Abstract
This study investigates the use of MnxOy/Al2O3 and Pt/Al2O3 catalysts for the decomposition of hydrogen peroxide in thrusters. It describes the purpose, procedures, performance, and conclusions coming from the test campaign of the catalyst lifetimes. In particular, eight different propellant samples with two different catalysts were tested twice (in order to exclude uncertainty). Similar operating and starting conditions were applied. All hot tests were performed in a thruster-like catalyst bed configuration with a propellant injector and outlet nozzle. Each bed was filled with the same mass of catalyst (for the same type of catalyst). The results show that platinum is a more effective catalyst than manganese oxides for the decomposition of hydrogen peroxide. The findings have important implications for the development of catalysts for “green” propellants.
1. Introduction
Hydrogen peroxide was one of the first propellants used in satellite propulsion [1]. Soon, the space race triggered the need for the development of higher-impulse propellants, without awareness of their environmental impact [2]. Consequently, this gave rise to the advancement of propellants, such as hydrazine and its derivatives. In the 21st century, space agencies and companies started looking for alternatives due to the growing interest in green propellants [3]. One of the alternatives is highly concentrated hydrogen peroxide, namely HTP (High-Test Peroxide). This propellant, used in the 1960s in propulsion, was usually concentrated up to approximately 80–90% [4,5]. The underlying causes laid in the high costs associated with obtaining higher concentrations and no catalysts that could decompose low and high concentrations equally well, while maintaining longevity. Such a low concentration was favourable for launcher and missile applications, since turbomachinery did not require ultra-high-temperature alloys for rotating parts of the hot section [6,7,8]. Pellet-based and wire mesh screen catalysts could easily handle minutes of rocket engine operation. In-space propulsion, however, demands high performance, achievable with highly concentrated hydrogen peroxide [9,10]. Density impulse increases with the HTP concentration [11]. This is a significant advantage in terms of the system requirements. However, the decomposition of higher concentrations exposes catalysts to higher temperatures and thermal shocks [12].
Hydrogen peroxide undergoes spontaneous decomposition, progressing over time and leading to a gradual decline in its concentration. Although it seems unintuitive, the stability of purified hydrogen peroxide improves with its concentration, leading to a decrease in its self-decomposition rate. All of the aforementioned factors demonstrate that, in order to compete with toxic fuels, it is essential to develop catalysts for the highest achievable concentrations of hydrogen peroxide. However, referring to HTP self-decomposition, which leads to a constant decrease in its concentration over a space mission lifetime, catalysts should also decompose lower-concentrated peroxide with the highest possible efficiency. Furthermore, the adiabatic decomposition temperature varies with the HTP concentration, as the remaining ingredient is water. In the real environment, namely the catalyst bed, the temperature changes along the bed with varying chemical species. A representative example of a one-dimensional model of decomposition of 87.5% HTP in a monolithic straight-channel bed, including temperature and species change, was described by Krejci et al. [13]. Since the boiling of both water and hydrogen peroxide consumes significant amounts of heat, two corresponding flat steps on temperature profiles along the channel occur. The maximum temperature is present in the plane of complete decomposition. The temperature drops downstream of this plane due to the heat flow from gas to the wall with no further exothermic reaction.
Many heterogeneous catalysts have been tested in the past. This specific type of catalyst is characterized by its limited lifetime, affected by certain factors: chemical, mechanical, and combinations of these two factors. Chemical factors are connected to the chemical reactions of propellant contents (e.g., stabilizers, impurities, propellant, and products of its decomposition) with catalysts and the deactivation of active phases [14]. Mechanical factors are based on the influence of the fluid flow on the catalyst surface and of the washout of the active phase. Another factor is the abrasion of the active layer, caused by the rubbing of particles (in the case of packed catalyst beds). The remaining issue limiting the catalyst lifetime is the effect of the support material which may react with propellant, abrade, or crack due to stress and thermal shocks.
The most active catalysts for hydrogen peroxide include platinum, silver, and manganese oxides [15,16]. Silver can be washed out, or may creep from the catalyst bed due to its melting temperature, which is close to the decomposition temperature of 98% hydrogen peroxide [17,18,19,20]. Despite attempts made by Runckel [21] to use silver wire mesh with various additives, the efforts to use this active material for hydrogen peroxide concentrations greater than 90% have since diminished.
A thruster maintains its design characteristics as long as the HTP concentration remains steady. The off-design peroxide concentration may appear during space missions lasting months to years. It will impact the thruster performance not only due to the concentration-related decomposition temperature, but also lower reactivity. The last phenomenon may affect the ability of the catalyst bed to fully decompose the propellant, resulting in further loss of performance. For this reason, it is important to investigate the impact of the propellant concentration on the fixed catalyst bed (the critical part of a thruster) performance.
Numerous papers, describing experimental work with certain HTP concentrations and thruster-like catalyst beds, were published recently. Koopmans et al. investigated the impact of various additively manufactured structures on catalyst bed performance [22]. The effect of injection patterns on the characteristics of a catalyst bed was presented by Kang et al. [23]. An et al. investigated the impact of pressure and aspect ratio [24] on the chugging instability of packed catalyst beds, whereas Jo et al. [25] reported the influence of catalyst reactivity and support sizes on the same phenomenon.
Among numerous publications reporting works with various classes of hydrogen peroxide, the authors have not identified any research devoted to the impact of HTP concentration on the performance of a fixed catalyst bed. An intuitive and qualitative assessment stands for the decay of performance with decreasing concentration. However, the experimental verification of this hypothesis would also be valuable in terms of the quantitative assessment. Therefore, the aim of this research is a preliminary characterization of the impact of hydrogen peroxide concentration on catalyst bed performance. In this first step of the work, a fixed-geometry catalyst bed was applied, and propellant concentration was varied from 85% to 99%. The expected impact of this variation, connected to the propellant–catalyst reaction rate, is the performance-related efficiency of the characteristic velocity (ηc*). The results of this investigation will help in the proper sizing of catalyst beds operating with variable-in-time concentrations of HTP. The additional goal of this investigation is the assessment of the catalyst degradation over the run time. However, it needs to be pointed out that the limited propellant throughput may appear to be insufficient for such an evaluation.
Due to the high cost of certain active materials, two-phase catalysts are usually applied, where the active phase consists of metals such as platinum or silver. Ceramic materials are commonly used as supports due to their low cost, high melting temperature, mechanical resistance, thermal shock resistance, and high specific surface area. Another essential factor is their adherence to metals [26,27,28].
Ceramic-supported catalysts, by means of active phases, may be represented either by precious metals or low-cost oxides. As one can learn from many publications, platinum is one of the most active materials for hydrogen peroxide decomposition. The high cost of platinum drives the search for alternatives or limitations of the active phase weight loading. Nevertheless, platinum should be considered primarily for catalysts of small HTP thrusters, where the cost of the catalyst hasa minor effect on the cost of the whole thruster [29,30,31]. Therefore, platinum and manganese oxides were selected as active phases for the purpose of this investigation.
2. Methods
2.1. Object of Testing
The experimental part of the research consisted of 32 single-run hot tests. Every test was performed with a fresh catalyst bed, in order to exclude any discussion on the impact of the catalyst health on test results. Platinum, supported on a γ-alumina carrier, is a standard, commercially available, product. The active phase content of the catalyst (see Figure 1) was 5% by mass. The second one (see Figure 2), manganese oxide catalyst (silica-doped α-alumina supported), was developed by Łukasiewicz—Institute of Aviation and reported in [32,33]. This catalyst was prepared by wet impregnation in a potassium permanganate water solution [34]. The active phase, containing manganese oxides, provides satisfying response time without preheating [35]. The active phase content of the catalyst, after the impregnation–drying–calcination process, reached 4.5% by mass.
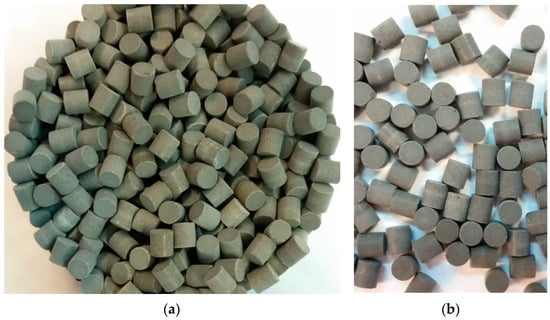
Figure 1.
Platinum catalyst, supported on the γ-alumina: (a) pre-test view and (b) post-test condition.

Figure 2.
Manganese oxide catalyst, supported on the α-alumina: (a) pre-test view and (b) post-test condition.
A catalyst decomposing 98% hydrogen peroxide usually demonstrates changes in its original colour or shade. A slight change in shade, concerning the Pt active layer, can be noticed in Figure 1b. A significant change in colour occurred with the MnxOy catalyst (see Figure 2b). These modifications are of mechanical and chemical natures, and their impact on the catalyst activity occurs on various levels.
The in-house-made MnxOy catalyst was characterized with X-ray diffraction (XRD), Scanning Electron Microscopy (SEM), Energy-Dispersive X-ray Spectroscopy (EDS) and Brunauer–Emmet–Teller (BET). This detail characterization served to report the comprehensive study, containing theoretical considerations and experimental investigation, presented in [32]. The supplier of the commercially available platinum catalyst provided the certificate of analysis, containing basic parameters. The detail characterization was not performed in this case. The most important parameters, characterizing these two catalysts, are presented in Table 1.

Table 1.
Basic parameters characterizing the catalysts.
2.2. Testing Method
Each catalyst was packed into the thruster-like decomposition chamber and tested for 8 different concentrations of HTP, as specified in Table 2. An in-house-developed standard packing method was applied. Every set of four tests was conducted with a single hydrogen peroxide concentration level: two using catalysts with manganese oxides and two using platinum. The density and temperature of hydrogen peroxide were measured using the DMA 35 BASIC density meter by Anton Paar. Subsequently, the concentration was calculated based on the density and temperature measurement results. The measurement error was ±0.2%.

Table 2.
HTP concentrations applied for tests, their adiabatic decomposition temperatures, and theoretical c* (characteristic velocity).
The adiabatic decomposition temperature and theoretical c* for each concentration of hydrogen peroxide were computed using CEA code (Chemical Equilibrium with Applications) [36]. The thermodynamic relation for the theoretical c* is expressed in Formula (1) [37]. Thereafter, based on test results, the real c* and its efficiency were calculated using Formulas (2) and (3). The efficiency of characteristic velocity determines the efficiency of chemical processes in the catalyst bed.
where:
—theoretical characteristic velocity, m/s;
—ratio of specific heats;
R—gas constant per unit weight, J/kg/K;
Tc—gas temperature in the chamber, K.
The value of characteristic velocity, obtained by testing, is calculated from:
where:
—characteristic velocity obtained experimentally, m/s;
pc—chamber pressure, Pa;
At—area of the nozzle throat, m2;
—propellant mass flow rate, kg/s.
The efficiency of c* (ηc*) is the ratio of the real to the theoretical value:
The profile of ηc* versus run time was taken as the main characteristic determining the performance of the catalyst bed, possibly changing with run time due to the chemical degradation of catalysts. Theoretical values of characteristic velocities were calculated for given test conditions, including the real concentration of hydrogen peroxide. The temperature was measured at 6 points along the catalyst bed. Temperature profiles, as functions of the run time, were carefully analysed in order to verify potential catalyst degradation. If the temperature is measured at a point, a thermocouple’s tip may either stay in contact with the catalyst (reaction zone) or may reside in a free flow, facing the flow channelling (depending on the random configuration of catalyst pellets or spheres). Taking into consideration an additional fact, described by Ponzo [38], thermocouple measurement, as a method used to assess monopropellant decomposition efficiency, is unreliable. When partially decomposed fog from peroxide/water touches a hot metal surface, it will decompose thermally. In case a shielded thermocouple is used, it reads the full decomposition temperature locally, while the global stream of fluid is decomposed only partially. Consequently, temperature measurement does not always provide reliable results on the catalyst bed efficiency. Nevertheless, the own experience proved that the analysis of temperature profiles offers a valuable set of outputs in terms of any evolution (namely degradation) of the catalyst bed performance.
The distance between subsequent measurement ports in the catalyst bed is constant and equal to 10 mm. The schematic view with measurement ports is presented in Figure 3.
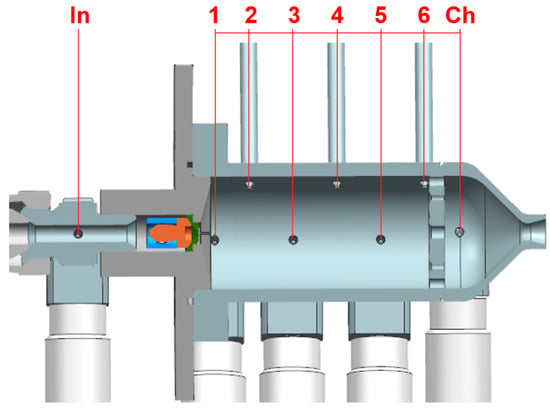
Figure 3.
Location of measurement ports (sections): In—inlet, 1–6—catalytic chamber, Ch—expansion chamber.
A full-cone swirl injector was used to achieve good atomization and uniform distribution of the propellant into the front surface of the catalyst bed. The overall length of the catalyst chamber, i.e., distance from the injector face to the retaining plate, was 50 mm. The standard length, filled with catalyst, equalled 40 mm. A preload with a spring, welded to the wire mesh holder, was applied to limit catalyst movement during hot-fire tests. The holder ensured the distance (approx. 10 mm) between the injector and the catalyst. The volume of the catalyst chamber (from the injector to the retaining plate) was 26.5 cm3. The usable volume, occupied by the catalyst, was 21.2 cm3. The main operating parameters of the thruster are presented in Table 3.

Table 3.
Main parameters of the thruster.
Each catalyst was packed with the assistance of a vibrational table, which helped to maintain equal bulk densities in individual beds. The only variation (approx. 8%) in bulk densities occurred between two different catalysts—platinum and manganese oxide—as different materials, sizes, and shapes were applied. Consequentially, Pt- and MnxOy-packed beds were characterized by different masses. Positive tolerances resulted from mass variation in individual pellets and spheres:
- Pt catalyst (Elemental Microanalysis): 25.2 g (+0.05 g of tolerance),
- MnxOy catalyst (own production): 25.4 g (+0.05 g of tolerance).
Apart from the temperature, pressure was measured at the inlet, expansion chamber, and at 6 locations in the catalyst bed. The Coriolis mass flow meter was applied as the primary equipment for measuring the propellant mass flow rate. According to the plan, as soon as the HTP throughput exceeded 5.3 kg, a hot test was stopped.
The load cell was applied to assess the axial thrust generated by the thruster-like test setup. It served as a reference to the chamber pressure. However, this study and its main goals did not require thrust for analysis, which is why this parameter was skipped from further consideration.
2.3. Test Stand
As depicted in Figure 4, the system comprised two 2 L tanks (1) and was equipped with several essential components to ensure proper fuelling and testing procedures. The vacuum line (2) served the purpose of evacuating the air during loading of the HTP, while the pressurization line (3) provided high-pressure nitrogen to the system. The gas relief valve line (4) allowed for safe depressurization of the system, while the fuelling line (5) was responsible for propellant filling. The liquid dump line (6) served to empty the system of the propellant, whenever needed. Additionally, the system featured measurement ports (7) in the feeding line to monitor pressure and temperature during testing. The feeding line was equipped with two flow meters, including a turbine meter (8) for volumetric flow measurement and a Coriolis mass flow meter (9), both of which were used to improve the accuracy of test results. All the hot tests were performed in the special internal facility at Łukasiewicz—Institute of Aviation (see Figure 5).
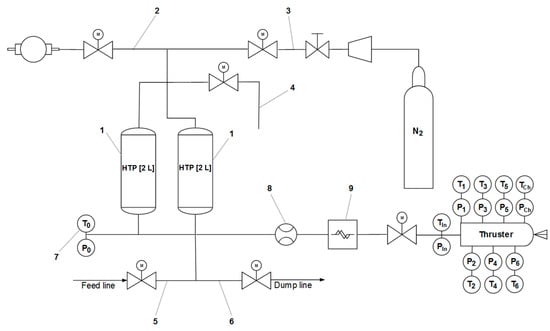
Figure 4.
Scheme of Ground Support Equipment.

Figure 5.
Łukasiewicz—Institute of Aviation Test Stand comprising the object of the investigation.
3. Results and Discussion
The test campaign assumed a total of 32 hot flows, in which each experiment utilized a fresh catalyst of 2 different types. The experiments were conducted using eight different concentrations of hydrogen peroxide. Both catalyst types were tested twice for each concentration, with a newly packed catalyst bed prepared for each run. The nominal duration of a single test was 530 s. However, different catalysts and various concentrations of hydrogen peroxide resulted in different pressure drops. Consequently, mass flows during the tests depended on actual operating conditions. In connection with a fixed HTP throughput of 5.3 kg, it led to differences in the duration of each test.
The catalyst bed performance may be analysed based on multiple measurable and unmeasurable outputs. A representative of the second group is a visual image of the thin-walled thruster and the outflow. As long as HTP decomposition products can be seen out of a sea-level nozzle, the decomposition remains incomplete. This phenomenon may be simply confirmed by the analysis applying ηc*. Moreover, a chamber made of steel (316L in this case) shines purple when heated with hot decomposition products. Thus, the area of the casing, shining purple at a steady state, indicates part of the catalyst bed being wetted with fully decomposed propellant. Furthermore, the shade of purple varies with the propellant concentration and the temperature of its decomposition products. An example of such a visual analysis is presented in Figure 6.
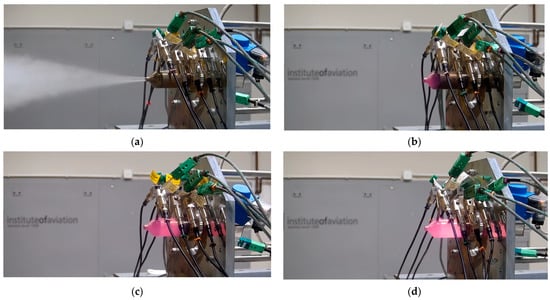
Figure 6.
Hot fire test of (a) 85.2%, (b) 90%, (c) 94.8%, and (d) 99% hydrogen peroxide; monopropellant thruster.
Since the adiabatic decomposition temperatures of 85.2% and 99% hydrogen peroxide were 891 K and 1235 K, respectively, and for the given nozzle, the outlet temperatures were 546 K and 781 K, respectively, complete decomposition should generate invisible outflow in every test. The visual image, presented in Figure 6a, suggests incomplete decomposition of the propellant. In contrast, images (c) and (d) suggest the location of the complete decomposition plane in upstream parts of these beds. The difference in the shade results from various gas temperatures.
The test results, presented in Figure 7, demonstrate that the rise time to 90% of the steady-state pressure was less than 10 s.
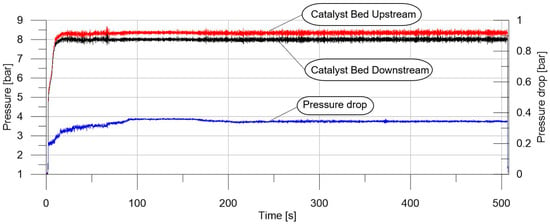
Figure 7.
Upstream and downstream pressure and pressure drop measured during the testing of a 5% Pt/Al2O3 catalyst for the decomposition of 97.8% hydrogen peroxide (test number 31).
The performance of the thruster was mostly satisfactory in our study. Steady-state test pressure remained constant, with no degradation observed. A low level of pressure instabilities was identified during testing. It indicated reliable and stable thruster performance. A pressure drop of less than 0.4 bar was observed in each test. Temperatures obtained during the tests were close to the adiabatic temperatures of hydrogen peroxide decomposition. It can be observed in Figure 8 that complete hydrogen peroxide decomposition already occurred between the third and the fourth section (10–20 mm of the catalyst bed length). This was observed in most of the tests with platinum and, in part, with manganese oxides. Moreover, a characteristic flat profile, indicating 480 K throughout the whole test (Section 2 in Figure 8), confirms the occurrence of a flat step on the temperature–axial distance chart, described previously in the Introduction. This temperature corresponds to the boiling point of water, under the pressure which was measured in the catalyst bed (approx. 8 bar). This finding confirms the results of the simulation provided by Krejci et al.
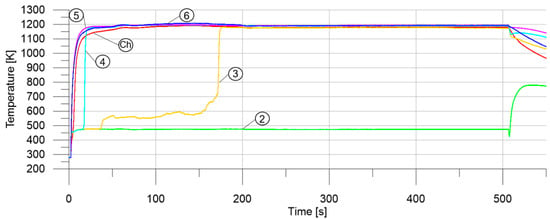
Figure 8.
Temperature in different sections of the thruster measured during the testing of a 5% Pt/Al2O3 catalyst for the decomposition of 97.8% hydrogen peroxide (test number 31): 2–6—subsequent sections of the catalyst bed, Ch—expansion chamber.
Apart from the visual (quality) assessment, the analysis, based on the efficiency of characteristic velocity, was performed. Results of this analysis are presented in Figure 9. In particular, the evolution of characteristic velocity in time was carefully analysed. The graph also presents an error analysis of the ηc*, considering the accuracy of the pressure sensors (0.25%), mass flow meter (0.1%), and hydrogen peroxide concentration measurement. The diameter of the nozzle throat remained constant before and after the tests, and the measurement error was negligible.
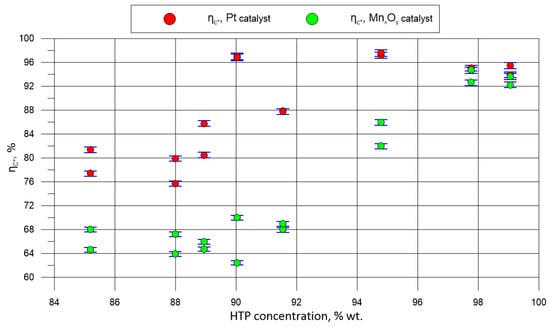
Figure 9.
Characteristics of ηc* with regard to the propellant concentration.
In general, it can be noticed that ηc* rises with the propellant concentration. The alumina-supported platinum catalyst, performing better than manganese oxides, reached its maximum efficiency with approximately 95% HTP. In the case of the other catalyst, the rising characteristics of ηc* vs. propellant concentration was maintained up to 99% peroxide. The explanation of this difference lays in the energy balance and heat loss from the catalyst bed to the environment, reaching its maximum with the highest decomposition temperature (for 99% HTP) and heat exchange area (related to the location of the full-decomposition plane). The higher the activity, the greater the area of the wall that reaches the maximum temperature. The complete decomposition of peroxide with the less active MnxOy-based catalyst occurred further downstream (with respect to the injector location) than with the platinum catalyst. Thus, the heat loss to the wall was always lower with manganese oxides for this design.
The impact of mechanical factors on the catalyst bed performance was negligible among subsequent tests because of the same packing (by means of amounts and procedures) of catalyst beds.
4. Conclusions
The presented research aimed at the identification of the relation between the concentration of hydrogen peroxide and the efficiency of the chemical process of its decomposition. Since a decrease in HTP concentration is expected during long-term space missions, the identification of its impact on thruster performance is of the great value. The right approach to this issue will help to exclude failures of propulsion systems resulting from the natural self-decomposition of hydrogen peroxide. The authors have not identified any results, available in the literature, leading to the answer to this question. Therefore, findings presented in this manuscript are original and usable for green space propulsion.
This experimental investigation was conducted using fixed-geometry catalyst beds and two catalytic materials of different activities with respect to hydrogen peroxide. Eight concentrations of hydrogen peroxide, from 85% wt. to 99% wt. were applied. A combination of 16 compositions (2 catalysts times 8 concentrations), multiplied by 2 redundant hot runs, resulted in 32 hot tests, lasting approx. 9 min each. The hot-fire test campaign provided reliable results for a comprehensive analysis.
In conclusion, for a fixed-geometry catalyst bed, designed for over 90% HTP, and a highly active catalyst, there is a clear relationship between the efficiency of decomposition and the propellant concentration. Different characteristics were obtained for platinum (highly active) and manganese oxides (medium active) catalysts. Whenever the complete decomposition of the propellant occurred in the catalyst bed, the location of its plane determined the heat loss and the efficiency of the characteristic velocity. When hydrogen peroxide of lower than 90% concentration was used with platinum catalyst, the decomposition efficiency dropped significantly, leaving part of the propellant non-decomposed. The other catalyst, based on manganese oxides, performed efficiently only with the highest-class peroxide. These experimental findings provide guidelines for the future design of thrusters and further verification tests. The catalyst bed, operating either with platinum or manganese oxides, needs to be lengthened to maintain its performance with a wide rage of the HTP concentration.
The time to reach steady-state chamber pressure was found to depend on the catalyst and hydrogen peroxide concentration, with the platinum catalyst proving to be more active than the one based on manganese oxides, and the higher concentration of peroxide led to a shorter transient time. Finally, higher propellant throughputs would be needed in order to assess the influence of the propellant specification on the catalyst lifetime with better precision.
Author Contributions
Conceptualization, A.P. and P.S.; methodology, A.P. and P.S.; software, A.P. and Z.G.; validation, A.P., P.S. and Z.G.; formal Analysis, A.P. and P.S.; investigation, A.P., P.S. and Z.G.; resources, A.P.; data curation, A.P. and P.S.; writing—original draft preparation, A.P. and P.S.; writing—review & editing, A.P., P.S. and Z.G.; visualization, A.P. and P.S.; supervision, P.S.; project administration, P.S.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Space Agency (ESA), Contract No. 4000118443/16/NL/PS.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions of the contract between the Contractor and the funding organization.
Acknowledgments
Developmental activities presented in this manuscript are parts of research and development (R&D) actions prepared for the ESA project: Hydrogen Peroxide Storability/Compatibility Verification. Moreover, the authors would like to express their gratitude to other team members of the Space Technology Department of Łukasiewicz Research Network—Institute of Aviation for their extensive support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ventura, M.; Garboden, G. A Brief History of Concentrated Hydrogen Peroxide Uses. In Proceedings of the 35th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exibit, Los Angeles, CA, USA, 20–24 June 1999. [Google Scholar] [CrossRef]
- Rarata, G.; Rokicka, K.; Surmacz, P. Hydrogen Peroxide as a High Energy Compound Optimal for Propulsive Applications. Cent. Eur. J. Energ. Mater. 2016, 13, 778–790. [Google Scholar] [CrossRef]
- Gotzig, U.; Wurdak, M.; Lauck, F. Development of a Flight Type 1N Hydrogen Peroxide Thruster. In Proceedings of the Space Propulsion Conference, Seville, Spain, 14–18 May 2018. [Google Scholar]
- Wernimont, E.; Ventura, M.; Garboden, G.; Mullens, P. Past and Present Uses of Rocket Grade Hydrogen Peroxide; General Kinetics: Huntington Beach, CA, USA, 2005. [Google Scholar]
- Ventura, M.; Wernimont, E.; Dillard, J. Hydrogen Peroxide—Optimal for Turbomachinery and Power Applications. In Proceedings of the 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007. [Google Scholar] [CrossRef]
- Neufeld, M.J. The Rocket and the Reich: Peenemünde and the Coming of the Ballistic Missile Era; Harvard University Press: Cambridge, MA, USA, 1996; pp. 38–75. [Google Scholar]
- Stokes, P.R. Hydrogen Peroxide for Power and Propulsion. Trans. Newcomen Soc. 1997, 69, 69–96. [Google Scholar] [CrossRef]
- Baker, D. The Rocket: The History and Development of Rocket & Missile Technology, 1st ed.; Crown: New York, NY, USA, 1978; pp. 5–21. [Google Scholar]
- Jones, C.W.; Clark, J.H. Applications of Hydrogen Peroxide and Derivatives; Royal Society of Chemistry: London, UK, 2000; pp. 232–233. [Google Scholar] [CrossRef]
- Cervone, A.; Torre, L.; d’Agostino, L.; Musker, A.J.; Roberts, G.T.; Bramanti, C.; Soccoccia, G. Development of Hydrogen Peroxide Monopropellant Rockets. In Proceedings of the 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exibit, Sacramento, CA, USA, 9–12 July 2006. [Google Scholar] [CrossRef]
- Rarata, G.; Surmacz, P. Hydrogen Peroxide 98% HTP-class—An Alternative for Hydrazine (in Polish). Pr. Inst. Lotn. 2014, 1, 25–33. [Google Scholar]
- Rarata, G.; Surmacz, P. The Safe Preparation of HTP and Concentrated H2O2 Samples. Trans. Inst. Aviat. 2011, 217, 116–124. [Google Scholar]
- Krejci, D.; Woschnak, A.; Scharlemann, C.; Ponweiser, K. Structural impact of honeycomb catalysts on hydrogen peroxide decomposition for micro propulsion. Chem. Eng. Res. Des. 2012, 90, 2302–2315. [Google Scholar] [CrossRef]
- Palmer, M.J. Experimental Evaluation of Hydrogen Peroxide Catalysts for Monopropellant Attitude Control Thrusters. Ph.D. Thesis, University of Southampton, Southampton, UK, 2014. [Google Scholar]
- Rusek, J.J. New Decomposition Catalysts and Characterization Techniques for Rocket-Grade Hydrogen Peroxide. J. Propul. Power 1996, 12, 574–579. [Google Scholar] [CrossRef]
- Romeo, L.; Torre, L.; Pasini, A.; Cervone, A.; d’Agostino, L.; Calderazzo, F. Performance of Different Catalysts Supported on Alumina Spheres for Hydrogen Peroxide Decomposition. In Proceedings of the 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007. [Google Scholar] [CrossRef]
- Wanhainen, J.P.; Ross, P.S.; DeWitt, R.L. Effect of Propellant and Catalyst Bed Temperatures on Thrust Buildup in Several Hydrogen Peroxide Reaction Control Rockets; Technical Note D-480; NASA: Washington, DC, USA, 1960. [Google Scholar]
- Lee, S.-L.; Lee, C.-W. Performance characteristics of silver catalyst bed for hydrogen peroxide. Aerosp. Sci. Technol. 2009, 13, 12–17. [Google Scholar] [CrossRef]
- Chan, Y.-A.; Liu, H.J.; Tseng, K.-C.; Kuo, T.C. Preliminary Development of a Hydrogen Peroxide Thruster. Int. J. Mech. Aerosp. Ind. Mechatron. Manuf. Eng. 2013, 7, 1226–1233. [Google Scholar]
- Amri, R.; Gibbon, D.; Rezoug, T. The design, development and test of one newton hydrogen peroxide monopropellant thruster. Aerosp. Sci. Technol. 2013, 25, 266–272. [Google Scholar] [CrossRef]
- Runckel, J.F.; Willis, C.M.; Salters, L.B. Investigation of Catalyst Beds for 98-Percent Concentration Hydrogen Peroxide; Technical Note, D-1808; NASA: Washington, DC, USA, 1963. [Google Scholar]
- Koopmans, R.-J.; Nandyala, V.R.; Pavesi, S.; Batonneau, Y.; Beauchet, R.; Maleix, C.; Schwentenwein, M.; Spitzbart, M.; Altun, A.A.; Scharlemann, C. Comparison of HTP catalyst performance for different internal monolith structures. Acta Astronaut. 2019, 164, 106–111. [Google Scholar] [CrossRef]
- Kang, H.; Lee, D.; Kang, S.; Kwon, S. Effect of H2O2 injection patterns on catalyst bed characteristics. Acta Astronaut. 2017, 130, 75–83. [Google Scholar] [CrossRef]
- An, S.; Jin, J.; Lee, J.; Jo, S.; Park, D.; Kwon, S. Chugging Instability of H2O2 Monopropellant Thrusters with Reactor Aspect Ratio and Pressures. J. Propuls. Power 2011, 27, 422. [Google Scholar] [CrossRef]
- Jo, S.; Jang, D.; An, S.; Kwon, S. Chugging Instability of H2O2 Monopropellant Thrusters with Catalyst Reactivity and Support Sizes. J. Propuls. Power 2011, 27, 920. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, T.; Sun, Z.; Liang, D.; Lin, L. Performance and deactivation of Ir/γ-Al2O3 catalyst in the hydrogen peroxide monopropellant thruster. Appl. Catal. A Gen. 2001, 210, 55–62. [Google Scholar] [CrossRef]
- An, S.; Kwon, S. Scaling and evaluation of Pt/Al2O3 catalytic reactor for hydrogen peroxide monopropellant thruster. J. Propuls. Power 2009, 25, 1041–1045. [Google Scholar] [CrossRef]
- An, S.; Lee, J.; Brahmi, R.; Kappenstein, C.; Kwon, S. Comparison of catalyst support between monolith and pellet in hydrogen peroxide thrusters. J. Propuls. Power 2010, 26, 439–445. [Google Scholar] [CrossRef]
- Huh, J.; Kwon, S. Design, fabrication and thrust measurement of a micro liquid monopropellant thruster. J. Micromech. Microeng. 2014, 24, 104001. [Google Scholar] [CrossRef]
- Pasini, A.; Torre, L.; Romeo, L.; Cervone, A.; d’Agostino, L. Performance characterization of pellet catalytic beds for hydrogen peroxide monopropellant rocket. J. Propuls. Power 2011, 27, 428–436. [Google Scholar] [CrossRef]
- Keating, K.B.; Rozner, A.G.; Youngblood, J.L. The effect of deformation on catalytic activity of platinum in the decomposition of hydrogen peroxide. J. Catal. 1965, 4, 608–619. [Google Scholar] [CrossRef]
- Surmacz, P.; Kostecki, M.; Gut, Z.; Olszyna, A. Aluminum Oxide–Supported Manganese Oxide Catalyst for a 98% Hydrogen Peroxide Thruster. J. Propuls. Power 2019, 35, 614–623. [Google Scholar] [CrossRef]
- Rarata, G.; Rokicka, K. The Manganese Oxides Decomposition Catalysts for Highly Concentrated Hydrogen Peroxide. Trans. Inst. Aviat. 2015, 240, 49. [Google Scholar] [CrossRef]
- Surmacz, P. Influence of Various Types of Al2O3/MnxOy Catalysts on Performance of a 100 mm Chamber for Decomposition of 98%+ Hydrogen Peroxide. Trans. Inst. Aviat. 2015, 240, 58. [Google Scholar] [CrossRef]
- Surmacz, P.; Gut, Z. The Experimental Investigation of a 98% Hydrogen Peroxide Monopropellant Thruster Comprising the Metal-Foam-Supported Manganese Oxide Catalyst. Aerospace 2023, 10, 215. [Google Scholar] [CrossRef]
- Gordon, S.; McBride, B.J. Computer Program for Calculation of Complex Chemical Equilibrium Compositions and Applications. Part 1: Analysis. NASA Technol. Memo. 1994. Available online: https://ntrs.nasa.gov/citations/19950013764 (accessed on 20 April 2023).
- Sutton, G.P.; Biblarz, O. Rocket Propulsion Elements; John Wiley & Sons: Hoboken, NJ, USA, 2016; ISBN 9781118753651. [Google Scholar]
- Ponzo, J.B. (Aerojet, Scramento, CA, USA) Monolithic Hydrogen Peroxide Catalyst Bed Development. 2003. Available online: https://ntrs.nasa.gov/citations/20030066364 (accessed on 19 April 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).