Proof of Principle of the Lunar Soil Volatile Measuring Instrument on Chang’ e-7: In Situ N Isotopic Analysis of Lunar Soil
Abstract
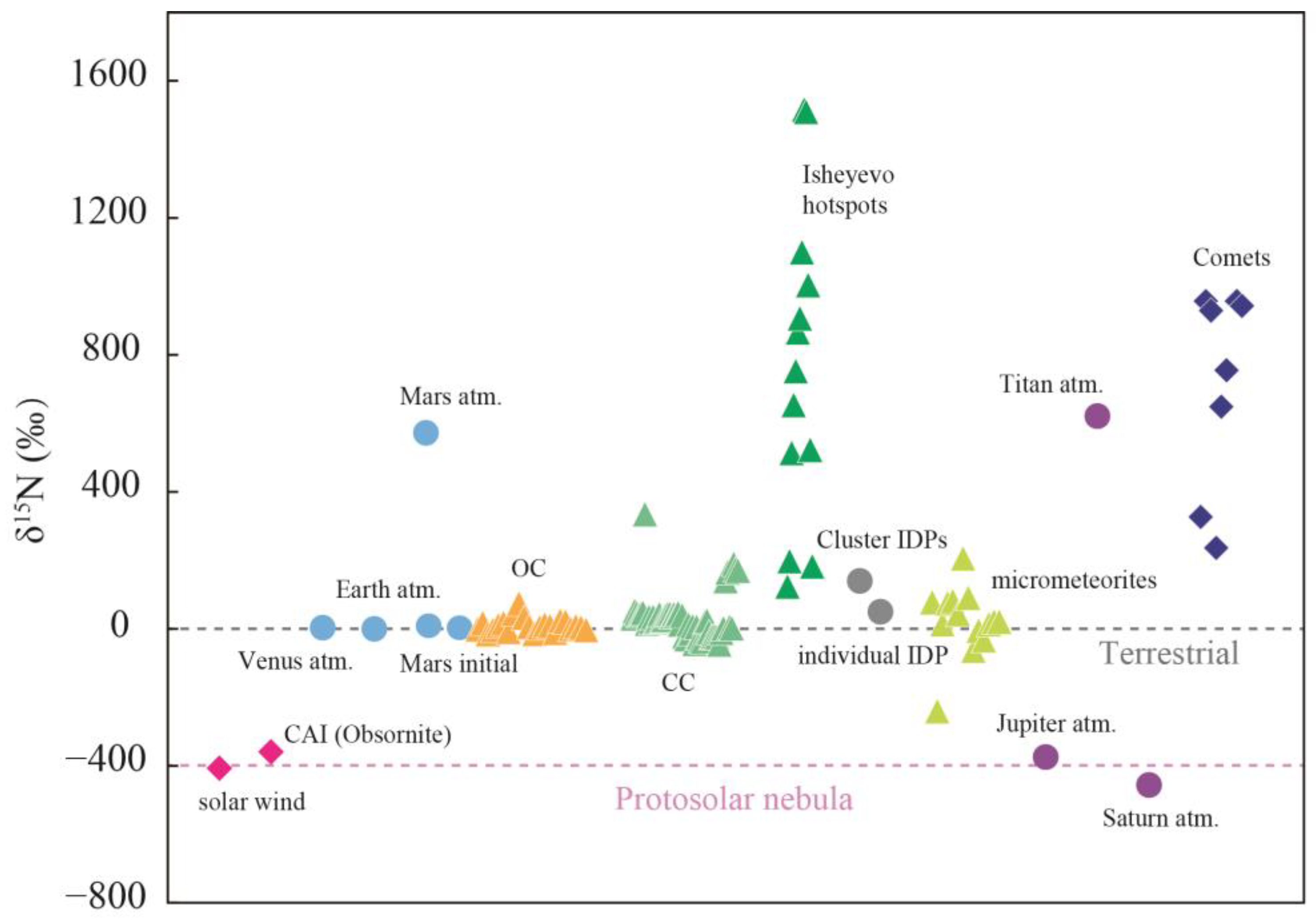
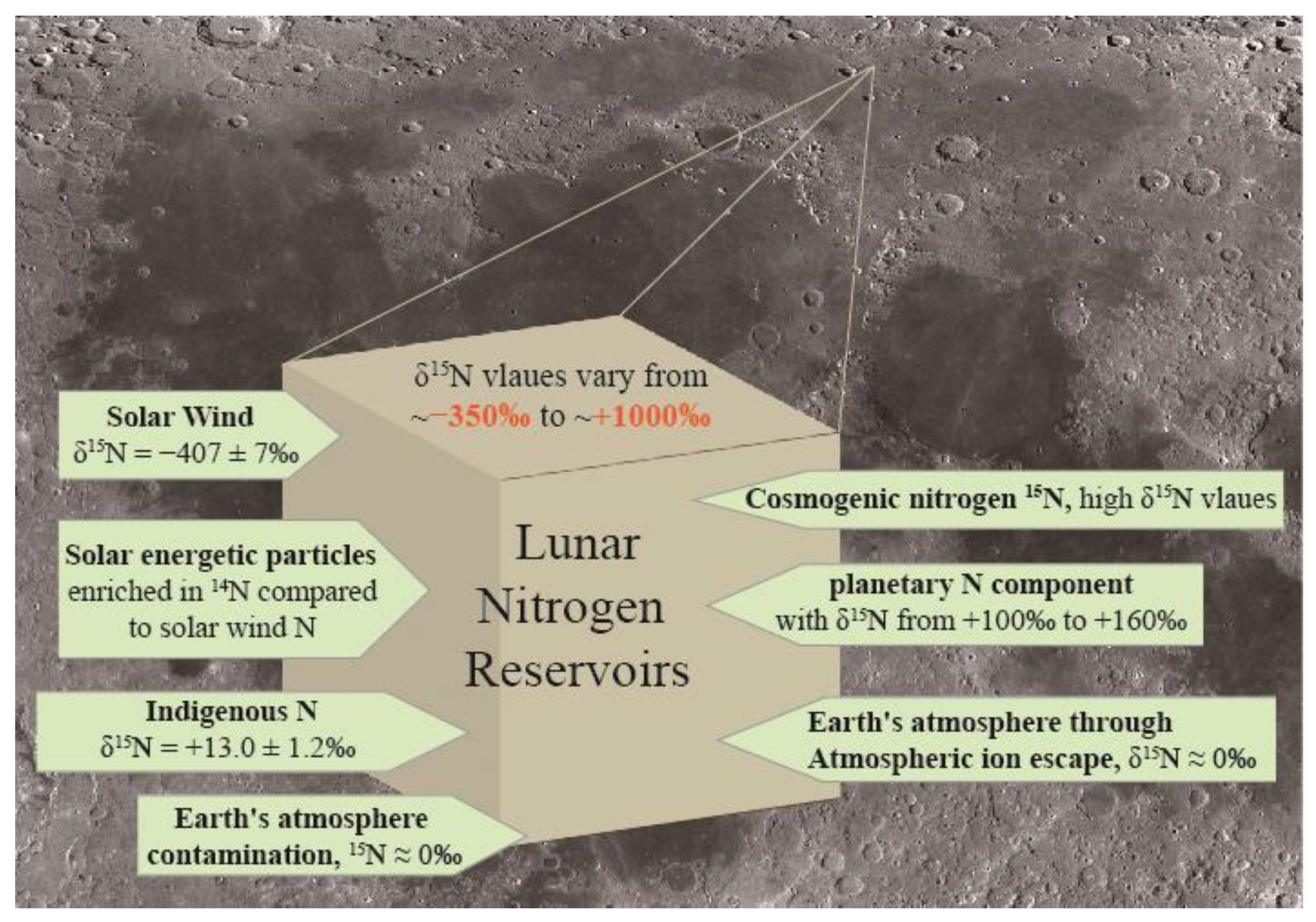
1. Introduction
- 1.
- Implantation of solar wind ions
- 2.
- Implantation of solar energetic particles (SEP)
- 3.
- A planetary nitrogen component
- 4.
- Implantation of Earth wind (EW) ions
- 5.
- Indigenous lunar nitrogen
- 6.
- Cosmogenic nitrogen
2. In Situ N Isotopic Analysis on Orbit
2.1. Development Status of In Situ N Isotopic Analysis on Orbit
2.2. Process Design of In Situ N Isotopic Analysis on Orbit
3. Devices and Procedures for the Ground Verification Test
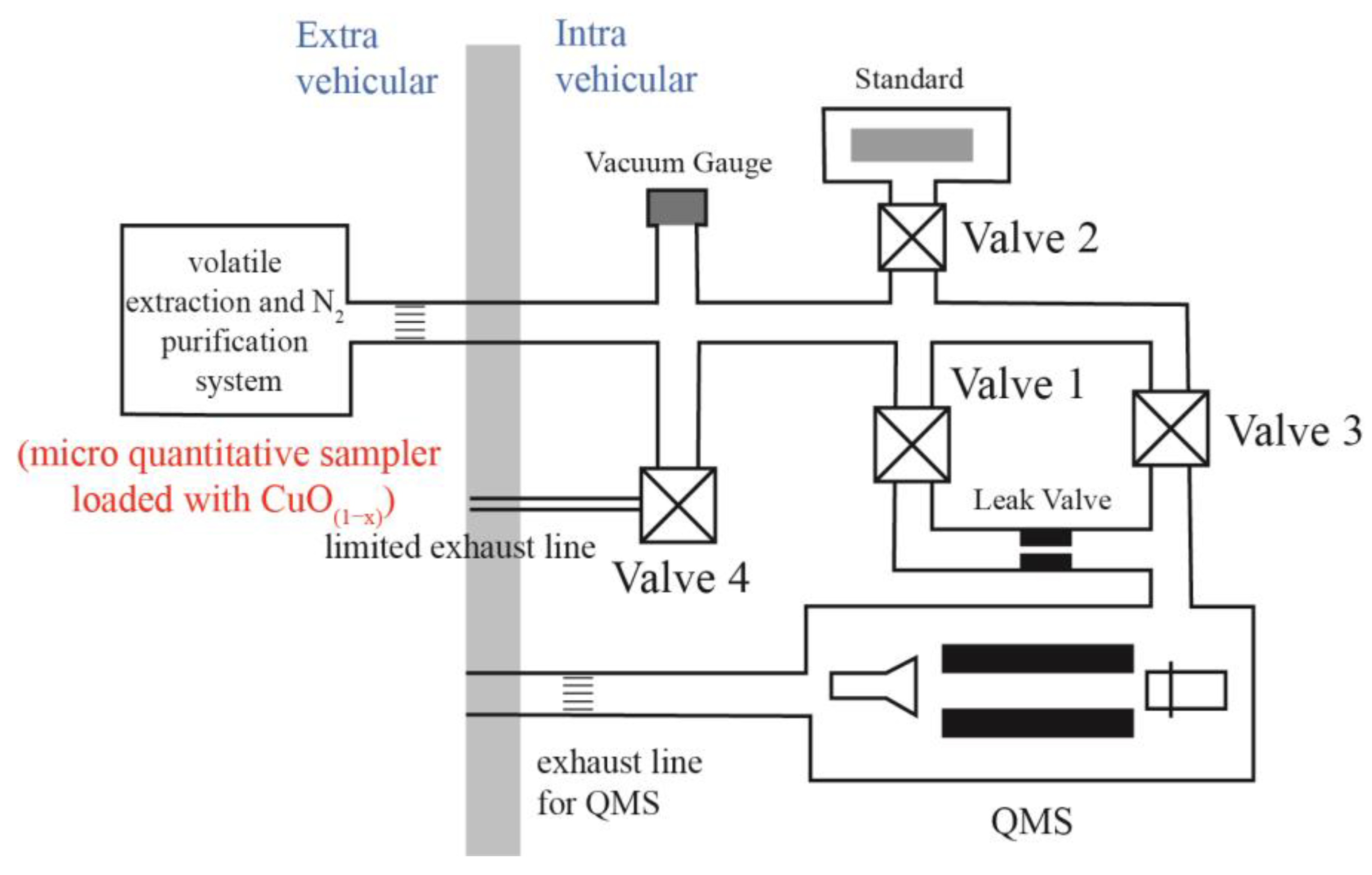
3.1. Test Devices
3.1.1. Gas Inlet Sections
3.1.2. Gas Purification Section
3.1.3. Gas Measurement Section
3.2. Test Procedural
3.2.1. Nitrogen Extraction
- (1)
- Open v5 so that the air in the stainless cylinder can diffuse into valve v6. Maintain for 2 min until the diffused gas reaches an equilibrium state;
- (2)
- Close v5 and open v6 to make the gas between valves v5 and v6 diffuse into the gas purification section. Maintain for 2 min; and
- (3)
- Close v6.
3.2.2. Nitrogen Purification
- (1)
- Once the gases from the gas inlet Section 2 have been introduced to the gas purification section, v1 is closed so that part of the gas is introduced into the CuO(1−x) furnace while the remains are directly introduced into the CT.
- (2)
- Open v3 and adsorb CO2, H2O, and other condensable gases for 10 min at liquid-nitrogen temperature. This step can be used to evaluate the effect of the nitrogen purification section.
- (3)
- Open Vc and then introduce the gas into the gas measurement section for nitrogen analysis.
- (4)
- Simultaneously with steps (2) and (3), the gases in the CuO(1−x) furnace are reacted for 10 min with pure O2, produced by heating the CuO(1−x) particle at 850 °C. During this process, carbonaceous gases, such as CO, CH4, and other hydrocarbons, are oxidized to CO2, hydrogen is oxidized to H2O, and sulfur is oxidized to SO2.
- (5)
- After 10 min, excess O2 needs to be reabsorbed back onto the CuO(1−x) particle, first at 600 °C for 15 min and then at 450 °C for 15 min, since in the mass spectrometer oxygen will react with carbon on the filament [73] so that the result will be the same as if the nitrogen is contaminated by CO.
- (6)
- The oxidized gases can then be introduced into the CT by opening v1 to let the condensable gases adsorbed in a glass tube CT held at liquid-nitrogen temperature for 10 min.
- (7)
- The purified nitrogen is then admitted to the mass spectrometer through a leak valve by opening Valve C for the N isotope analysis.
3.2.3. Nitrogen Isotope Analysis
4. Standards, Blanks, and Corrections
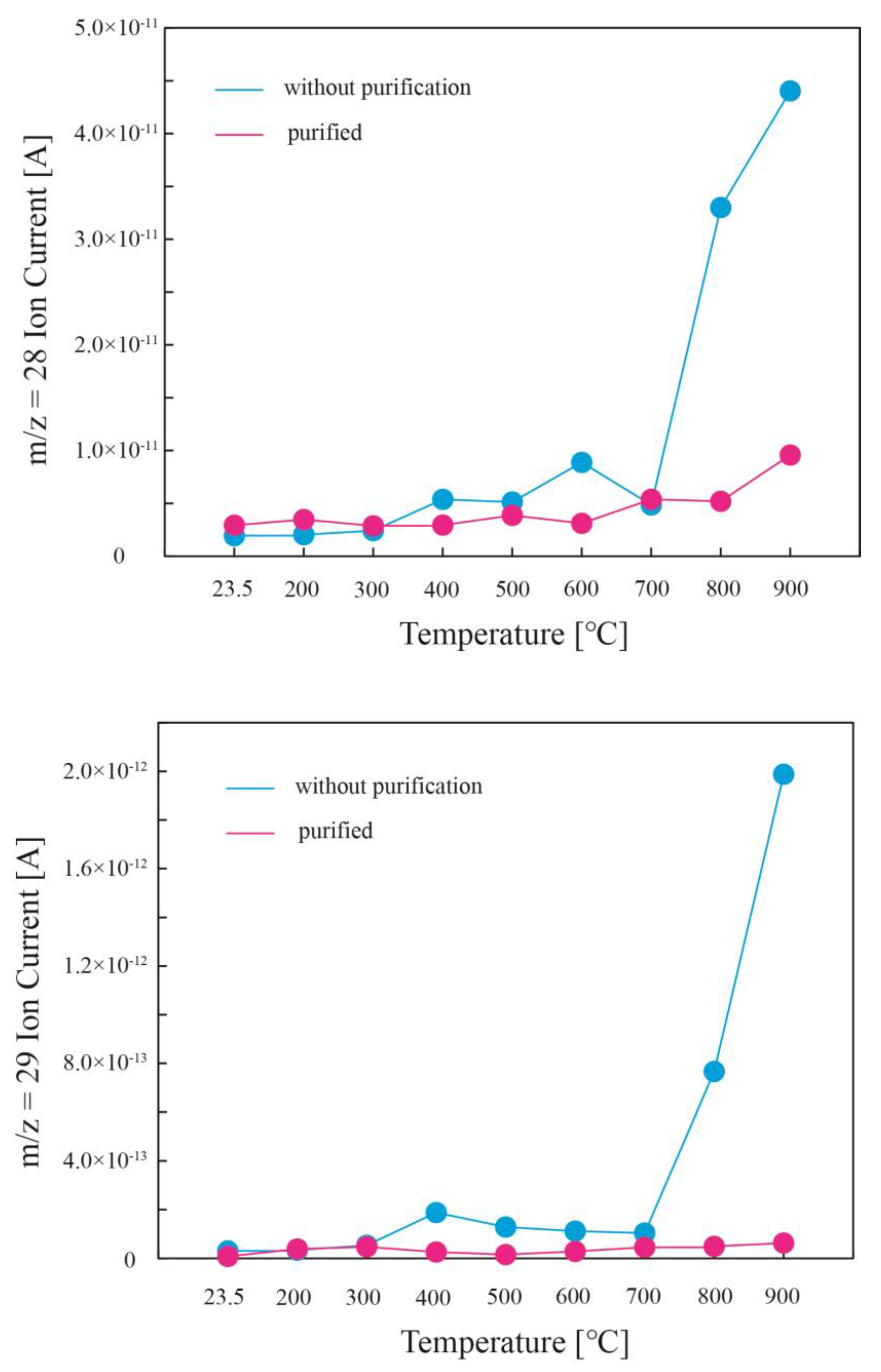
4.1. Blank Contribution and Correction
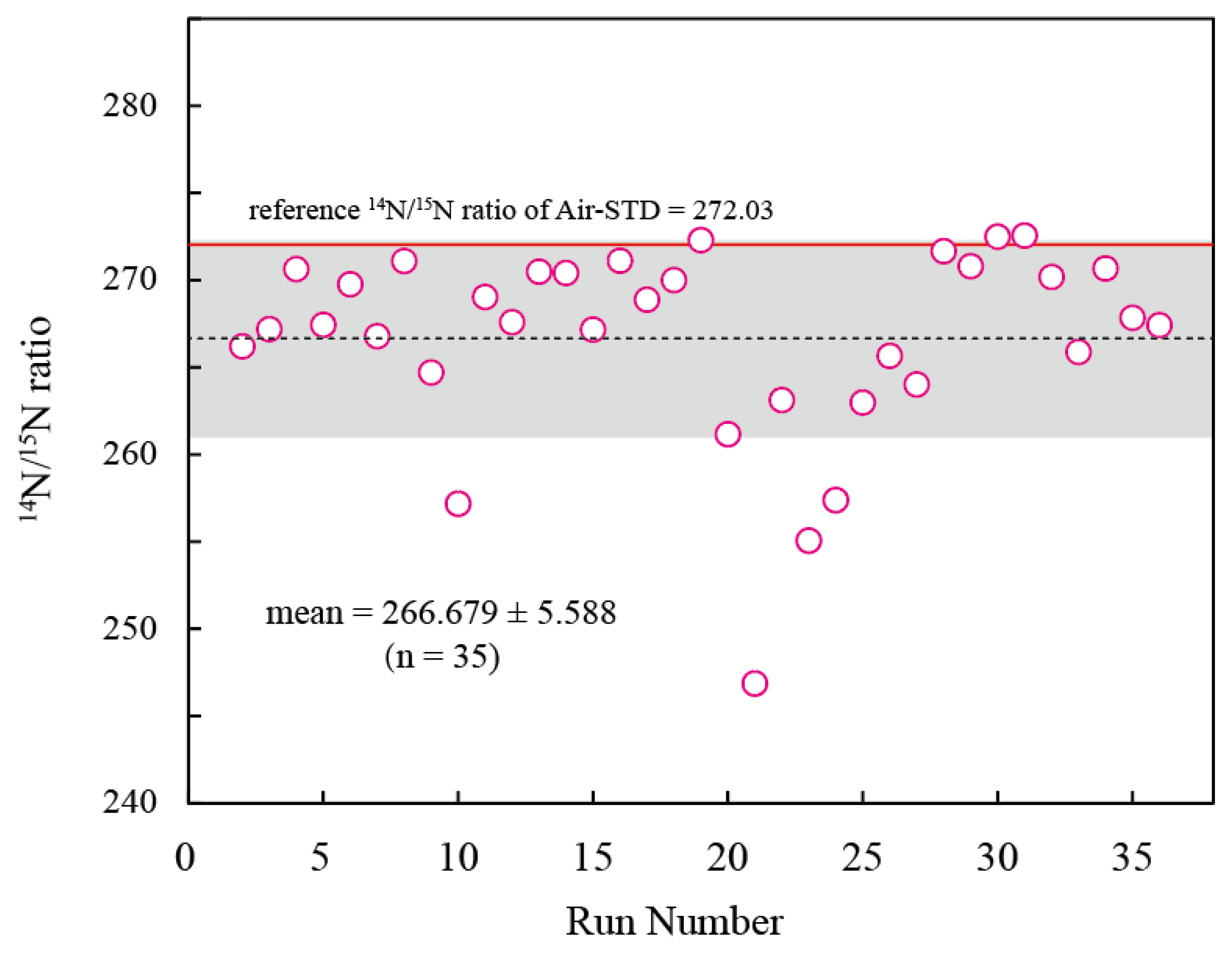
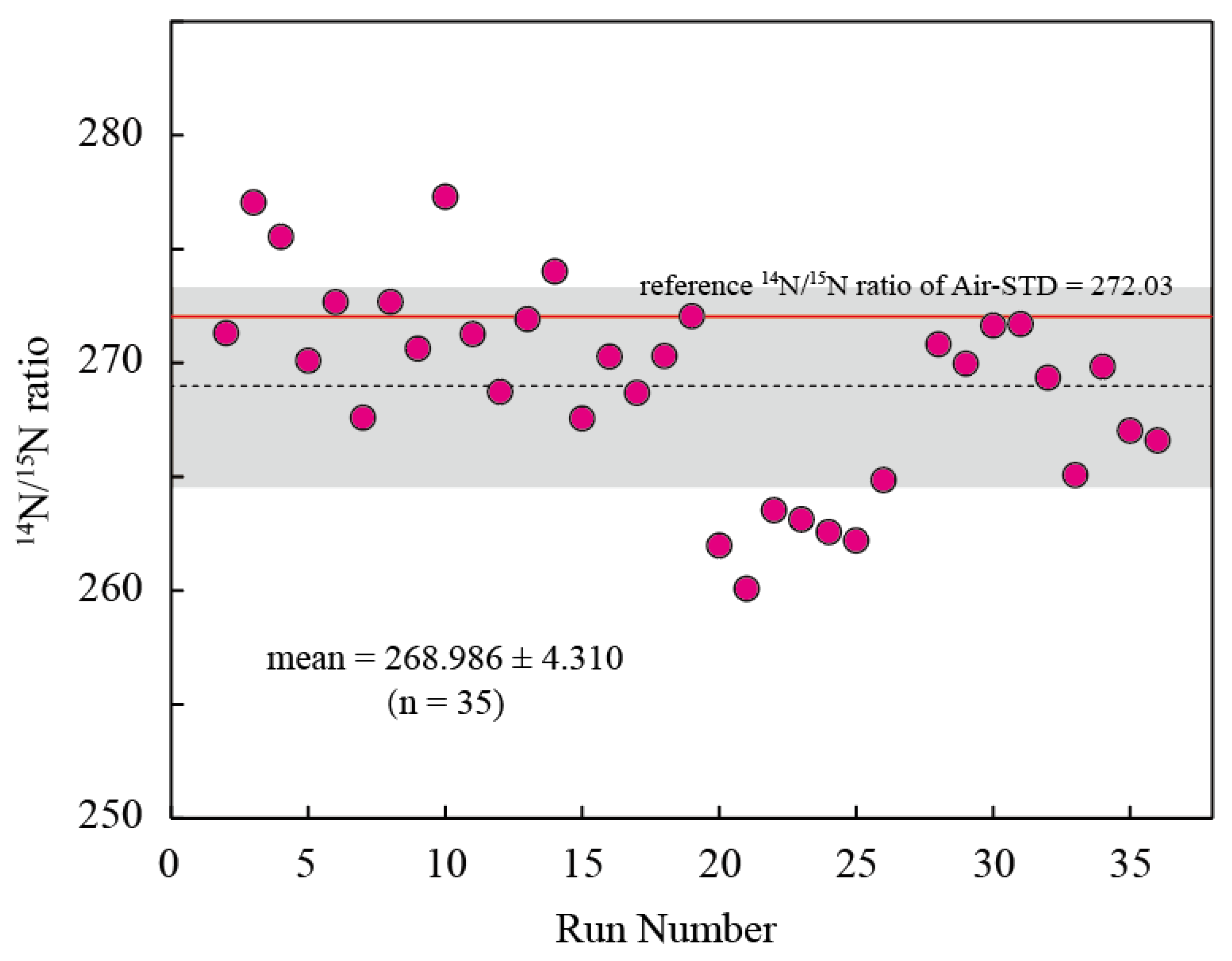
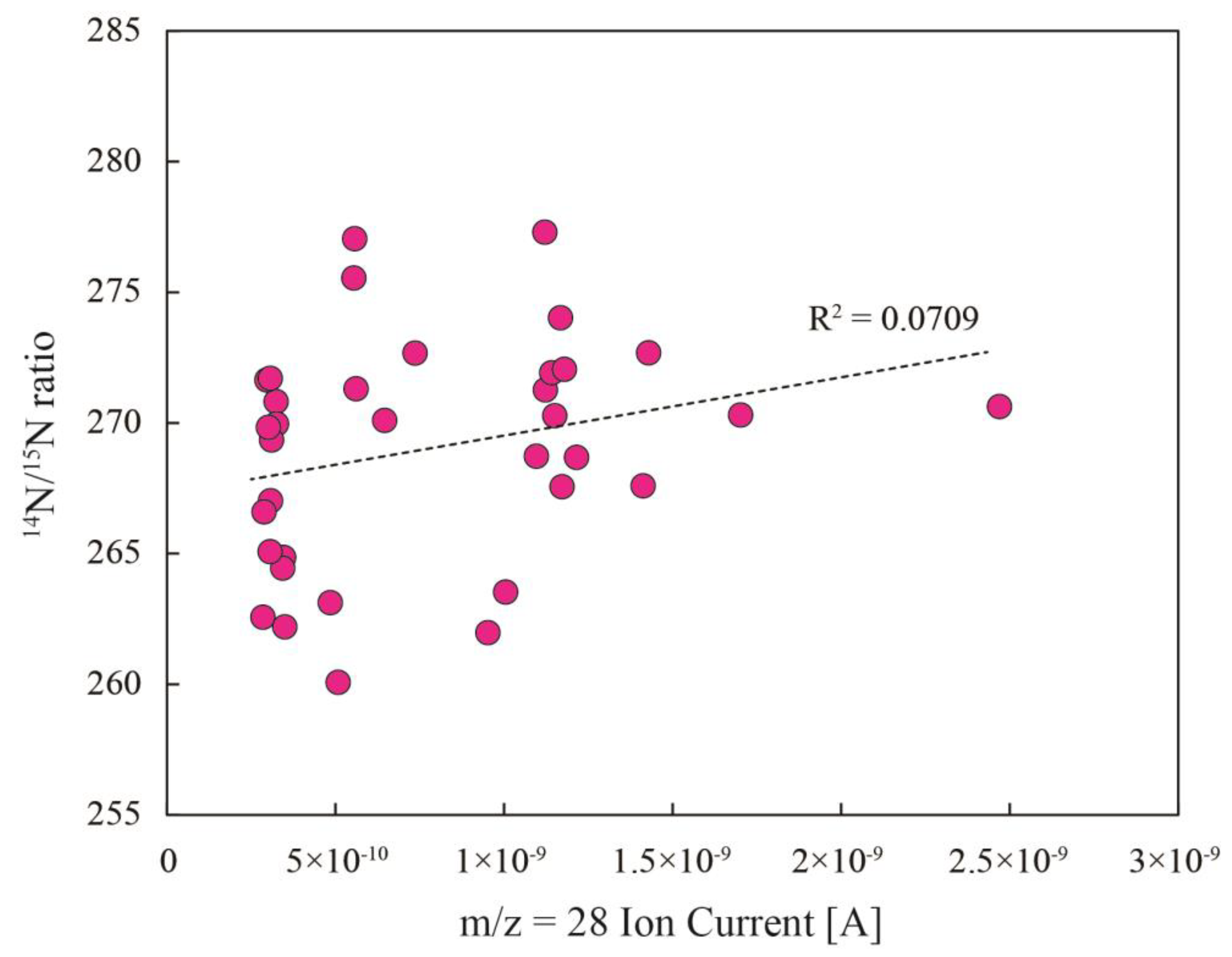
4.2. Air Standards
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stevenson, D.J. Origin of the Moon-The Collision Hypothesis. Annu. Rev. Earth Planet. Sci. 1987, 15, 271–315. [Google Scholar] [CrossRef]
- Canup, R.M. Forming a Moon with an Earth-like Composition via a Giant Impact. Science 2012, 338, 1052–1055. [Google Scholar] [CrossRef]
- Hartmann, W.K.; Davis, D.R. Satellite-sized planetesimals and lunar origin. Icarus 1975, 24, 504–515. [Google Scholar] [CrossRef]
- Becker, R.H.; Clayton, R.N. Nitrogen abundances and isotopic compositions in lunar samples. Lunar Planet. Sci. Conf. Proc. 1975, 2, 2131–2149. [Google Scholar]
- Kerridge, J.F. Solar Nitrogen: Evidence for a Secular Increase in the Ratio of Nitrogen-15 to Nitrogen-14. Science 1975, 188, 162–164. [Google Scholar] [CrossRef]
- Kerridge, J.F. Long-term compositional variation in solar corpuscular radiation: Evidence from nitrogen isotopes in the lunar regolith. Rev. Geophys. 1993, 31, 423–437. [Google Scholar] [CrossRef]
- Thiemens, M.H.; Clayton, R.N. Ancient solar wind in lunar microbreccias. Earth Planet. Sci. Lett. 1980, 47, 34–42. [Google Scholar] [CrossRef]
- Brilliant, D.R.; Franchi, I.A.; Pillinger, C.T. Nitrogen components in lunar soil 12023: Complex grains are not the carrier of isotopically light nitrogen. Meteoritics 1994, 29, 718–723. [Google Scholar] [CrossRef]
- Frick, U.; Becker, R.H.; Pepin, R.O. Solar wind record in the lunar regolith: Nitrogen and noble gases. Lunar Planet. Sci. Conf. Proc. 1988, 18, 87–120. [Google Scholar]
- Ozima, M.; Seki, K.; Terada, N.; Miura, Y.N.; Podosek, F.A.; Shinagawa, H. Terrestrial nitrogen and noble gases in lunar soils. Nature 2005, 436, 655–659. [Google Scholar] [CrossRef]
- Füri, E.; Marty, B. Nitrogen isotope variations in the Solar System. Nat. Geosci. 2015, 8, 515–522. [Google Scholar] [CrossRef]
- Nier, A.O. A Redetermination of the Relative Abundances of the Isotopes of Carbon, Nitrogen, Oxygen, Argon, and Potassium. Phys. Rev. 1950, 77, 789–793. [Google Scholar] [CrossRef]
- Marty, B.; Chaussidon, M.; Wiens, R.C.; Jurewicz, A.J.; Burnett, D.S. A 15N-poor isotopic composition for the solar system as shown by Genesis solar wind samples. Science 2011, 332, 1533–1536. [Google Scholar] [CrossRef]
- Meibom, A.; Krot, A.N.; Robert, F.; Mostefaoui, S.; Russell, S.S.; Petaev, M.I.; Gounelle, M. Nitrogen and Carbon Isotopic Composition of the Sun Inferred from a High-Temperature Solar Nebular Condensate. Astrophys. J. 2017, 656, L33. [Google Scholar] [CrossRef]
- Hoffman, J.H.; Hodges, R.R.; McElroy, M.B.; Donahue, T.M.; Kolpin, M. Composition and Structure of the Venus Atmosphere: Results from Pioneer Venus. Science 1979, 205, 49–52. [Google Scholar] [CrossRef]
- Mathew, K.J.; Marti, K. Early evolution of Martian volatiles: Nitrogen and noble gas components in ALH84001 and Chassigny. J. Geophys. Res. 2001, 106, 1401–1422. [Google Scholar] [CrossRef]
- Mathew, K.J.; Marti, K. Evolutionary trends in volatiles of the nakhlite source region of Mars. J. Geophys. Res. 2005, 110, E12S05. [Google Scholar] [CrossRef]
- Wong, M.H.; Atreya, S.K.; Mahaffy, P.N.; Franz, H.B.; Malespin, C.; Trainer, M.G.; Stern, J.C.; Conrad, P.G.; Manning, H.L.K.; Pepin, R.O.; et al. Isotopes of nitrogen on Mars: Atmospheric measurements by Curiosity’s mass spectrometer. Geophys. Res. Lett. 2013, 40, 6033–6037. [Google Scholar] [CrossRef]
- Hashizume, K.; Sugiura, N. Nitrogen isotopes in bulk ordinary chondrites. Geochim. Cosmochim. Acta 1995, 59, 4057–4069. [Google Scholar] [CrossRef]
- Kerridge, J.F. Carbon, hydrogen and nitrogen in carbonaceous chondrites: Abundances and isotopic compositions in bulk samples. Geochim. Cosmochim. Acta 1985, 49, 1707–1714. [Google Scholar] [CrossRef]
- Ivanova, M.A.; Kononkova, N.N.; Krot, A.N.; Greenwood, R.C.; Franchi, I.A.; Verchovsky, A.B.; Trieloff, M.; Korochantseva, E.V.; Brandstatter, F. The Isheyevo meteorite: Mineralogy, petrology, bulk chemistry, oxygen, nitrogen, carbon isotopic compositions, and 40Ar-39Ar ages. Meteorit. Planet. Sci. 2008, 43, 915–940. [Google Scholar] [CrossRef]
- Messenger, S. Identification of molecular-cloud material in interplanetary dust particles. Nature 2000, 404, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Marty, B.; Robert, P.; Zimmermann, L. Nitrogen and noble gases in micrometeorites. Meteorit. Planet. Sci. 2005, 40, 881–894. [Google Scholar] [CrossRef]
- Owen, T.; Mahaffy, P.R.; Niemann, H.B.; Atreya, S.; Wong, M. Protosolar Nitrogen. Astrophys. J. 2001, 553, L77. [Google Scholar] [CrossRef]
- Niemann, H.B.; Atreya, S.K.; Demick, J.E.; Gautier, D.; Haberman, J.A.; Harpold, D.N.; Kasprzak, W.T.; Lunine, J.I.; Owen, T.C.; Raulin, F. Composition of Titan’s lower atmosphere and simple surface volatiles as measured by the Cassini-Huygens probe gas chromatograph mass spectrometer experiment. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef]
- Fletcher, L.N.; Greathouse, T.K.; Orton, G.S.; Irwin, P.G.J.; Mousis, O.; Sinclair, J.A.; Giles, R.S. The origin of nitrogen on Jupiter and Saturn from the 15N/14N ratio. Icarus 2014, 238, 170–190. [Google Scholar] [CrossRef]
- Bockelée-Morvan, D.; Calmonte, U.; Charnley, S.; Duprat, J.; Engrand, C.; Gicquel, A.; Hässig, M.; Jehin, E.; Kawakita, H.; Marty, B.; et al. Cometary Isotopic Measurements. Space Sci. Rev. 2015, 197, 47–83. [Google Scholar] [CrossRef]
- Mathew, K.J.; Marti, K. Solar wind and other gases in the regoliths of the Pesyanoe parent object and the moon. Meteorit. Planet. Sci. 2003, 38, 627–643. [Google Scholar] [CrossRef]
- Becker, R.H. Nitrogen on the Moon. Science 2000, 5494, 1110–1111. [Google Scholar] [CrossRef]
- Garrick-Bethell, I.; Weiss, B.P.; Shuster, D.L.; Buz, J. Early lunar magnetism. Science 2009, 323, 356–359. [Google Scholar] [CrossRef]
- Weiss, B.P.; Tikoo, S.M. The lunar dynamo. Science 2014, 346, 1246753. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.B.; Gibson, E.K.; Larimer, J.W.; Lewis, C.F.; Nichiporuk, W. Total carbon and nitrogen abundances in Apollo 11 lunar samples and selected achondrites and basalts. Lunar Planet. Sci. Conf. Proc. 1970, 2, 1375–1382. [Google Scholar]
- Abel1, P.I.; Cadogan, P.H.; Eglinton, G.; Maxwell, J.R.; Pillinger, C.T. Survey of lunar carbon compounds. I. The presence of indigenous gases and hydrolysable carbon compounds in Apollo 11 and Apollo 12 samples. Lunar Planet. Sci. Conf. Proc. 1971, 2, 1843–1863. [Google Scholar]
- Mulller, O. Chemically bound nitrogen contents of Apollo 16 and Apollo 15 lunar fines. Lunar Planet. Sci. Conf. Proc. 1973, 2, 1625–1634. [Google Scholar]
- Mulller, O. Solar wind nitrogen and indigenous nitrogen in Apollo 17 lunar samples. Lunar Planet. Sci. Conf. Proc. 1974, 2, 1907–1918. [Google Scholar]
- Mathew, K.J.; Kerridge, J.F.; Marti, K. Nitrogen in solar energetic particles: Isotopically distinct from solar wind. Geophys. Res. Lett. 1998, 25, 4293–4296. [Google Scholar] [CrossRef] [PubMed]
- Geiss, J.; Bochsler, P. Nitrogen isotopes in the solar system. Geochim. Cosmochim. Acta 1982, 46, 529–548. [Google Scholar] [CrossRef]
- Wieler, R.; Humbert, F.; Marty, B. Evidence for a predominantly non-solar origin of nitrogen in the lunar regolith revealed by single grain analyses. Earth Planet. Sci. Lett. 1999, 167, 47–60. [Google Scholar] [CrossRef]
- Hashizume, K.; Chaussidon, M.; Marty, B.; Robert, F. Solar Wind Record on the Moon: Deciphering Presolar from Planetary Nitrogen. Science 2000, 290, 1142–1145. [Google Scholar] [CrossRef]
- Hashizume, K.; Marty, B.; Wieler, R. Analyses of nitrogen and argon in single lunar grains: Towards a quantification of the asteroidal contribution to planetary surfaces. Earth Planet. Sci. Lett. 2002, 202, 201–216. [Google Scholar] [CrossRef]
- Assonov, S.S.; Franchi, I.A.; Pillinger, C.T.; Semenova, A.S.; Shukolyukov, Y.A.; Verchovsky, A.B.; Iassevitch, A.N. Nitrogen and argon release profiles in Luna 16 and Luna 24 regolith samples: The effects of regolith reworking. Meteorit. Planet. Sci. 2002, 37, 27–48. [Google Scholar] [CrossRef]
- Marty, B.; Hashizume, K.; Chaussidon, M.; Wieler, R. Nitrogen Isotopes on the Moon: Archives of the Solar and Planetary Contributions to the Inner Solar System. Space Sci. Rev. 2003, 106, 175–196. [Google Scholar] [CrossRef]
- Füri, E.; Marty, B.; Assonov, S.S. Constraints on the flux of meteoritic and cometary water on the Moon from volatile element (N-Ar) analyses of single lunar soil grains, Luna 24 core. Icarus 2012, 218, 220–229. [Google Scholar] [CrossRef]
- Wei, Y.; Zhong, J.; Hui, H.; Shi, Q.; Cui, J.; He, H.; Zhang, H.; Yao, Z.; Yue, X.; Rong, Z.; et al. Implantation of Earth’s Atmospheric Ions Into the Nearside and Farside Lunar Soil: Implications to Geodynamo Evolution. Geophys. Res. Lett. 2020, 47, e2019GL086208. [Google Scholar] [CrossRef]
- Becker, R.H.; Clayton, R.N.; Mayeda, T.K. Characterization of lunar nitrogen components. Lunar Planet. Sci. Conf. Proc. 1976, 1, 441–458. [Google Scholar]
- DesMarais, D.J. Carbon, nitrogen and sulfur in Apollo 15, 16 and 17 rocks. Lunar Planet. Sci. Conf. Proc. 1978, 9, 2451–2467. [Google Scholar]
- DesMarais, D.J. Light element geochemistry and spallogenesis in lunar rocks. Geochim. Cosmochim. Acta 1983, 47, 1769–1781. [Google Scholar]
- Funkhouser, J.; Jessberger, E.; Müller, O.; Zähringer, J. Active and inert gases in Apollo 12 and Apollo 11 samples released by crushing at room temperature and by heating a low temperatures. Lunar Planet. Sci. Conf. Proc. 1971, 2, 1381–1396. [Google Scholar]
- Mathew, K.J.; Marti, K. Lunar nitrogen: Indigenous signature and cosmic-ray production rate. Earth Planet. Sci. Lett. 2001, 184, 659–669. [Google Scholar] [CrossRef]
- Mortimer, J.; Verchovsky, A.B.; Anand, M.; Gilmour, I.; Pillinger, C.T. Simultaneous analysis of abundance and isotopic composition of nitrogen, carbon, and noble gases in lunar basalts: Insights into interior and surface processes on the Moon. Icarus 2015, 255, 3–17. [Google Scholar] [CrossRef]
- Müller, O.; Grallath, E.; Tölg, G. Nitrogen in lunar igneous rocks. Lunar Planet. Sci. Conf. Proc. 1976, 7, 1615–1622. [Google Scholar]
- Wieler, R. Noble gases in the Solar System. Rev. Mineral. Geochem. 2002, 47, 21–70. [Google Scholar] [CrossRef]
- Clayton, D.; Dwek, E.; Woosley, S. Isotopic anamolies and proton irradiation in the early Solar System. Astrophys. J. 1977, 214, 300–315. [Google Scholar] [CrossRef][Green Version]
- Mathew, K.J.; Murty, S.V.S. Cosmic ray produced nitrogen in extra terrestrial matter. Proc. Indian Acad. Sci. -Earth Planet. Sci. 1993, 102, 415–437. [Google Scholar] [CrossRef]
- Murty, S.V.S.; Goswami, J.N. Nitrogen, noble gases, and nuclear tracks in lunar meteorites MAC88104/105. Lunar Planet. Sci. Conf. Proc. 1992, 22, 225–237. [Google Scholar]
- Kerridge, J.F.; Eugster, O.; Kim, J.S.; Marti, K. Nitrogen isotopes in the 74001/74002 double-drive tube from Shorty Crater, Apollo 17. Lunar Planet. Sci. Conf. Proc. 1991, 21, 291–299. [Google Scholar]
- Füri, E.; Barry, P.H.; Taylor, L.A.; Marty, B. Indigenous nitrogen in the Moon: Constraints from coupled nitrogen-noble gas analyses of mare basalts. Earth Planet. Sci. Lett. 2015, 431, 195–205. [Google Scholar] [CrossRef]
- Cartigny, P.; Marty, B. Nitrogen Isotopes and Mantle Geodynamics: The Emergence of Life and the Atmosphere-Crust-Mantle Connection. Elements 2013, 9, 359–366. [Google Scholar] [CrossRef]
- Kung, C.C.; Clayton, R.N. Nitrogen abundances and isotopic compositions in stony meteorites. Earth Planet. Sci. Lett. 1978, 38, 421–435. [Google Scholar] [CrossRef]
- Marty, B. The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet. Sci. Lett. 2012, 313, 56–66. [Google Scholar] [CrossRef]
- Anderson, D.M.; Biemann, K.; Orgel, L.E.; Oro, J.; Owen, T.; Shulman, G.P.; Toulmin, P.; Urey, H.C. Mass spectrometric analysis of organic compounds, water and volatile constituents in the atmosphere and surface of Mars: The Viking Mars Lander. Icarus 1972, 16, 111–138. [Google Scholar] [CrossRef]
- Owen, T.; Biemann, K.; Rushneck, D.R.; Biller, J.E.; Howarth, D.W.; Lafleur, A.L. The composition of the atmosphere at the surface of Mars. J. Geophys. Res. 1977, 82, 4635–4639. [Google Scholar] [CrossRef]
- Wong, M.H.; Mahaffy, P.R.; Atreya, S.K.; Niemann, H.B.; Owen, T.C. Updated Galileo probe mass spectrometer measurements of carbon, oxygen, nitrogen, and sulfur on Jupiter. Icarus 2004, 171, 153–170. [Google Scholar] [CrossRef]
- Balsiger, H.; Altwegg, K.; Arijs, E.; Bertaux, J.L.; Berthelier, J.J.; Bochsler, P.; Carignan, G.R.; Eberhardt, P.; Fisk, L.A.; Fuselier, S.A.; et al. Rosetta Orbiter Spectrometer for Ion and Neutral Analysis—ROSINA. Advanves Space Res. 1998, 21, 1527–1535. [Google Scholar] [CrossRef]
- Rubin, M.; Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J.J.; Bieler, A.; Bochsler, P.; Briois, C.; Calmonte, U.; Combi, M.; et al. Molecular nitrogen in comet 67P/Churyumov-Gerasimenko indicates a low formation temperature. Science 2015, 348, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Mahaffy, P.R.; Webster, C.R.; Cabane, M.; Conrad, P.G.; Coll, P.; Atreya, S.K.; Arvey, R.; Barciniak, M.; Benna, M.; Bleacher, L.; et al. The Sample Analysis at Mars Investigation and Instrument Suite. Space Sci. Rev. 2012, 170, 401–478. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Tang, J.Y.; Zhang, W.W.; Sun, F. Micro Quantitative Sampler for Lunar Regolith: Design and Validation. J. Deep. Space Explor. 2022, 9, 165–172. [Google Scholar]
- Liu, Z.; He, H.; Li, J.; Hao, J.; Tang, J.; Zhang, Z.; Jiang, S.; Chi, G.; Liu, R.; Wang, L.; et al. Measurement and Uncertainty Analysis of Lunar Soil Water Content via Heating Flux Method. Aerospace 2023, 10, 657. [Google Scholar] [CrossRef]
- Boyd, S.R.; Wright, I.P.; Franchi, I.A.; Pillinger, C.T. Preparation of sub-nanomole quantities of nitrogen gas for stable isotopic analysis. J. Phys. E Sci. Instrum. 1988, 21, 876–885. [Google Scholar] [CrossRef]
- Takahata, N.; Nishio, Y.; Yoshida, N.; Sano, Y. Precise Isotopic Measurements of Nitrogen at the Sub-Nanomole Level. Anal. Sci. 1998, 14, 485–491. [Google Scholar] [CrossRef]
- Marty, B. Nitrogen content of the mantle inferred from N2-Ar correlation in oceanic basalts. Nature 1995, 377, 326–329. [Google Scholar] [CrossRef]
- Barry, P.H.; Hilton, D.R.; Halldórsson, S.A.; Hahm, D.; Marti, K. High precision nitrogen isotope measurements in oceanic basalts using a static triple collection noble gas mass spectrometer. Geochem. Geophys. Geosystems 2012, 13, Q01019. [Google Scholar] [CrossRef]
- Young, J.R. Interaction of Oxygen with Incandescent Filaments. J. Appl. Phys. 1959, 30, 1671–1673. [Google Scholar] [CrossRef]

| Viking Lander | Galileo Probe Mass Spectrometer (GPMS) | Rosetta Orbiter Spectrometer for Ion and Neutral Analysis Mass Spectrometer (ROSINA) | The Sample Analysis at Mars (SAM) | |
|---|---|---|---|---|
| NASA | NASA | ESA | NASA | |
| Research object | Martian atmosphere | Jovian atmosphere | Jupiter family comet 67P/Churyumov-Gerasimenko | Martian atmosphere |
| Type of mass spectrometer | Double-focusing mass spectrometer | Quadrupole Mass spectrometer | Double-focusing mass spectrometer | Quadrupole Mass spectrometer |
| Measurement of nitrogen isotopic compositions | Remove the interfering substances, CO and CO2, by a chemical filter, which employs silver oxide to oxidize CO to CO2, lithium hydroxide-oxide mixture to absorb all the CO2, and magnesium perchlorate to remove H2O. | Obtain from the doubly charged ratio of 15NH3++/14NH3++ at 9 and 8.5 amu. The contribution of 14NDH2++ at 9 amu will be negligible, as will the signal from H2O++. | The mass spectrometer has a high mass resolution of m/Δm about 3000 at the 1% level at atmoic mass per unit of charge 28 u/e, allowing the separation of N2 from CO (Δm = 0.011 u) by numerical peak fitting. | Derive 14N/15N from direct atmospheric experiments using the m/z 14/14.5 count ratio. In enrichment experiments, chemical scrubbers remove CO2, H2O, and other species with chemical affinity to the scrubber material. |
| Mass range | 12–200 amu | 2–150 amu | from 1 amu to >300 amu | 2–535 amu |
| Result | 14N/15N ratio of 168 ± 17 | 15N/14N ratio of (2.3 ± 0.3) × 10−3 | N2/CO ratio of (5.70 ± 0.66) × 10−3 | 14N/15N ratio of 173 ± 11 |
| Date | Run Number | m/z = 28 Ion Current (A) | 14N14N/14N15N Blank Corrected | 14N14N/14N15N CO and Blank Corrected | 14N/15N Corrected | δ15N Corrected (‰) | Blank Contribution on Mass 28 (%) |
|---|---|---|---|---|---|---|---|
| 31 October 2022 | 1 | 5.61 × 10−10 | 133.103 | 135.654 | 271.308 | 2.7 | 0.32 |
| 2 | 5.57 × 10−10 | 133.596 | 138.524 | 277.048 | −18.1 | 0.32 | |
| 1 November 2022 | 1 | 5.54 × 10−10 | 135.318 | 137.773 | 275.546 | −12.7 | 0.31 |
| 2 November 2022 | 1 | 6.46 × 10−10 | 133.719 | 135.050 | 270.099 | 7.2 | 0.27 |
| 3 November 2022 | 1 | 7.36 × 10−10 | 134.882 | 136.337 | 272.673 | −2.3 | 0.28 |
| 2 | 1.41 × 10−9 | 133.393 | 133.798 | 267.596 | 16.6 | 0.16 | |
| 4 November 2022 | 1 | 1.43 × 10−9 | 135.550 | 136.342 | 272.684 | -2.4 | 0.15 |
| 2 | 2.47 × 10−9 | 132.353 | 135.314 | 270.628 | 5.2 | 0.08 | |
| 7 November 2022 | 1 | 1.12 × 10−9 | 128.589 | 138.649 | 277.299 | −19.0 | 0.12 |
| 2 | 1.12 × 10−9 | 134.518 | 135.632 | 271.264 | 2.8 | 0.12 | |
| 9 November 2022 | 1 | 1.10 × 10−9 | 133.795 | 134.364 | 268.728 | 12.3 | 0.20 |
| 2 | 1.14 × 10−9 | 135.243 | 135.960 | 271.921 | 0.4 | 0.16 | |
| 10 November 2022 | 1 | 1.17 × 10−9 | 135.208 | 137.012 | 274.024 | −7.3 | 0.13 |
| 14 November 2022 | 1 | 1.17 × 10−9 | 133.583 | 133.781 | 267.561 | 16.7 | 0.11 |
| 15 November 2022 | 1 | 1.15 × 10−9 | 135.558 | 135.139 | 270.278 | 6.5 | 0.11 |
| 2 | 1.21 × 10−9 | 134.442 | 134.342 | 268.684 | 12.5 | 0.12 | |
| 16 November 2022 | 1 | 1.70 × 10−9 | 135.002 | 135.153 | 270.306 | 6.4 | 0.11 |
| 17 November 2022 | 1 | 1.18 × 10−9 | 136.147 | 136.026 | 272.053 | -0.1 | 0.11 |
| 6 February 2023 | 1 | 9.52 × 10−10 | 130.584 | 130.990 | 261.979 | 38.4 | 0.23 |
| 7 February 2023 | 1 | 5.08 × 10−10 | 123.434 | 130.040 | 260.080 | 46.0 | 0.17 |
| 2 | 1.00 × 10−9 | 131.562 | 131.766 | 263.533 | 32.3 | 0.22 | |
| 2 March 2023 | 1 | 4.84 × 10−10 | 127.530 | 131.564 | 263.128 | 33.8 | 1.01 |
| 2 | 2.85 × 10−10 | 128.692 | 131.286 | 262.571 | 36.0 | 1.34 | |
| 6 March 2023 | 1 | 3.49 × 10−10 | 131.492 | 131.100 | 262.201 | 37.5 | 0.94 |
| 6 April 2023 | 1 | 3.46 × 10−10 | 132.830 | 132.429 | 264.858 | 27.1 | 0.56 |
| 7 April 2023 | 1 | 3.43 × 10−10 | 132.010 | 132.224 | 264.448 | 28.7 | 0.60 |
| 14 April 2023 | 1 | 3.24 × 10−10 | 135.831 | 135.409 | 270.819 | 4.5 | 0.42 |
| 17 April 2023 | 1 | 3.25 × 10−10 | 135.401 | 134.983 | 269.966 | 7.7 | 0.68 |
| 18 April 2023 | 1 | 2.96 × 10−10 | 136.247 | 135.822 | 271.645 | 1.4 | 0.55 |
| 2 | 3.06 × 10−10 | 136.278 | 135.854 | 271.707 | 1.2 | 0.45 | |
| 21 April 2023 | 1 | 3.10 × 10−10 | 135.092 | 134.675 | 269.351 | 10.0 | 0.79 |
| 5 May 2023 | 1 | 3.06 × 10−10 | 132.941 | 132.539 | 265.078 | 26.2 | 2.80 |
| 9 May 2023 | 1 | 3.01 × 10−10 | 135.335 | 134.917 | 269.834 | 8.2 | 0.50 |
| 19 May 2023 | 1 | 3.07 × 10−10 | 133.922 | 133.513 | 267.026 | 18.8 | 0.66 |
| 23 May 2023 | 1 | 2.88 × 10−10 | 133.707 | 133.300 | 266.600 | 20.4 | 0.44 |
| mean | 133.340 | 134.493 | 268.986 | 11.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; He, H.; Liu, Z.; Su, F.; Li, J.; Zhang, Y.; Li, R.; Huang, X.; Zhang, X.; Lu, C.; et al. Proof of Principle of the Lunar Soil Volatile Measuring Instrument on Chang’ e-7: In Situ N Isotopic Analysis of Lunar Soil. Aerospace 2024, 11, 114. https://doi.org/10.3390/aerospace11020114
He Y, He H, Liu Z, Su F, Li J, Zhang Y, Li R, Huang X, Zhang X, Lu C, et al. Proof of Principle of the Lunar Soil Volatile Measuring Instrument on Chang’ e-7: In Situ N Isotopic Analysis of Lunar Soil. Aerospace. 2024; 11(2):114. https://doi.org/10.3390/aerospace11020114
Chicago/Turabian StyleHe, Ye, Huaiyu He, Ziheng Liu, Fei Su, Jiannan Li, Yanan Zhang, Rongji Li, Xinyu Huang, Xuhang Zhang, Chao Lu, and et al. 2024. "Proof of Principle of the Lunar Soil Volatile Measuring Instrument on Chang’ e-7: In Situ N Isotopic Analysis of Lunar Soil" Aerospace 11, no. 2: 114. https://doi.org/10.3390/aerospace11020114
APA StyleHe, Y., He, H., Liu, Z., Su, F., Li, J., Zhang, Y., Li, R., Huang, X., Zhang, X., Lu, C., Jiang, S., Tang, J., & Liu, R. (2024). Proof of Principle of the Lunar Soil Volatile Measuring Instrument on Chang’ e-7: In Situ N Isotopic Analysis of Lunar Soil. Aerospace, 11(2), 114. https://doi.org/10.3390/aerospace11020114







