Integrated Design and Simulation of Helicopter Nuclear, Biological, and Chemical Protection System
Abstract
1. Introduction
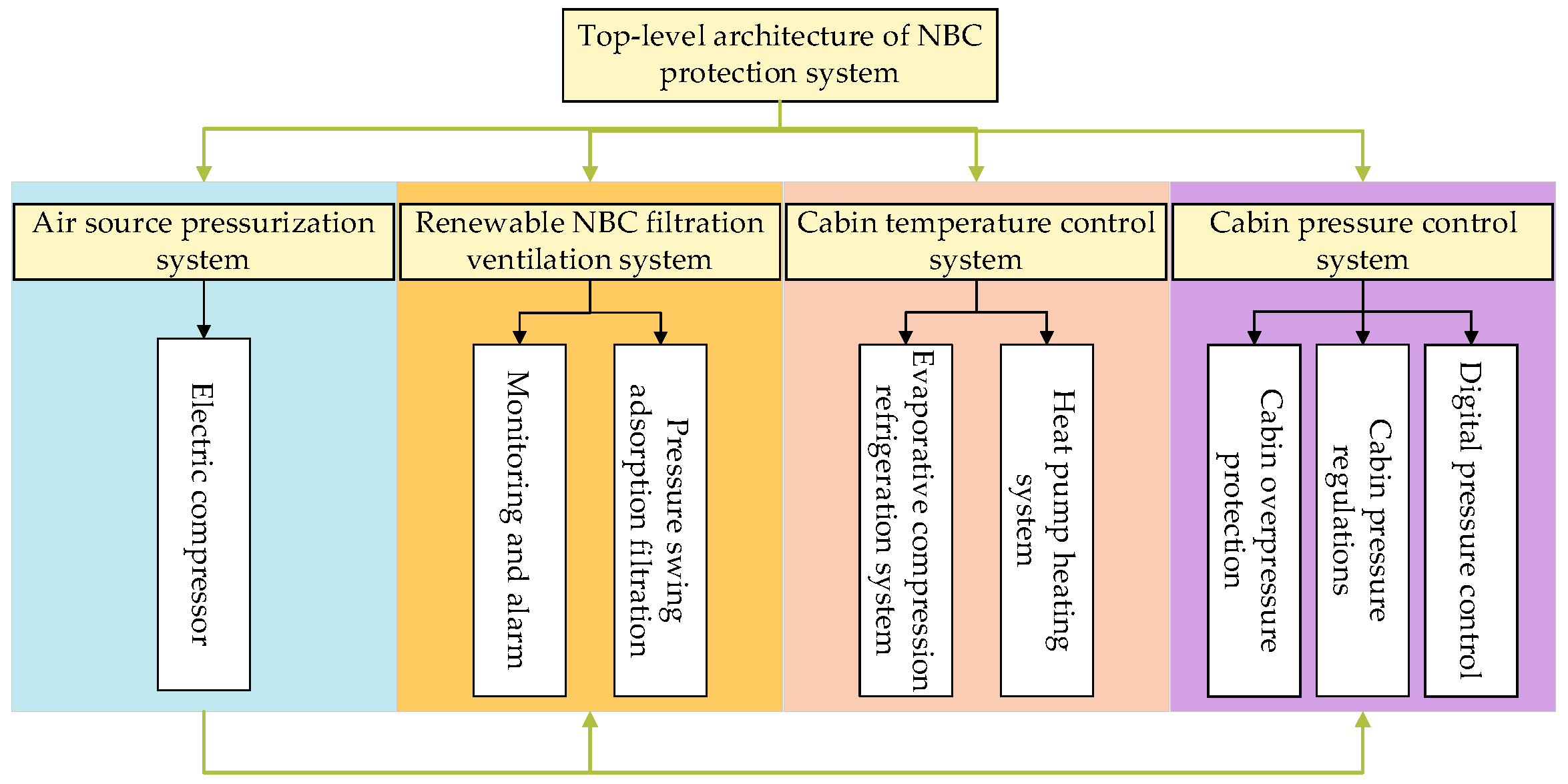
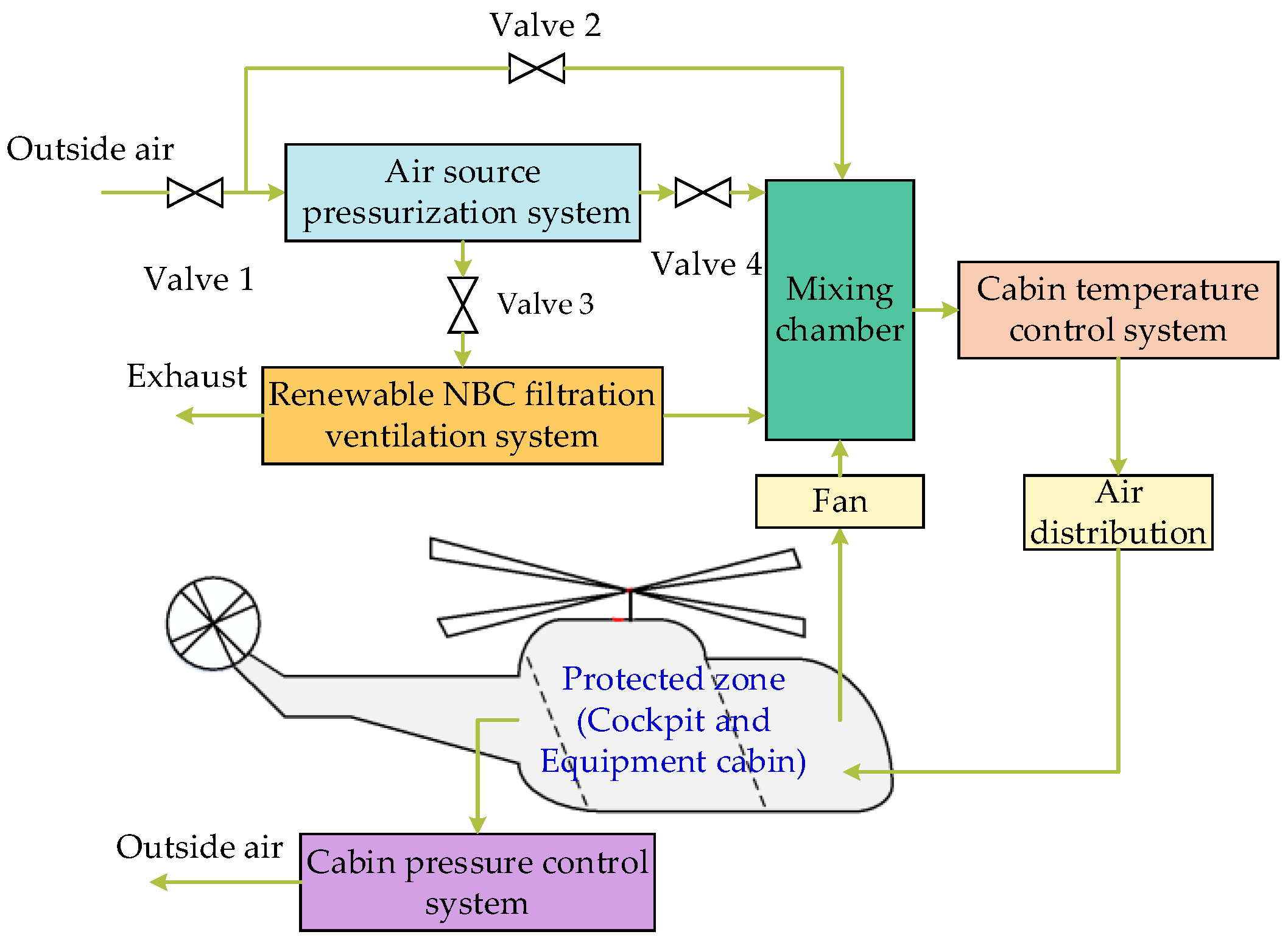
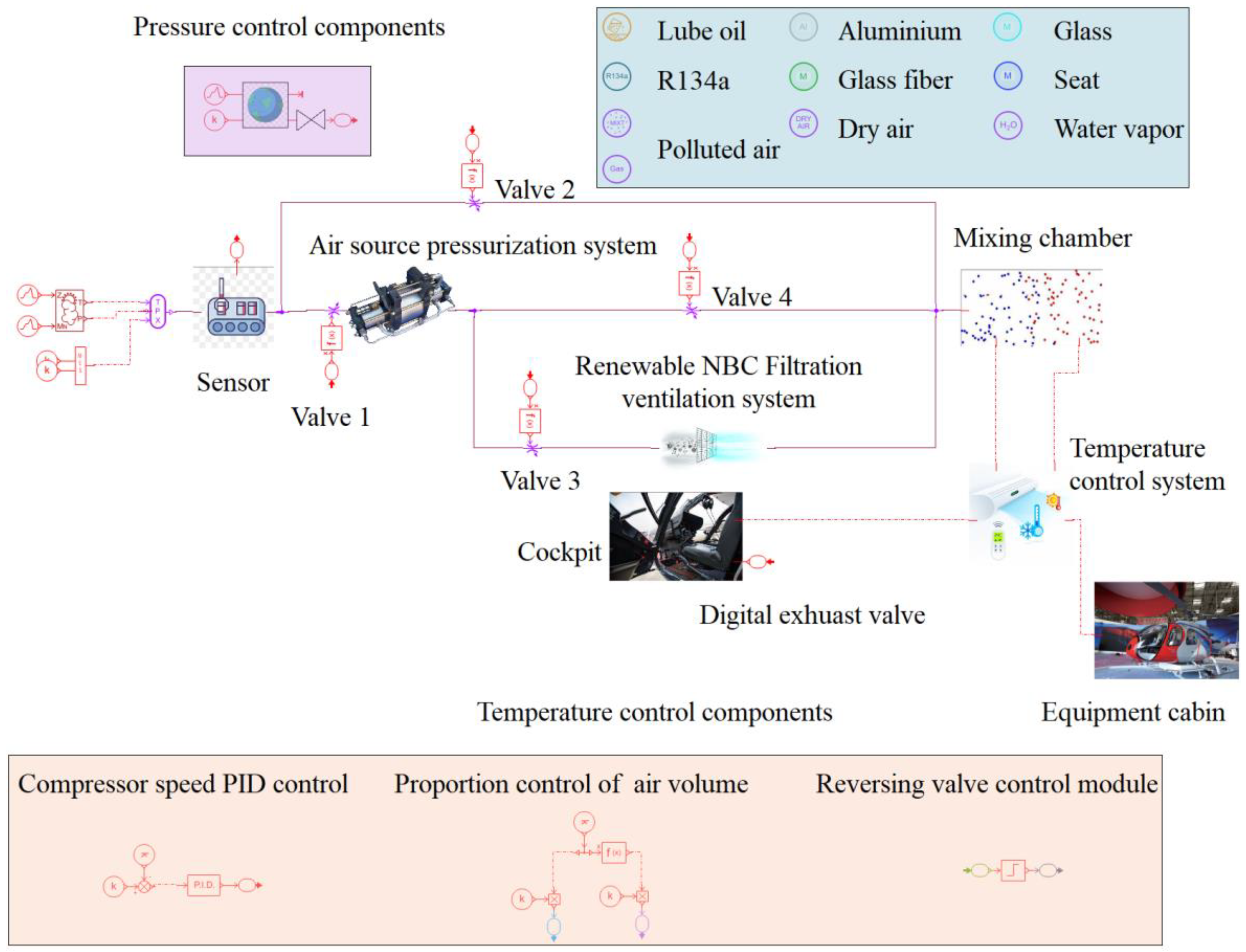
2. The Top-Level Architecture of the System
3. Subsystem Design
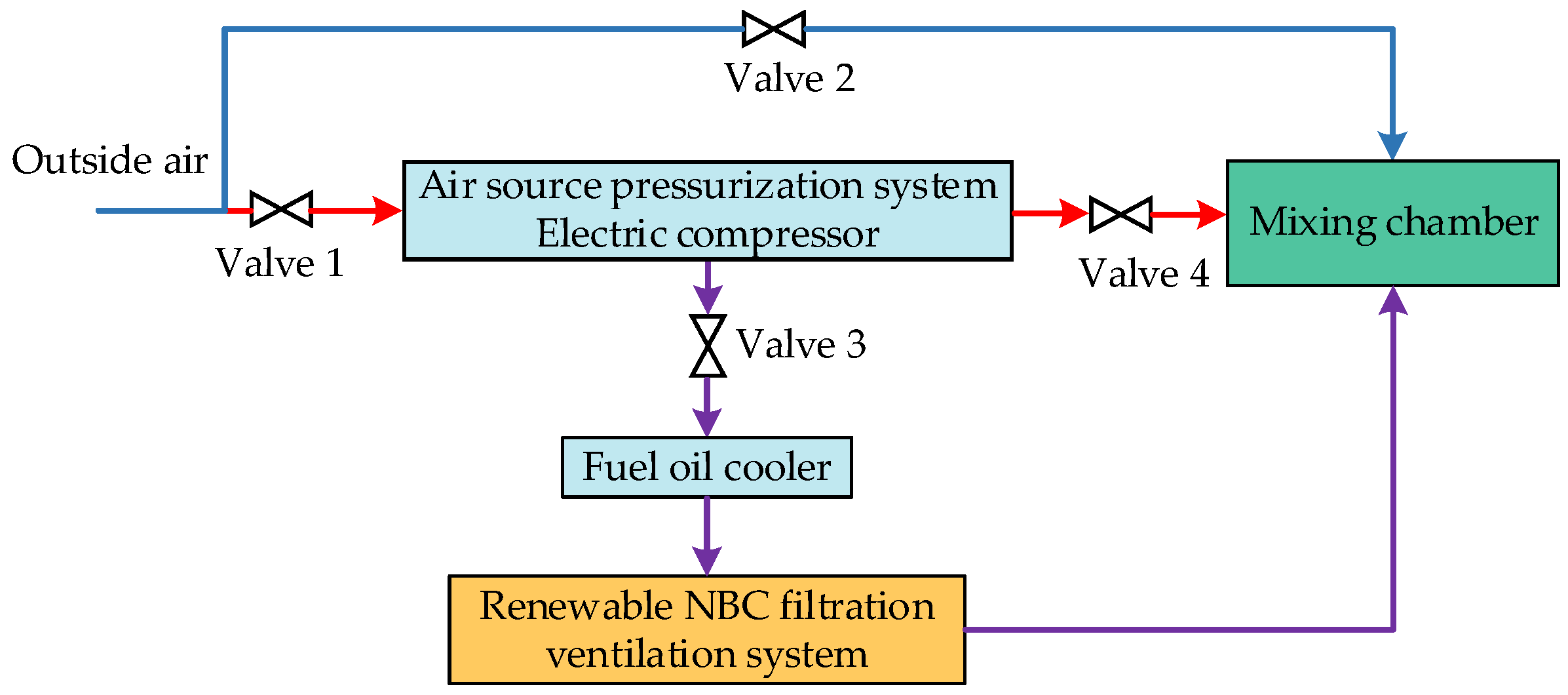
3.1. Air Source Pressurization System
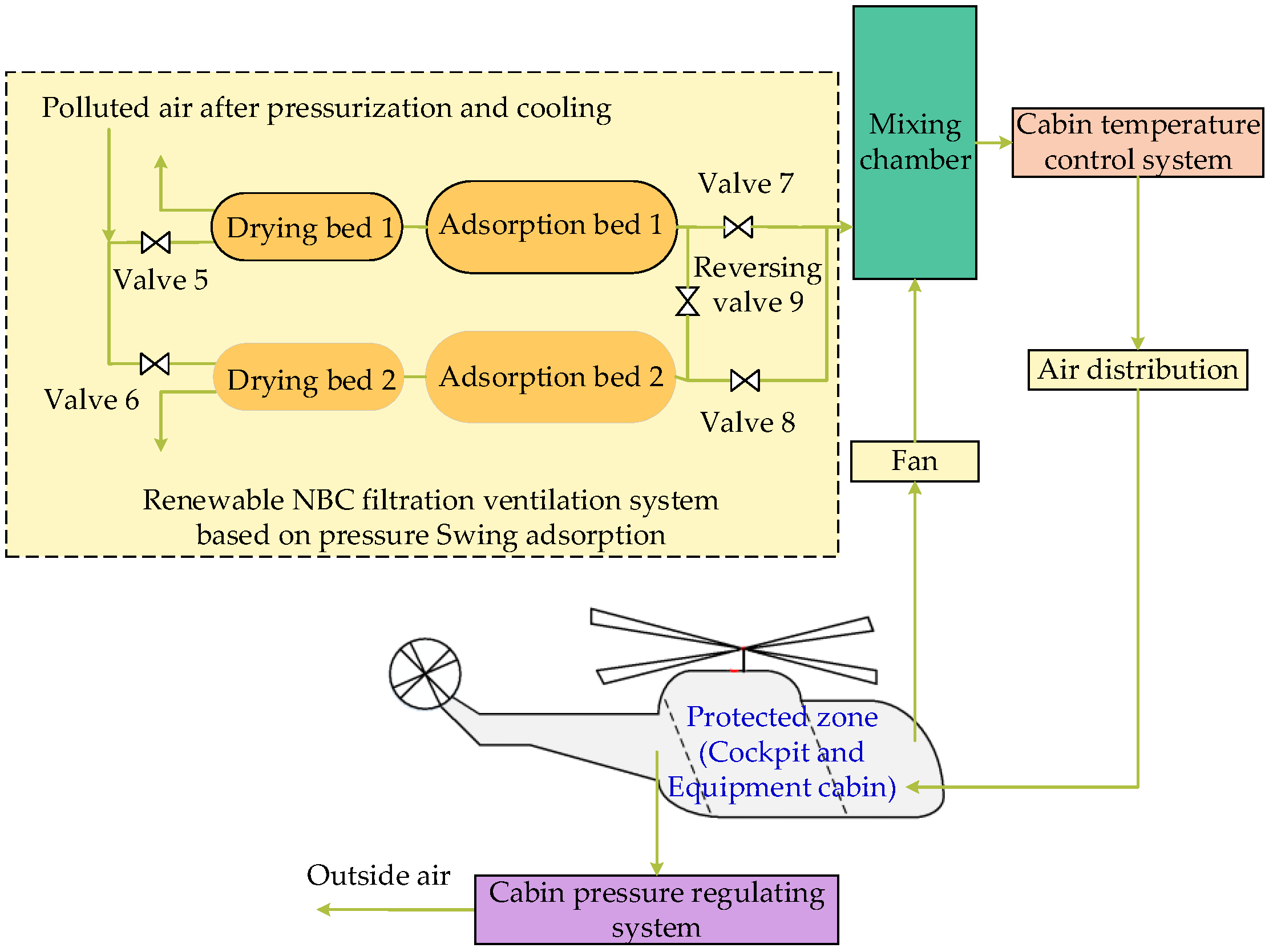
3.2. Renewable NBC Filtration Ventilation System
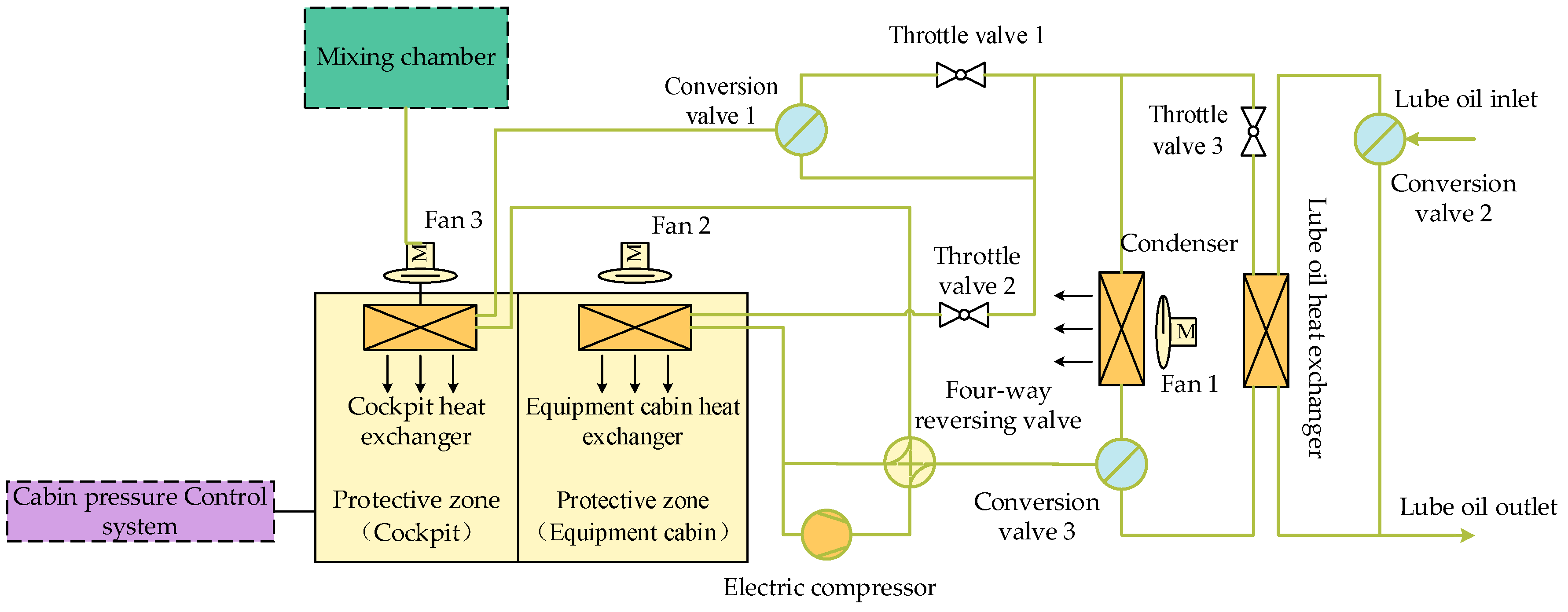
3.3. Cabin Temperature Control System
3.4. Cabin Pressure Control System
4. Mathematical Model
4.1. Air Source Pressurization System
4.1.1. Compressor
4.1.2. Fuel Oil Cooler
4.2. Renewable NBC Filtration Ventilation System
4.2.1. Poison
4.2.2. Adsorption Bed
4.2.3. Drying Bed
4.3. Cabin Temperature Control System
4.3.1. Two-Phase Heat Exchanger
4.3.2. Throttle Valve
4.4. Cabin Pressure Control System
4.4.1. Exhaust Valve
4.4.2. Cabin Overpressure Protection Threshold
5. Dynamic Simulation of Helicopter NBC Protection System
5.1. Simulation Model
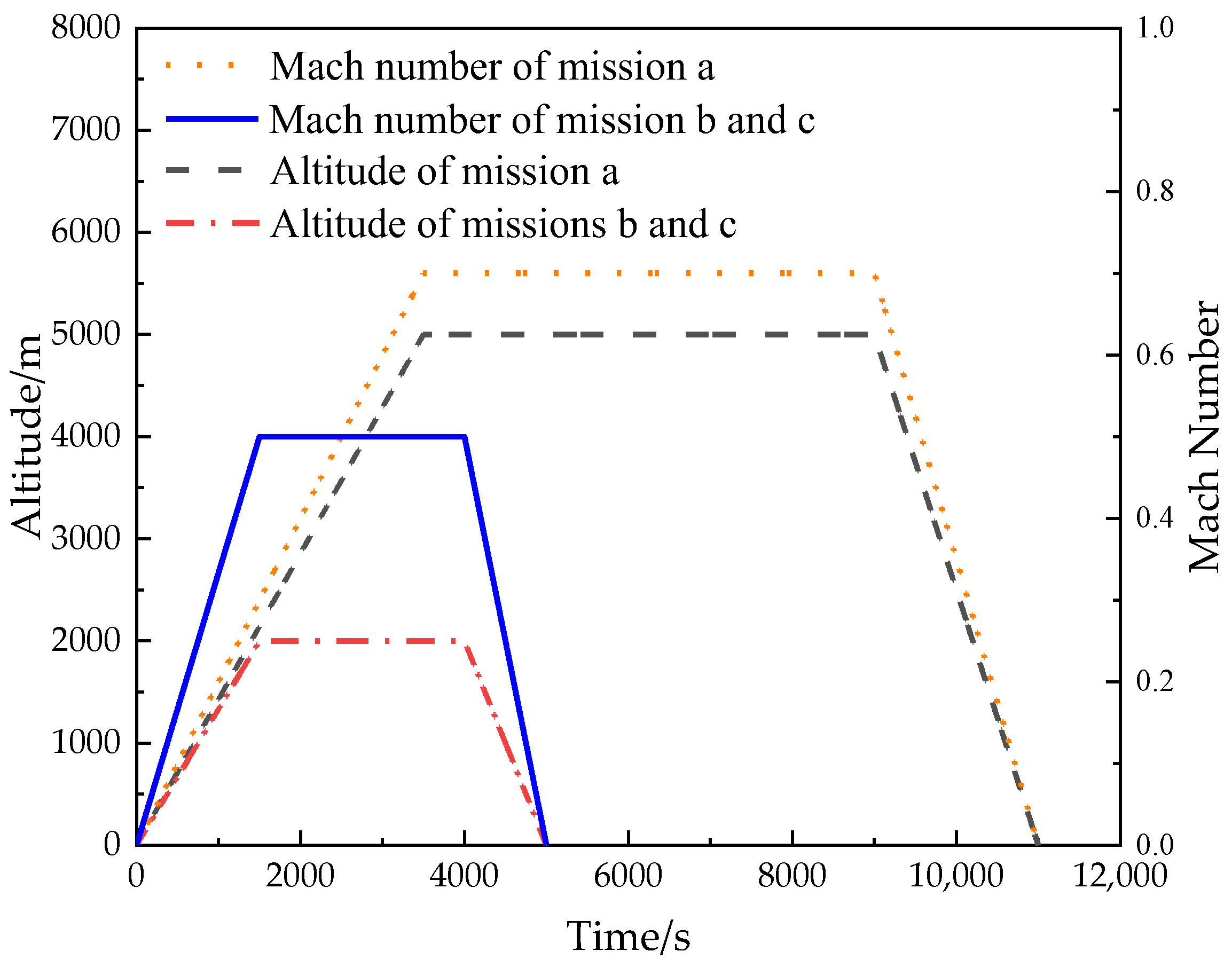
5.2. Simulation Parameter Setting
6. Results and Discussion
6.1. Pressure Control System Results and Discussion
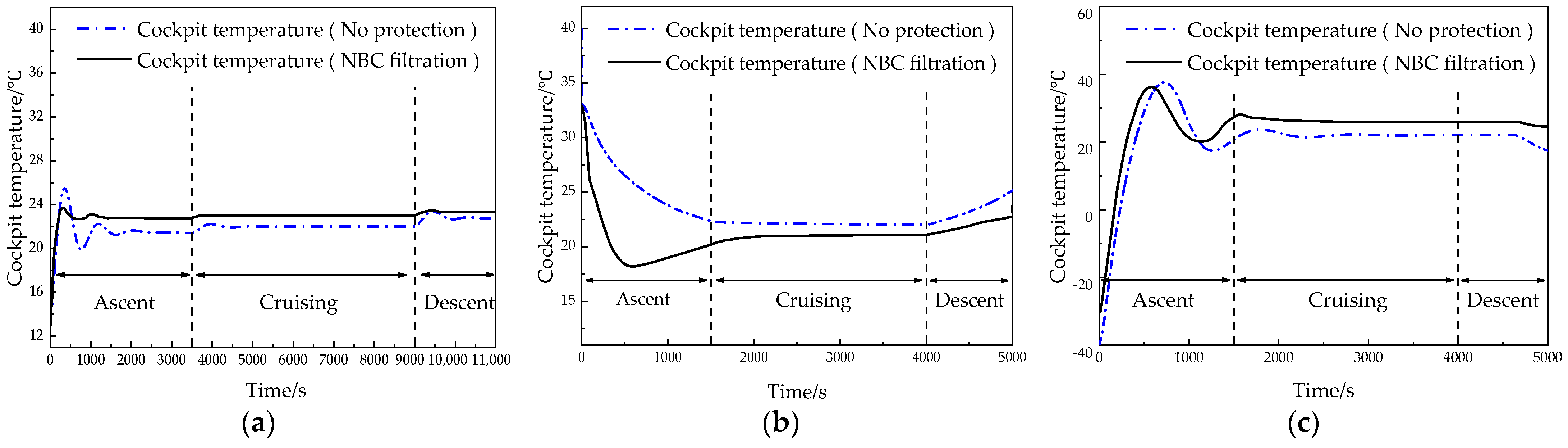
6.2. Temperature Control System Results and Discussion
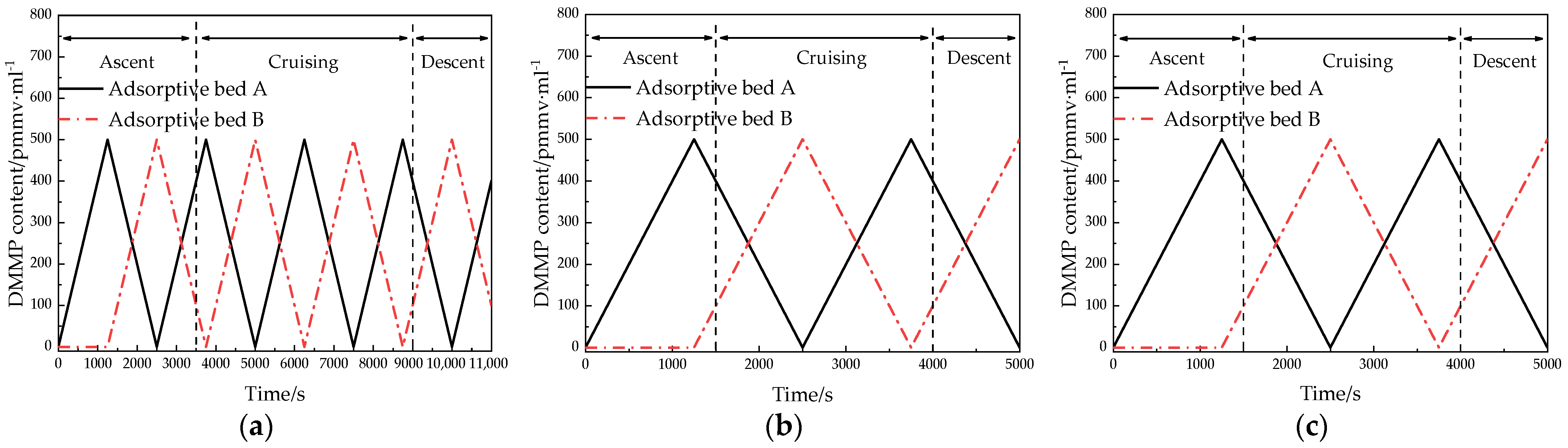
6.3. Renewable NBC Filtration Ventilation System Results and Discussion
7. Conclusions
- A helicopter NBC protection system was designed from a top-level architecture with an advanced system-integrated approach that can comprehensively achieve functions including air source pressurization, renewable NBC filtration ventilation, as well as cabin temperature and pressure control. The system considers the characteristics of comfort and safety and provides a strong safeguard for the helicopter aircrew to work in the NBC environment.
- Due to the short working lifespan of the traditional CPS and the need to replace the filter frequently, real-time renewable technology is adopted, which not only realizes the helicopter airborne renewable but also effectively prolongs the operational lifespan of the helicopter in the NBC environment.
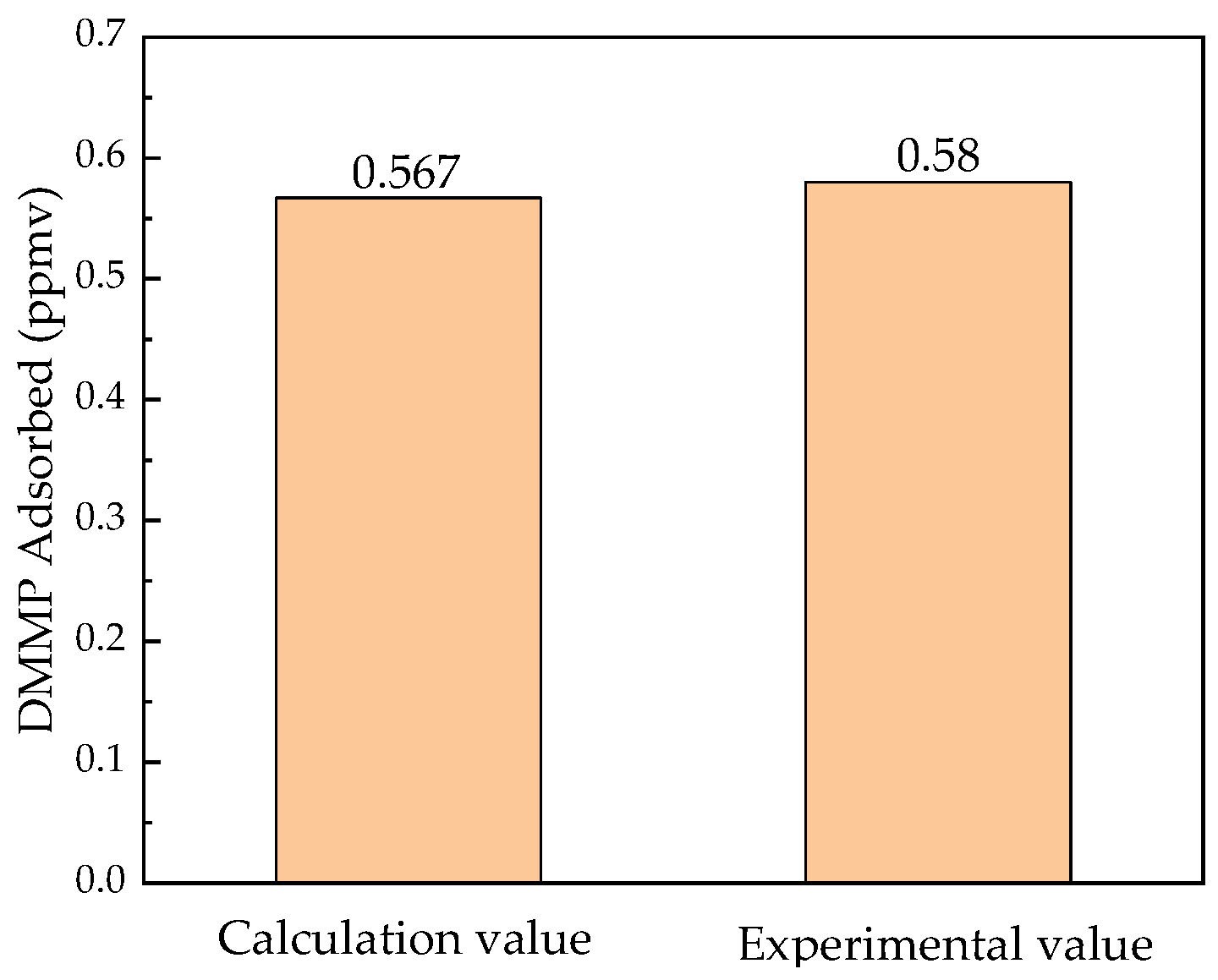
- The dynamic performance of the helicopter NBC protection system designed has been validated. The results demonstrate that the cockpit temperature and pressure can well satisfy the needs of aircrew with a helicopter; the renewable NBC filtration ventilation system realizes real-time airborne regeneration, and the maximum adsorption rate reaches 99.6%. The helicopter NBC protection system exhibits robust operation and can effectively adapt to significant changes in flight conditions.
- This study provides a novel idea for the design of helicopter NBC protection systems so as to further optimize the design of helicopter NBC protection systems.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Liu, H.; Wu, G.; Zhou, Y. Training effectiveness evaluation of helicopter emergency relief based on virtual simulation. Chin. J. Aeronaut. 2018, 31, 2000–2012. [Google Scholar] [CrossRef]
- Sun, X.; Liu, H.; Tian, Y.; Wu, G.; Gao, Y. Team effectiveness evaluation and virtual reality scenario mapping model for helicopter emergency rescue. Chin. J. Aeronaut. 2020, 33, 3306–3317. [Google Scholar] [CrossRef]
- Yi, Y.; Chen, R.; Ji, H. An active flow control over the ship deck for helicopter shipboard operations. Aerospace 2024, 11, 119. [Google Scholar] [CrossRef]
- Russell, R.L.; Hoskins, R.S. Compatibility of a pressure breathing for G system with aircrew chemical defense. In Proceedings of the 29th Annual Symposium, Las Vegas, NV, USA, 9–11 April 1991; SAFE Association: Las Vegas, NV, USA, 1991; pp. 258–263. [Google Scholar]
- James, C.H. US Navy Marine Corps programs for aircrew chemical biological (CB) protection. In Proceedings of the 29th Annual Symposium, Las Vegas, NV, USA, 9–11 April 1991; SAFE Association: Las Vegas, NV, USA, 1991; pp. 156–160. [Google Scholar]
- Self, B.P. A comparison of the field of view of three chemical defense masks. SAFE J. 1999, 29, 79–84. [Google Scholar]
- Władysław, H.; Zbigniew, S.; Marian, S.; Adam, B. Collective protection measures-methods to ensure clean air. Cent. Rzeczoznawstwa Bud. 2023, 5, 53–71. [Google Scholar]
- Vicary, A.G.; Wilson, J. Nuclear biological and chemical defense. RUSI J. 1981, 126, 7–12. [Google Scholar] [CrossRef]
- Miller, R.E.; Lamontagne, R.A. Test and Analysis of Missile Exhaust Effects on the Collective Protection System. Nav. Eng. J. 1996, 108, 299–314. [Google Scholar] [CrossRef]
- Boerum, R.E.; Buck, K.P. Naval Ship Design Implications of the Collective Protection System. Nav. Eng. J. 1984, 96, 29–32. [Google Scholar] [CrossRef]
- Liu, L.; Luo, Q. Exploration and Thinking on Collective Protection System of Floating Nuclear Power Plant. J. Phys. Conf. Ser. 2021, 1802, 022030. [Google Scholar] [CrossRef]
- Morrison, R.W. Overview of current collective protection filtration technology. In Proceedings of the NBC Defense Collective Protection Conference, Baltimore, MD, USA, 30 October 2002. [Google Scholar]
- Li, C.; Tang, S.; Xu, Y.; Liu, F.; Li, M.; Zhi, X.; Ma, Y. Ultrasonic-assisted activated carbon separation removing bacterial endotoxin from salvia miltiorrhizae injection. Ultrason. Sonochem. 2024, 103, 106781. [Google Scholar] [CrossRef]
- Jerez, F.; Ramos, P.B.; Córdoba, V.E.; Ponce, M.F.; Acosta, G.G.; Bavio, M.A. Yerba mate: From waste to activated carbon for supercapacitors. J. Environ. Manag. 2023, 330, 117158. [Google Scholar] [CrossRef] [PubMed]
- Skoczko, I.; Szatyłowicz, E.; Tabor, A.; Gumiński, R. Manufacturing Options for Activated Carbons with Selected Synthetic Polymers as Binders. Materials 2024, 17, 1753. [Google Scholar] [CrossRef] [PubMed]
- Swetha, G.; Gopi, T.; Shekar, S.C.; Ramakrishna, C.; Saini, B.; Krishna, R. Breakthrough studies of Methyl salicylate and DMMP Mixed in Methyl salicylate with Pressure swing adsorption composed of 13X Molecular sieves. Def. Life Sci. J. 2016, 1, 155–161. [Google Scholar] [CrossRef]
- Carr, R.; Comer, J.; Ginsberg, M.D.; Aksimentiev, A. Microscopic Perspective on the Adsorption Isotherm of a Heterogeneous Surface. J. Phys. Chem. Lett. 2011, 2, 1804–1807. [Google Scholar] [CrossRef] [PubMed]
- Khaaday, W.A.; Majid, S.A.; Shekar, S.C.; Tomar, R. Synthesis and characterization of various zeolites and study of dynamic adsorption of dimethyl methyl phosphate over them. Mater. Res. Bull 2013, 48, 79–86. [Google Scholar]
- Horning, H.K. Performance Assessment of the DDG-51 Collective Protection System. Nav. Eng. J. 1994, 106, 118–125. [Google Scholar] [CrossRef]
- Pang, L.; Luo, K.; Yuan, Y.; Mao, X.; Fang, Y. Thermal performance of helicopter air conditioning system with lube oil source (LOS) heat pump. Energy 2020, 190, 116446. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Shetty, S.; Kramlich, J.C.; Reinhall, P.G.; Novosselov, I.V. Hydrolysis of Dimethyl Methyl phosphonate (DMMP) in Hot-Compressed Water. J. Phys. Chem. 2020, 124, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Schoenitz, M.; Dreizin, E.L. Vapor-phase decomposition of dimethyl methylphosphonate (DMMP), a sarin surrogate, in presence of metal oxides. Def. Technol. 2021, 17, 1095–1114. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Gupta, A.K. Determination of thermodynamic parameters from Langmuir isotherm constant-revisited. J. Mol. Liq. 2017, 225, 137–146. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. Comparison of linearization methods for modeling the Langmuir adsorption isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Vieira, J.C.; Soares, L.C.; Froes-Silva, R.E.S. Comparing chemometric and Langmuir isotherm for determination of maximum capacity adsorption of arsenic by a biosorbent. Microchem. J. 2018, 137, 324–328. [Google Scholar] [CrossRef]
- Pang, L.; Zhao, M.; Luo, K.; Yin, Y.; Yue, Z. Dynamic temperature prediction of electronic equipment under high altitude long endurance conditions. Chin. J. Aeronaut. 2018, 31, 1189–1197. [Google Scholar] [CrossRef]
- Gorgy, E.; Eckels, S. Convective boiling of R-134a on enhanced-tube bundles. Int. J. Refrig. 2016, 68, 145–160. [Google Scholar] [CrossRef]
- Zajaczkowski, B.; Halon, T.; Krolicki, Z. Experimental verification of heat transfer coefficient for nucleate boiling at sub-atmospheric pressure and small heat fluxes. Int. J. Heat Mass Tran. 2016, 52, 205–215. [Google Scholar] [CrossRef]
- Sun, J.B.; Hyun, S.P.; Sung, J.Y.; Song, C.-H.; Choi, Y.-D.; Lee, S.-H.; Shin, J.-K. Study on the optimal heat supply control algorithm for district heating distribution network in response to outdoor air temperature. Energy 2015, 86, 247–256. [Google Scholar]
- Fu, L.; Tian, G.; Liang, B.; Shi, Q. Real Time Modeling and Simulation of Aircraft Cabin Pressure Control system. Computer Simulation. 2016, 2, 61–64+82. [Google Scholar]
- Fang, Y. Analysis of Overpressure and its Fluctuation of Naval Ships’ Collective Protection System. Chem. Def. Ships 2013, 3, 48–51. [Google Scholar]
- Qin, F.; Xue, Q.; Velez, G.M.A.; Zhang, G.; Zou, H.; Tian, C. Experimental investigation on heating performance of heat pump for electric vehicles at −20 °C ambient temperature. Energy Convers. Manag. 2015, 102, 39–49. [Google Scholar] [CrossRef]



| Parameter | Value |
|---|---|
| Adsorption temperature/K | 298.15 |
| Adsorption pressure/bar | 5 |
| Desorption temperature/K | 298.15 |
| Desorption pressure/bar | 1.013 |
| Title | Parameter | Value |
|---|---|---|
| Compressor | Displacement/cc·rev−1 | 180 |
| Volumetric efficiency | 0.6 | |
| Isentropic efficiency | 0.75 | |
| Mechanical efficiency | 0.9 | |
| Cockpit | Total air volume of the/(kg/s) | 1.79 |
| Size of the heat exchanger/mm | 737 × 240 × 70 | |
| Air-side heat exchange area/m2 | 14.2 | |
| Refrigerant-side heat exchange area/m2 | 0.59 | |
| The ratio between fresh and return air | 1.5:7.5 | |
| Equipment cabin | Total air volume/(kg/s) | 0.5 |
| Size of the heat exchanger/mm | 478 × 240 × 70 | |
| Air-side heat exchange area/m2 | 9.2 | |
| Refrigerant-side heat exchange area/m2 | 0.38 | |
| Adsorption bed | Adsorption rate/mL/s | 0.4 ppmv |
| Maximum adsorption capacity/ml | 500 ppmv | |
| Desorption bed | Desorption rate/mL/s | 0.4 ppmv |
| Materials | Wall materials | Aluminum, Glass Fiber |
| Refrigerant | R410A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Mao, X.; Wang, H.; Pang, L.; Zhao, Q. Integrated Design and Simulation of Helicopter Nuclear, Biological, and Chemical Protection System. Aerospace 2024, 11, 633. https://doi.org/10.3390/aerospace11080633
Wen Y, Mao X, Wang H, Pang L, Zhao Q. Integrated Design and Simulation of Helicopter Nuclear, Biological, and Chemical Protection System. Aerospace. 2024; 11(8):633. https://doi.org/10.3390/aerospace11080633
Chicago/Turabian StyleWen, Yilong, Xiaodong Mao, Hexiang Wang, Liping Pang, and Quanyu Zhao. 2024. "Integrated Design and Simulation of Helicopter Nuclear, Biological, and Chemical Protection System" Aerospace 11, no. 8: 633. https://doi.org/10.3390/aerospace11080633
APA StyleWen, Y., Mao, X., Wang, H., Pang, L., & Zhao, Q. (2024). Integrated Design and Simulation of Helicopter Nuclear, Biological, and Chemical Protection System. Aerospace, 11(8), 633. https://doi.org/10.3390/aerospace11080633







