4.2. Fuel Cell and Fuel Cell Stack
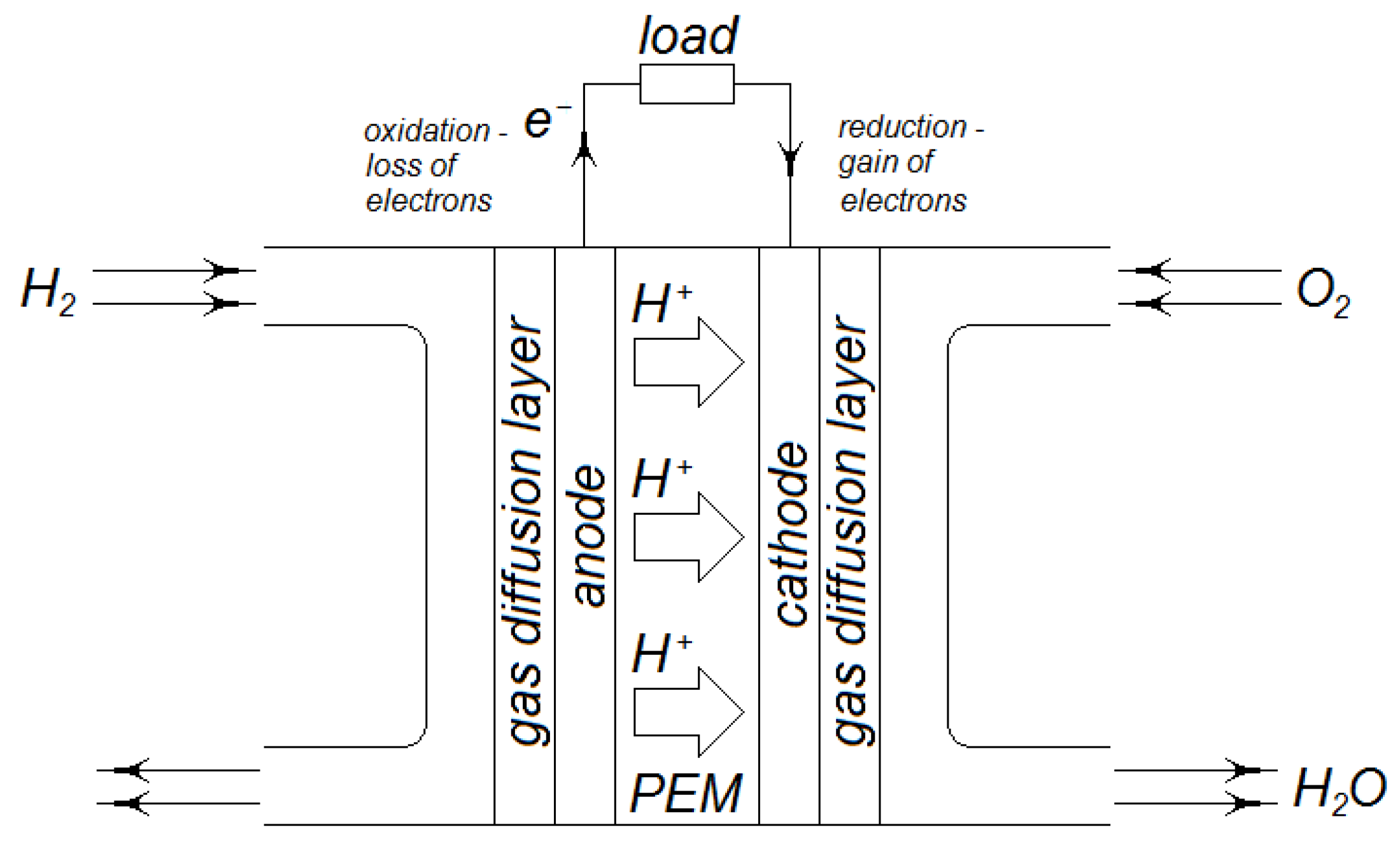
Figure 4 shows the layout of a basic individual proton exchange membrane (PEM) fuel cell. Hydrogen gas,
enters on the anode side of the cell, while compressed air at pressure
enters on the cathode side. Air consists of
oxygen gas
and
nitrogen gas
expressed as mole fractions. Therefore, the partial pressure of the atmospheric oxygen is
. A compressor controls the pressure of oxygen at the cathode, which can change the shape of the polarity curve and also compensate for variations in aircraft altitude. As the fuel cell operates at a temperature of approximately
, the nitrogen remains essentially inert, which means no oxide of nitrogen emissions. Liquid water
and the nitrogen gas
exit the cell on the cathode side.
Hydrogen molecules flow through a gas diffusion layer to the activation layer of the anode, which may contain platinum. This platinum acts as a catalyst, forming a triple-phase boundary (see ref. [
8]) which facilitates the breakup of hydrogen molecules into individual hydrogen atoms and then aids the hydrogen atoms in ionizing, becoming individual protons,
. The resulting free electrons are free to enter the anode. Therefore, at the anode, the following half-cell reaction occurs:
This reaction is an oxidization reaction (loss of electrons).
The protons migrate under the influence of an electrostatic force (see ref. [
9]) through the proton exchange membrane to the cathode. Oxygen molecules flow through a gas diffusion layer to the activation layer of the cathode, which may contain nickel. This nickel acts as a catalyst, again forming a triple-phase boundary, which facilitates the breakup of oxygen molecules into individual oxygen atoms, then aids the oxygen atoms, hydrogen ions, and electrons to form water. Therefore, at the cathode, another half-cell reaction occurs:
This reaction involves a reduction (gain of electrons).
The total chemical reaction taking place across the fuel cell is therefore as follows:
However, given that we induce air into the fuel cell stack rather than pure oxygen, our stoichiometric chemical reaction is as follows:
The stoichiometric air-to-fuel ratio for this reaction is .
During steady-state operation, as mentioned, hydrogen ions (protons) migrate from the anode under the influence of an electrochemical potential field through the membrane to the cathode. The polymer electrolyte membrane allows for the transportation of ions (protons) but does not allow for the transportation of hydrogen or electrons. When a conductor establishes an external bridge between the anode and the cathode, we meet the last requirement of the cathode half-cell reaction. This conductor allows the electrons produced by the oxidation of hydrogen to travel from the anode to the cathode, thus establishing an electric circuit. The resulting voltage between the anode and cathode can drive an electrical load.
The molar specific electrical work
that a polymer (proton) exchange membrane fuel cell can produce is
:
where the change in the molar Gibb’s function is the difference between the specific heat input to the cell and the change in the specific heat output of the cell. Note that
represents the change in the molar enthalpy for formation at standard conditions,
represents the absolute temperature, and
represents the change in molar entropy at standard conditions. For our reaction, the fact that
is negative indicates that the total chemical reaction taking place across the fuel cell (see above) has a strong driving force to proceed in the direction indicated by the arrow, i.e., to produce water.
The reversible potential difference
between the anode and the cathode as a voltage (remembering that voltage has the units of
) is the change in the molar Gibb’s function divided by both
, which represents the multiple of the number of electrons transferred for each mole of hydrogen (in this case
), and
, which represents Faraday’s constant, which is the charge of one mole of electrons.
The open cell voltage
is as follows:
where
is the local atmospheric pressure. Note that
is the pressure of the hydrogen,
is the pressure of the air, and
is the mole fraction of the oxygen in the air (see ref. [
7] for more details).
Figure 5 shows the open cell voltage. It is constant, independent of the ionic current density. The cell voltage is, however, not independent of the ionic current density. It is subject to losses called overvoltages (see ref. [
9]), namely the activation overvoltage
, the ohmic overvoltage
, and the concentration overvoltage
.
Figure 5 also shows these overvoltages as a function of the ionic current density.
The activation overvoltage occurs at low current densities. In any reaction, we require a certain activation energy to break existing chemical bonds before the chemical bonds of the final product/s release energy. It is the energy required to break the existing bonds that absorbs energy, which is therefore the source of the activation overvoltage. Ref. [
10] writes an equation for this overvoltage as a function of not just the current density but also as a function of the fuel cell temperature, the Faraday constant, the universal gas constant, the multiple of the number of electrons transferred for each mole of hydrogen, the oxygen partial pressure, and the vapor saturation pressure. We can therefore adjust the shape of the activation overvoltage by changing the pressure of the air entering the cathode side of the fuel cell.
The proton exchange membrane provides an electrical resistance to the flow of protons (or ionic current), which causes the voltage across the membrane to increase linearly with the current density.
Finally, when the current density is high, the concentration of reactants decreases. This reduces the voltage across the fuel cell at high current densities and can terminate the production of voltage. We sometimes call the concentration overvoltage the mass transport overvoltage. Like the activation overvoltage, [
10] also writes this equation as a function of the oxygen partial pressure. We can therefore adjust the shape of the concentration overvoltage by changing the pressure of the air entering the cathode side of the fuel cell.
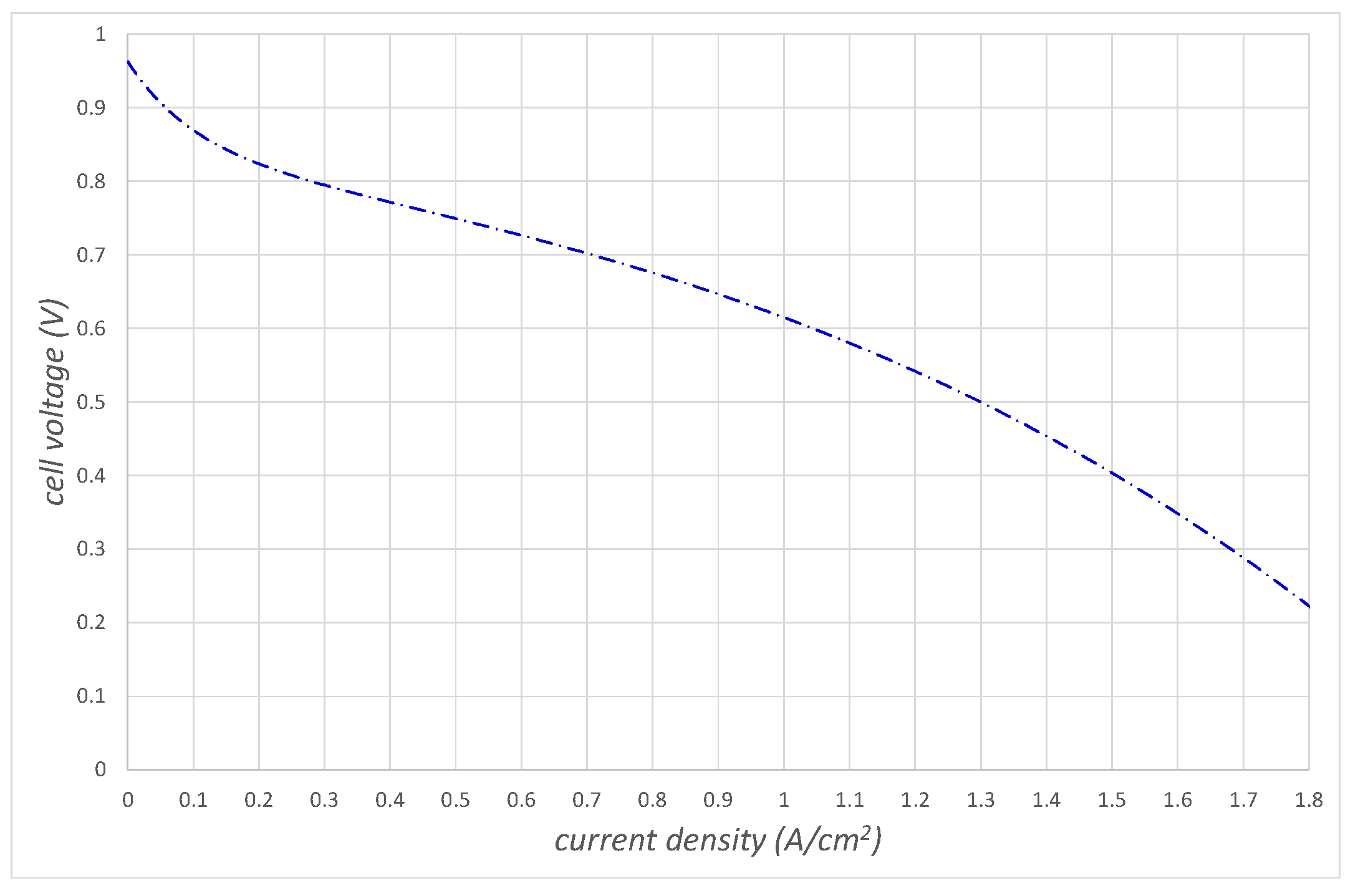
Figure 6 shows the cell voltage
as a function of the ionic current density, which we call the polarization curve, when we subtract these losses from the open cell voltage:
To generate these detailed curves, in this work, we have used the expressions for
,
,
, and
found in [
10].
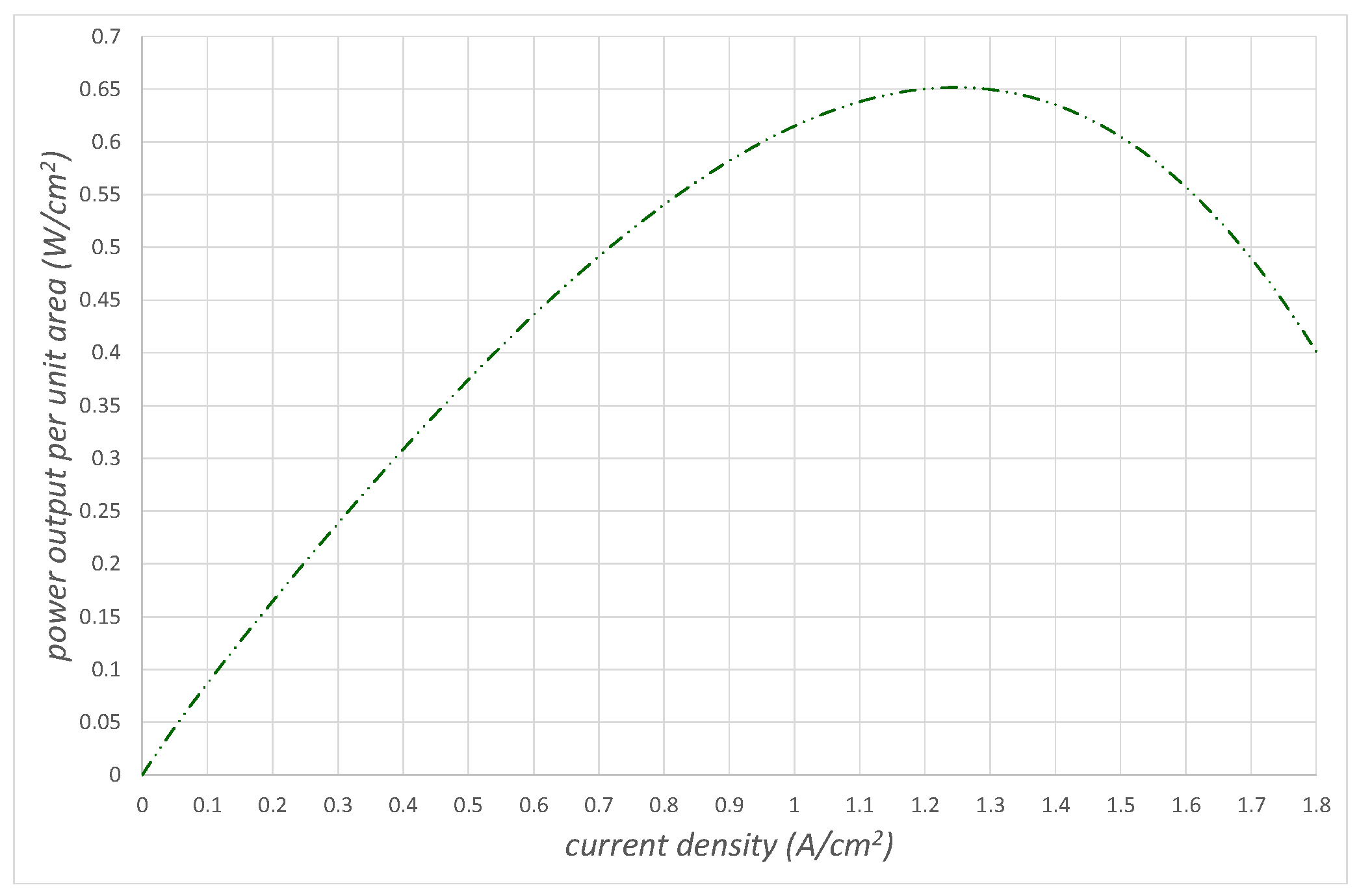
Figure 7 shows the power produced by the fuel cell
, which is the product of
and
, again as a function of the ionic current density. The power climbs to a maximum when the ionic current density is
before dropping off.
It is important to realize that we can change the shape of the activation and concentration overvoltages by changing the pressure of the air entering the cathode side of the fuel cell. This can have a significant effect on the shape of the polarization curve (see
Figure 6) and the power produced curve (see
Figure 7).
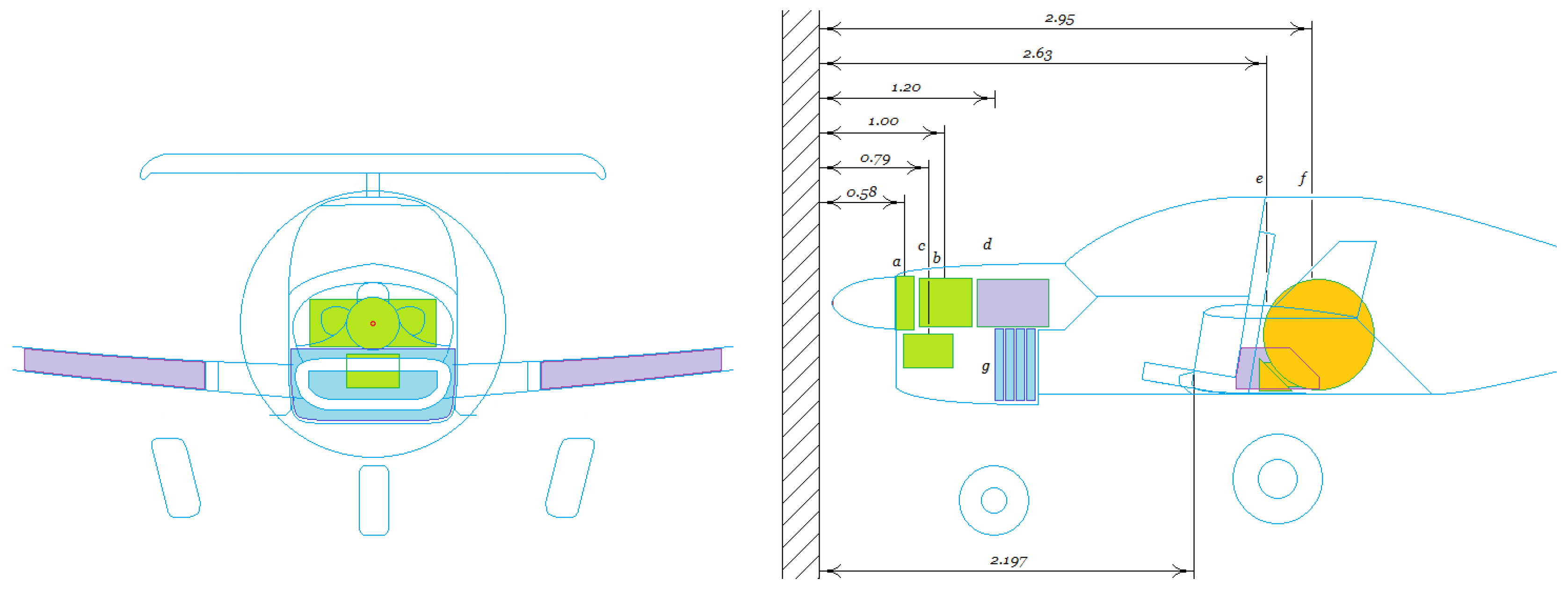
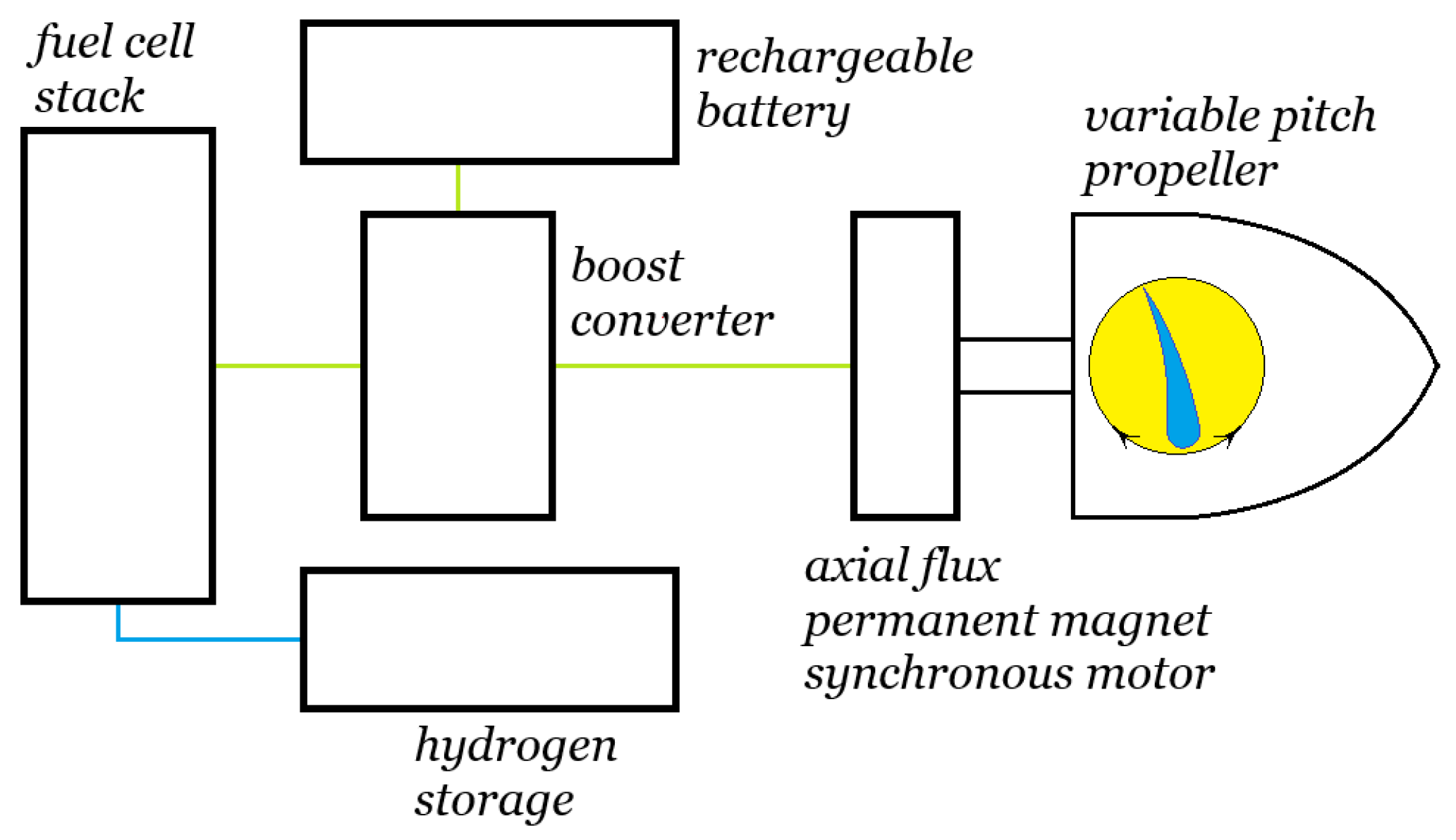
Figure 8 shows the fuel cell stack as the heart of the powertrain. It typically operates at a temperature of around
. Our fuel cell stack consists of
individual proton exchange membrane (PEM) fuel cells stacked side by side. The fuel cell stack’s voltage output
is the sum of the voltages across each of the individual fuel cell voltages. The flow of electrons through the external circuit
is the product of the current density across the cell
(due to the flow of protons across the polymer membrane) and the cross-sectional area
of the proton exchange membrane, i.e.,
, to balance the net charge entering the cathode.
To estimate the mass flow rate of hydrogen, we note that there are Avogadro’s number of molecules in a mole of , while there are two protons and two electrons in a hydrogen molecule. There are therefore electrons in a mole of hydrogen. If we multiply this by the charge on each electron, we obtain the charge liberated by a mole of at . This is twice the value of the Faraday constant.
If our fuel cell operates at a current density of
in cruise and
, then the current it produces is
or
. We can find the molar flow rate of
by dividing this current by the charge liberated by a mole of
. If we multiply this value by the molecular mass of
, we obtain the mass flow rate of
per cell. As there are
cells, the mass flow rate of
is
or
:
As our aircraft can initially store of hydrogen, its endurance is just over 2.5 h.
The mass flow rate of air required for stoichiometric conditions is therefore as follows:
The power required to compress this air from 0.540 bar at the service ceiling up to 2.5 bar is therefore as follows:
At , this power goes up to .
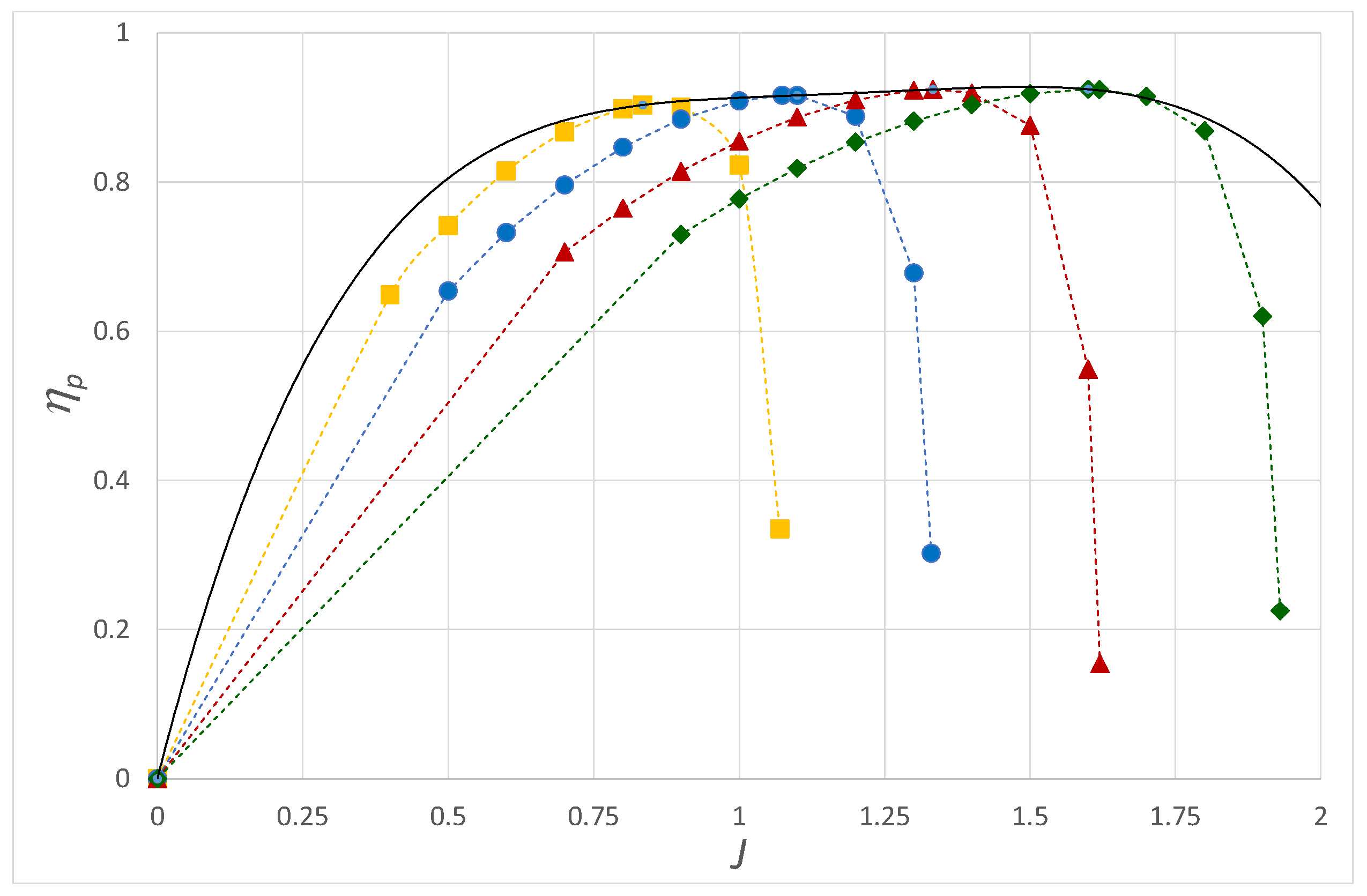
Figure 9 shows the efficiency of the fuel cell stack system
, which is equal to:
where
is the external battery power that drives the air compressor (see [
11]). The power that the battery provides to the compressor and the heat exchanger determines the extent to which we reduce the efficiency of the fuel cell stack system. These maxima occur at higher current densities as the battery power requirement increases.
In practice, we would like to run the fuel cell stack close to these maxima when the aircraft cruises near its design point. In doing so, it might be possible for the fuel cell system to operate when the aircraft cruises at an efficiency more than double that of an internal combustion engine running of AVGAS.
We can use the energy lost from both the fuel cell stack to promote the evaporation and subsequent heating of the cryogenic LH2, as its temperature needs to be the same as that of the fuel cell stack prior to entering the stack. At full power conditions for cruise, we estimate (see
Table 3) that the power required will be
. The efficiency of the fuel cell stack under these conditions is
. Therefore, the energy lost from the fuel cell stack is
. We estimate that the mass flow rate of hydrogen is
(
).
For hydrogen at
of pressure, the change in specific enthalpy required to change the hydrogen from a saturated liquid to a saturated vapor is
. Therefore, the rate at which we transfer heat to the saturated liquid to change it to saturated vapor is as follows:
To further raise the temperature of the hydrogen to
(
), we estimate from the data on the specific heat capacity of normal hydrogen (NIST) that the change in enthalpy of the hydrogen is
.
The energy required through heat transfer to the cryogenic hydrogen to raise it to a suitable temperature to enter the fuel cell stack is therefore . This represents a of the waste energy that we need to remove from the fuel cell stack.
Note also that we must keep the proton exchange membranes of each individual fuel cell humid to prevent permanent damage to the membranes, so a humidifier to humidify the hydrogen and air is a necessary component.
Figure 8 shows that we supply the humidifier with the water that is a product of the hydrogen–oxygen reaction in the fuel cell stack. Our water tank can be located so that it is passively heated by waste heat from the stack to prevent icing.
4.3. Thermal Efficiency and Heat Dissipation
At cruise, the powertrain needs to transfer of shaft power to the electric motor. The current density of the fuel cell stack will be set to , so the efficiency of the fuel cell stack will be (we ignore the boost controller efficiency). Therefore, we need to dissipate of heat to the surroundings. We have already determined that we will require of power to evaporate saturated liquid hydrogen and heat it to . Therefore, we will need a radiator to dissipate the remaining at cruise.
We will demonstrate at the end of this section that this is possible. However, if we can only dissipate this amount of power, we should limit the operation of the fuel cell to a current density of less than . This means that we cannot use the fuel cell to produce the “excess power” required for climb/maneuver. However, we can use the energy stored in the batteries to provide the “excess power” required for climb/maneuver.
Rechargeable lithium-ion batteries packs (as distinct from battery cells) currently have a gravimetric specific power of
[
12,
13] and a gravimetric specific energy of
or
. As we are carrying
of lithium-ion batteries, our batteries should be able to supply
of power to the system for
or 23 min and 20 s. Therefore, battery power will save us from having to run the fuel cell stack too hard.
Ideally, our aircraft can operate anywhere between sea-level at and the service ceiling at . Compared to their sea-level values, at the service ceiling, the local temperature can drop by nearly , while the local atmospheric static pressure can drop by . The local air density will drop from to , which means that at a given airspeed, the mass flow rate of a duct of fixed area (and hence the cooling power of the air) will drop by at the service ceiling. The saturation temperature of water is 99.6 at sea-level and 83.2 at the service ceiling.
Now, our fuel cell stack should not operate above , as the materials involved cannot cope with higher temperatures. Therefore, the water produced by the fuel cell stack always exits the fuel cell stack as a condensed liquid, even at the service ceiling. We can use this product water to humidify the fuel cell stack. However, our ability to cool the fuel cell stack with this product water is limited, owing to the fact that it cannot reach its saturation temperature.
The mass flow rate of the product water will be as follows:
A small battery-powered pump could spray this water to aid the cooling process in short bursts when deemed necessary. Note that the cooling effect of this water is . For our fuel cell stack, we need to cool the coolant water, the air entering the fuel cell stack and coolant from the electrical motor, and if necessary, the boost controller.
Ref. [
14] examined five different configurations of radiators used in the side pods of Formula One cars. These radiators cool water, oil, and charge air (from the turbocharger). The results of [
14] suggest that at a mass flow rate of air at
, these radiator configurations dissipate between
and
of power from the best configuration (con 1) to the worst configuration (con 2). The cooling demands of fuel cell aircraft are similar to the demands of a Formula One car.
The cross-sectional area of a Formula One radiator is approximately
If we utilize a radiator of this size and divide the radiator in two to form a vee, we have an area for each radiator of
. The size of the radiator inlet is
. Dividing by two, we obtain an inlet area for each radiator of
, so the area ratio for each duct will be
. Please note that this radiator size was already considered in
Section 3 above.
Negating the effects of the propeller wake, the mass flow rate of air approaching each radiator inlet at cruise speed of and at an altitude corresponding to the service ceiling of () is Given that we need to dissipate under these conditions, our radiators should be sufficient. To reduce the load on the coolant, we will place fins on all the fuel cell stack surfaces to increase the dissipation of energy to the surrounding air. To reduce the size of the radiator, one approach is to run the coolant through a heat pump. The heat pump compressor raises the pressure of the coolant. The coolant flows through the condenser radiator, which causes the refrigerant to condense first to a saturated vapor and then to a saturated liquid. We throttle the coolant in an isenthalpic process until it reaches the fuel cell stack (or evaporator) temperature and pressure. The azeotropic coolant causes the temperature of the fuel cell stack to remain constant , while the temperature of the refrigerant drops by a small amount before the phase of the refrigerant changes from liquid to saturated vapor. This approach is heavy, but a more detailed analysis will be required to assess this.
4.4. DC/DC Boost Converter
The design propeller speed for our light aircraft is set at , which is normal for this class of aircraft. The back electromotive force is likely to be in around , which means that the motor voltage must be greater than . The problem is that is often lower than half of this voltage depending on the operating point of the fuel cell stack.
Transformers step-up and step-down the voltages of alternating currents. An inverter can convert the direct current into an alternating current, the transformer can step-up the voltage, and a rectifier can convert the output alternating current into a direct current. We call such a converter an isolated DC/DC boost converter. Such a boost controller is likely to be very heavy [
15], so a non-isolated DC/DC boost converter is a better option.
Figure 10 shows the most basic type.
The MOSFET shown in
Figure 10 is a very high-speed switch. A pulse width modulator (PWM) gate driver controlled by a feedback mechanism activates this switch. The MOSFET has a cycle time denoted as
. The time period over which the signal switches the MOSFET “on” is
. The time period over which the signal switches the MOSFET “off” is
. We define the duty cycle as
.
Neglecting losses, in steady-state operation:
and:
so that the power exiting the circuit is equal to the power entering the circuit. The longer the duty cycle, the greater the voltage gain, but the longer the MOSFET has to cope with the input current.
The simple DC/DC boost converter circuit contains an inductor and a capacitor. Inductors store energy within a magnetic field, and capacitors store energy within an electric field. In steady-state operation, the rate at which the capacitor transfers energy to the electric motor is equal to the rate at which the inductor transfers energy to the capacitor.
What follows refers to steady-state operation. After start-up, increases with respect to until , where is a specified gain. This happens quickly in practice. Note that the higher the gain, the higher the value of that can be applied to the electric motor for a given , or the lower the value of for a given value of . This could lead to a reduction in the number of fuel cells required in the stack.
When the MOSFET is on, the inductor’s magnetic field stores energy. Meanwhile, the energy stored within the capacitor’s electric field drives the electric motor. For our aircraft, as 2.5, the voltage on the cathode side of the diode is very much higher than on the anode side, so no current flows across the diode. When the MOSFET is off, the collapse of the inductor’s magnetic field induces a high voltage across the inductor. In steady-state operation, the voltage across the inductor is approximately . The collapse of the inductor’s magnetic field produces a high voltage, which allows current to flow through the diode, thus enabling its energy transfer to the capacitor.
Figure 11 shows a more sophisticated two-phase interleaved boost controller. An interleaved boost controller can have multiple phases. The Toyota Mirai uses a four-phase interleaved boost controller [
15]. The advantage of interleaving is that it reduces the time period over which the input current passes through any individual MOSFET.
4.6. The Electric Motor
Old-fashioned brushed DC motors rely on mechanical commutation, whereas brushless DC motors use electronic commutation. The brushless DC motor is therefore more dependable than the brushed DC motor, as the brushes wear over time. Wilson & Trickey invented the brushless DC motor in the early 1960s [
17] after the invention of the transistor in the late 1940s made high-speed switching of electrical currents possible.
The brushless DC motor consists of a rotor incorporating permanent magnets and a stator which holds multiple coils (sometimes interconnected), which we call stator windings. The rotor is responsible for the transfer of rotational work from the machine, while the stator is attached to the motor’s frame.
Most brushless DC motors employ three-phase inverter systems and make use of three half-bridge circuits. We can connect the three windings in star or delta arrangements, but most prefer the star arrangement as it allows for lower currents. Each half-bridge contains two high-speed switches, one on the high side and the other on the low side of the electrical input. In
Figure 12, we show these switches as metal oxide silicon field effect transistors (MOSFETs), as they can manage higher currents than transistors.
Most brushless DC motors use Hall-effect sensors to enable the controller to know the rotational position of the rotor. Based on this knowledge, the controller signals the correct high and low-side MOSFET drivers to direct current to the appropriate stator windings. As the rotor turns, the sensors continually update the controller on the rotor position so that it can change which of the high and low-side MOSFET drivers to signal. By doing this, the stator windings create a magnetic field that rotates around the axis of the machine.
If we opt for trapezoidal control (see
Figure 13), the rotation of the magnetic field is not continuous but discreetly jumps. It is associated with torque output ripple, which may be detrimental when driving a propeller. If the propeller has a high rotational inertia, it may not affect the airflow in the vicinity of the blades but could cause propeller shaft fatigue problems. If the propeller has a low rotational inertia, it could cause the airflow to separate multiple times during each revolution. Pulse width modulation can reduce the voltage applied to the stator windings from that applied between the high-side and low-side voltages and is often employed to the high-side MOSFETs.
If we opt for sinusoidal control (see
Figure 14), we can expect negligible torque ripple, thus overcoming these problems. However, we need to know the spatial resolution rotor to a much greater extent for the triangular wave. This is also a form of pulse width modulation (see ref. [
18]).
Forces of attraction between “unlike” magnetic poles across the rotor and stator and forces of repulsion between “like” poles across the rotor and stator create a turning force that enables the rotor to synchronize with the rotating magnetic field. We maximize this turning force when the rotating poles of the magnetic field and the permanent magnetic field are perpendicular. Thus, the timing currents fed to the stator windings are such that they initiate 60 degrees before these fields become perpendicular.
The synchronous (or rotor shaft) speed of a brushless DC motor (in
) is:
where
is the number of poles on the rotor and
is the frequency of the alternating current supplied to the stator windings (or electromagnets). Alternatively, we can write:
where
is the number of pole pairs on the rotor. Therefore, for example, if the stator windings operate at a frequency of
, a two-pole machine, i.e.,
(
) will turn at
. Similarly, if the stator windings operate at a frequency of
, a six-pole machine, i.e.,
(
) will also turn at
.
The ideal type of brushless electric motor required for our aircraft is one that has a high power density, high efficiency, high heat dissipation characteristics, and negligible torque ripple. The main candidates are the radial flux, brushless direct current motor, and the axial flux brushless direct current motor. The direction of the magnetic flux lines differentiates these two machines. In the radial flux variant, the magnetic flux lines point in the radial direction with respect to the rotational axis of the machine, whereas in the axial flux machine, the magnetic flux lines point perpendicular to the axis of the machine.
Ref. [
19] compares both radial flux and axial flux brushless direct current motors. They compare two types of radial flux brushless DC motors, namely slotted and unslotted. They also compare six types of axial flux brushless DC motors, namely single-stator, single-rotor, single-stator, double-rotor (
SSDR or TORUS), double-stator, single-rotor (
DSSR), slotted, and unslotted. They do not mention the multi-stator, multi-rotor (
MSMR) machines, but they are outside the scope of this work and are meant more for a high-speed aircraft like the Rolls Royce “Spirit of Innovation” [
20].
Ref. [
19] rates the single-stator, double-rotor (
SSDR) axial flux BLDC machine “highest” under the categories of power density, efficiency, and torque ripple and “normal” for heat dissipation. It is the best type of motor for our aircraft.
Note that the power output of an electrical motor is independent of the local air density and hence altitude, unlike a reciprocating-piston, spark-ignition, internal combustion engine, which struggles to breathe at high altitudes. This is a major advantage of electrical motors, which has the potential to significantly increase the aircraft’s flight ceiling, or in our case, increase the power available at the existing flight ceiling. The air density at altitude limits cooling.
4.7. The Variable Pitch Propeller
Our aircraft will be propelled by a variable-pitch propeller that rotates at a constant angular velocity, namely which corresponds to under all flight conditions, e.g., take-off, climb, cruise, turn, descend, and landing. The electric motor provides this constant rotational speed. The advance ratio , where represents airspeed and is the propeller tip diameter, specifies these different flight conditions.
Figure 15 shows that the propulsive efficiency of a propeller blade with a fixed geometric pitch angle is a function of
. As
increases, the propulsive efficiency increases, reaching a maximum and then rapidly decreasing.
changes during flight, which means that if we use a fixed-pitch propeller, we are not always maximizing our propulsive efficiency
.
The figure shows four fixed geometric propeller pitch angles, i.e., , , , and , measured at of the blade radius. There is a locus of points for each geometric blade angle which connects each geometric blade angle’s with the advance ratio . Typically, a sixth-order polynomial is sufficient to connect this locus of points, giving us an equation which expresses as the subject of and hence . If we can vary the geometric pitch of our propeller blades by rotating them during flight, we can maximize our propulsive efficiency for any chosen advance ratio.
The power made available at the propeller
to match the power required by the aircraft is equal to the product of the power absorbed by the propeller
(or the motor output shaft power
) and the propulsive efficiency.
Once we specify the number
and geometry of the propeller blades, we can calculate the power absorbed by the propeller for a given geometric blade angle
. And as we will know
and
, we can calculate the power available at the propeller (see [
21]).