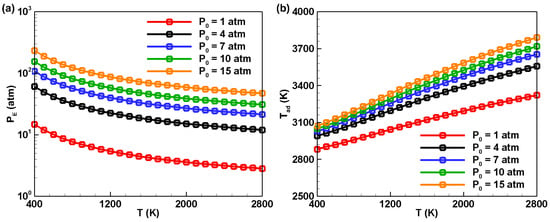
Figure 4.
Variation curves of the effects of initial temperature on the (a) equilibrium pressure and (b) adiabatic combustion temperature of ADN-based propellants.
Subsequently, the impact of different initial pressures on the PE and Tad of ADN-based propellants was studied. As shown in Figure 5a, research indicates that, with a constant initial temperature, the PE of ADN-based propellants after ignition increases with an increase in initial pressure P0, and a clear positive correlation is observed between PE and P0. The slope of the curve representing the change in PE with P0 decreases as the initial temperature of the mixture rises. Figure 5b displays the variation in the adiabatic combustion temperature Tad of the mixture with initial pressure. The results show that the adiabatic combustion temperature increases with an increase in initial pressure and the effect of initial temperature on the adiabatic combustion temperature of the mixture is more pronounced at higher temperatures (T0 = 2800 K) compared to lower temperatures (T0 = 400 K).
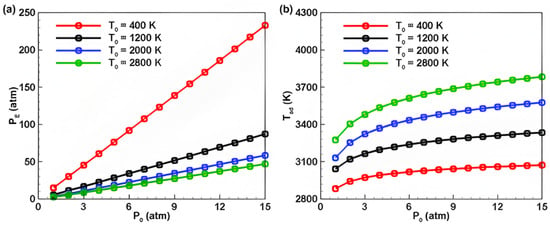
Figure 5.
Variation curves of the effects of pressure on the (a) equilibrium pressure and (b) adiabatic combustion temperature of ADN-based propellants.
3.4. Ignition Delay Time Analysis of ADN-Based Propellant
Figure 6 displays the curves illustrating the impact of various initial temperatures and pressures on the ignition delay time (τ) of ADN-based propellants. As shown in Figure 6a, under constant initial pressure conditions, the ignition delay time decreases as the initial temperature increases. This is because a higher initial temperature facilitates the achievement of ignition conditions for ADN propellants and accelerates the rate of combustion chemical reactions. For different initial pressures, the ignition delay times of ADN-based propellants at the same temperature are similar, indicating that the sensitivity of the ignition delay time of ADN fuels to initial temperature is significantly higher than to initial pressure. Figure 6b depicts the effect of different initial pressures on the ignition delay time. The calculation results show that the ignition delay time decreases slightly with an increase in initial pressure. However, for the same initial pressure, the ignition delay time of the mixture decreases significantly as the temperature increases. Taking P0 = 10 atm as an example, when the initial temperature of the mixture increases from 400 K to 2800 K, the ignition delay time decreases from 3.5 ms to 0.6 μs. These results indicate that the sensitivity of the ignition delay time of ADN-based propellants to initial temperature is higher than to initial pressure.
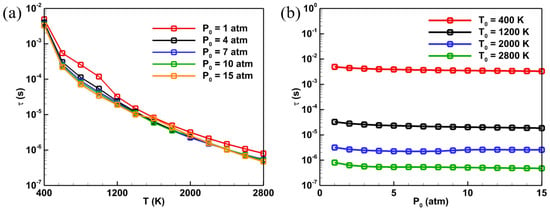
Figure 6.
Variation curves of the effects of different (a) temperatures and (b) pressures on the ignition delay time of ADN-based propellants.
4. Conclusions
In summary, this paper investigates the impact of various initial conditions on the combustion characteristics of ADN-based propellants, encompassing combustion products, equilibrium pressure, adiabatic temperature, and ignition delay time. Our findings contribute to enhancing the performance of aircraft propulsion systems and promotes the development of future aircraft designs towards higher performance and greater sustainability. The main conclusions are as follows:
- ADN-based propellants exhibit distinct two-stage combustion characteristics under low-temperature and low-pressure conditions. However, under high-temperature and high-pressure conditions, these two combustion stages occur concurrently and cannot be clearly distinguished.
- An increase in temperature effectively shortened the ignition delay time and accelerated the combustion reactions. When the initial temperature rises from 400 K to 2800 K, the ignition delay time decreases from 3.5 ms to 0.6 μs at a pressure of P0 = 10 atm.
- Compared to changes in initial temperature, an increase in initial pressure has a relatively minor effect on the ignition delay time. These results indicate that temperature has a more significant impact on the combustion characteristics of ADN-based propellants than pressure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/aerospace12040295/s1, Table S1: Simplified reaction mechanism of ADN-based propellant (18 species, 39 elementary reactions). Figure S1: Validation of the one-dimensional multi-component diffusion problem model under different initial conditions. Figure S2: Validation of the one-dimensional shock tube problem model.
Author Contributions
Conceptualization, J.H.; methodology, L.J. and B.D.; validation, G.F.; formal analysis, M.W.; investigation, J.H. and L.J.; resources, Y.H.; data curation, H.C.; writing—original draft preparation, J.H.; writing—review and editing, J.H. and J.S.; visualization, L.J. and J.S.; supervision, M.W.; project administration, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nagamachi, M.-Y.; Oliveira, J.-I.; Kawamoto, A.M.; Dutra, R.-C. ADN-The new oxidizer around the corner for an environmentally friendly smokeless propellant. J. Aerosp. Technol. Manag. 2009, 1, 153–160. [Google Scholar]
- Lee, D.; Kim, J.; Kwon, S. High performance microthruster with ammonium-dinitramide-based monopropellant. Sens. Actuators A Phys. 2018, 283, 211–219. [Google Scholar]
- Cheng, J.; Cao, J.; Li, F.; Zhang, Z.; Xu, J.; Ouyang, K.; Rossi, C.; Ye, Y.; Shen, R. Microwave controlled ignition and combustion characteristics of ADN-based ionic liquid propellant with fast response and environmental friendliness. Chem. Eng. J. 2023, 471, 144412. [Google Scholar] [CrossRef]
- Han, J.-H.; Jiang, L.-Y.; Ye, J.-F.; Song, J.-L.; Cui, H.-C.; Du, B.-S.; Feng, G.-P. Effects of nozzle type on combustion characteristics of Ammonium dinitramide-based energetic propellant. Aerospace 2024, 11, 935. [Google Scholar] [CrossRef]
- Jiang, L.-Y.; Mao, C.-T.; Han, J.-H.; Cui, H.-C.; Du, B.-S.; Zheng, Y.-Z.; Ye, J.-F.; Hong, Y.-J. Effects of Different initial conditions on combustion process of Ammonium dinitramide-based energetic propellant in Straight nozzle. Aerospace 2024, 11, 437. [Google Scholar] [CrossRef]
- Bottaro, J.C.; Penwell, P.E.; Schmitt, R.J. 1,1,3,3-Tetraoxo-1,2,3-triazapropene Anion, a New Oxy Anion of Nitrogen: The Dinitramide Anion and Its Salts. J. Am. Chem. Soc. 1997, 119, 9405–9410. [Google Scholar] [CrossRef]
- Christe, K.O.; Wilson, W.W.; Petrie, M.A.; Michels, H.H.; Bottaro, J.C.; Gilardi, R. The Dinitramide Anion, N(NO2)2−. Inorg. Chem. 1996, 35, 5068–5071. [Google Scholar] [CrossRef] [PubMed]
- Östmark, H.; Bemm, U.; Langlet, A.; Sandén, R.; Wingborg, N. The Properties of Ammonium Dinitramide (ADN): Part 1, Basic Properties and Spectroscopic Data. J. Energ. Mater. 2000, 18, 123–138. [Google Scholar] [CrossRef]
- Golden, D.M.; McMillen, D.F.; Rossi, M.J. Low Pressure Thermal Decomposition Studies Selected Nitramine and Dinitramine Energetic Materials; ADA247972; SRI International: Menlo Park, CA, USA, 1992. [Google Scholar]
- VA, T.; OA, L.Y. Synthesis of Dinitramide Salts. In Proceedings of the 25th International Annual Conference of ICT, Karlsruhe, Germany, 28 June–1 July 1994; p. 13. [Google Scholar]
- Li, H.-M.; Li, G.-X.; Li, L.; Yao, Z.-P. Experimental study on thermal ignition and combustion of droplet of ammonium dinitramide based liquid propellant in different oxidizing gas atmospheres. Acta Astronaut. 2020, 169, 40–49. [Google Scholar]
- Ide, Y.; Takahashi, T.; Iwai, K.; Nozoe, K.; Habu, H.; Tokudome, S. Potential of ADN-based Ionic Liquid Propellant for Spacecraft Propulsion. Procedia Eng. 2015, 99, 332–337. [Google Scholar]
- Matsunaga, H.; Habu, H.; Miyake, A. Preparation and thermal decomposition behavior of ammonium dinitramide-based energetic ionic liquid propellant. Sci. Technol. Energ. Mater. 2017, 78, 65–70. [Google Scholar]
- Matsunaga, H.; Katoh, K.; Habu, H.; Noda, M.; Miyake, A. Thermal behavior of ammonium dinitramide and amine nitrate mixtures. J. Therm. Anal. Calorim. 2018, 135, 2677–2685. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, W.; Wang, M.; Chen, J.; Shen, Y.; Wei, Y.; Yu, X.; Li, F.; Zeng, H. Tunable diode laser absorption spectroscopy measurements of high-pressure ammonium dinitramide combustion. Aerosp. Sci. Technol. 2015, 45, 140–149. [Google Scholar] [CrossRef]
- Zeng, H.; Li, F.; Yu, X.-L.; Chen, L.-Z.; Yao, Z.-P.; Zhang, W. Mid-infrared absorption combustion diagnostics for an ADN based thruster. J. Exp. Fluid Mech. 2017, 31, 47–53. [Google Scholar]
- Wang, F.-Y.; Zhang, S.-H.; Yu, X.-L. Spectroscopic Study of Plasma Generate by Laser-Induced Breakdown of ADN-based Propellant. Phys. Gases 2020, 5, 38–46. [Google Scholar]
- Shen, J.-N.; Yu, Y.-S.; Liu, X.-H.; Cao, J. Experimental research on microwave ignition and combustion characteristics of ADN-based liquid propellant. Micromachines 2022, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-S.; Li, G.-X.; Zhang, T.; Chen, J.; Wang, M. Effects of Catalyst-Bed’s Structure Parameters on Decomposition and Combustion Characteristics of an Ammonium Dinitramide (ADN)-Based Thruster. Energy Conv. Manag. 2015, 106, 566–575. [Google Scholar] [CrossRef]
- Hou, Y.-Y.; Yu, Y.-S.; Li, Y.-L. Experimental study on microwave-induced puffing, micro-explosion, and combustion characteristics of ammonium dinitramide-based liquid propellant droplets. Phys. Fluids 2023, 35, 117122. [Google Scholar]
- Jiang, L.-Y.; Chen, Y.-T.; Mao, C.-T.; Han, J.-H.; Chen, A.-M.; Ye, J.-F. Research on the performance of ammonium dinitramide-based liquid propellant in pulse laser ablation micro propulsion system based on LIBS. Plasma Sci. Technol. 2024, 27, 015503. [Google Scholar] [CrossRef]
- Du, B.; Zheng, Y.; Mao, C.; Cui, H.; Han, J.; Jiang, L.; Ye, J.; Hong, Y. Transmissive Mode Laser Micro-Ablation Performance of Ammonium Dinitramide-Based Liquid Propellant for Laser Micro-Thruster. Micromachines 2023, 14, 1219. [Google Scholar] [CrossRef]
- Velardez, G.F.; Alavi, S.; Thompson, D.L. Molecular Dynamics Studies of Melting and Liquid Properties of Ammonium Dinitramide. J. Chem. Phys. 2003, 119, 6698–6708. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, W.-X.; Fan, X.-Z. Energetic characteristics and comprehensive properties of propellants containing ADN. Chin. J. Explos. Prop. 2015, 38, 81–85. [Google Scholar]
- Jing, L.; You, X.; Huo, J.; Zhu, M.; Yao, Z. Experimental and Numerical Studies of Ammonium Dinitramide Based Liquid Propellant Combustion in Space Thruster. Aerosp. Sci. Technol. 2017, 69, 161–170. [Google Scholar] [CrossRef]
- Jing, L.; Huo, J.; Wang, H.; You, X.; Zhu, M.; Yang, Y.; Yao, Z. Experimental Investigation on the Evaporation and Combustion Processes of Ammonium-Dinitramide-Based Liquid Propellant. J. Propuls. Power 2017, 33, 343–349. [Google Scholar] [CrossRef]
- Liu, X.; Su, G.; Yao, Z.; Yan, Z.; Yu, Y. Numerical Study of Flow Boiling of ADN-Based Liquid Propellant in a Capillary. Materials 2023, 16, 1858. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhao, M.; Xu, Y.; Li, G.; Zhang, H. Eulerian-Lagrangian Modelling of Detonative Combustion in Two-phase Gas-droplet Mixtures with OpenFOAM: Validations and Verifications. Fuel 2021, 286, 119402. [Google Scholar] [CrossRef]
- Gutiérrez Marcantoni, L.F.; Tamagno, J.; Elaskar, S. Rhocentralrffoam: An Openfoam Solver for High Speed Chemically Active Flows—Simulation of Planar Detonations–. Comput. Phys. Commun. 2017, 219, 209–222. [Google Scholar] [CrossRef]
- Dasgupta, A.; Gonzalez-Juez, E.; Haworth, D.C. Flame Simulations with an Open-source Code. Comput. Phys. Commun. 2019, 237, 219–229. [Google Scholar] [CrossRef]
- Poinsot, T.; Veynante, D. Theoretical and Numerical Combustion, 3rd ed.; R.T. Edwards, Inc.: Philadelphia, PA, USA, 2012. [Google Scholar]
- Issa, R.I. Solution of the Implicitly Discretised Fluid Flow Equations by Operator-Splitting. J. Comput. Phys. 1986, 62, 40–65. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).