Bottom-Up Drivers for Global Fish Catch Assessed with Reconstructed Ocean Biogeochemistry from an Earth System Model
Abstract
1. Introduction
2. Data and Methods
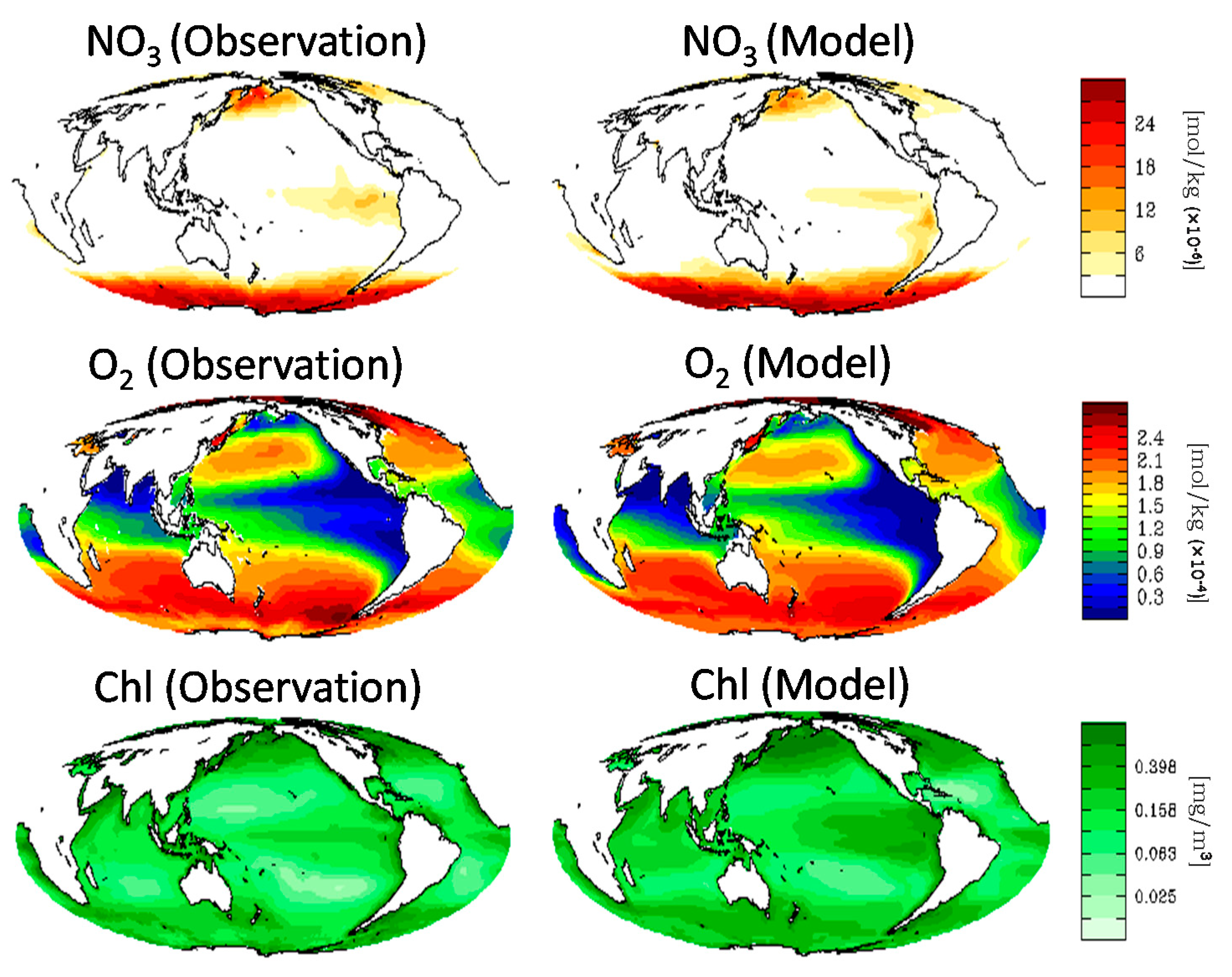
2.1. Reconstructed Ocean Physical-Biogeochemical Field
2.2. Fisheries Production Data
2.3. Decision Tree Analysis with Monte Carlo Samples
3. Results
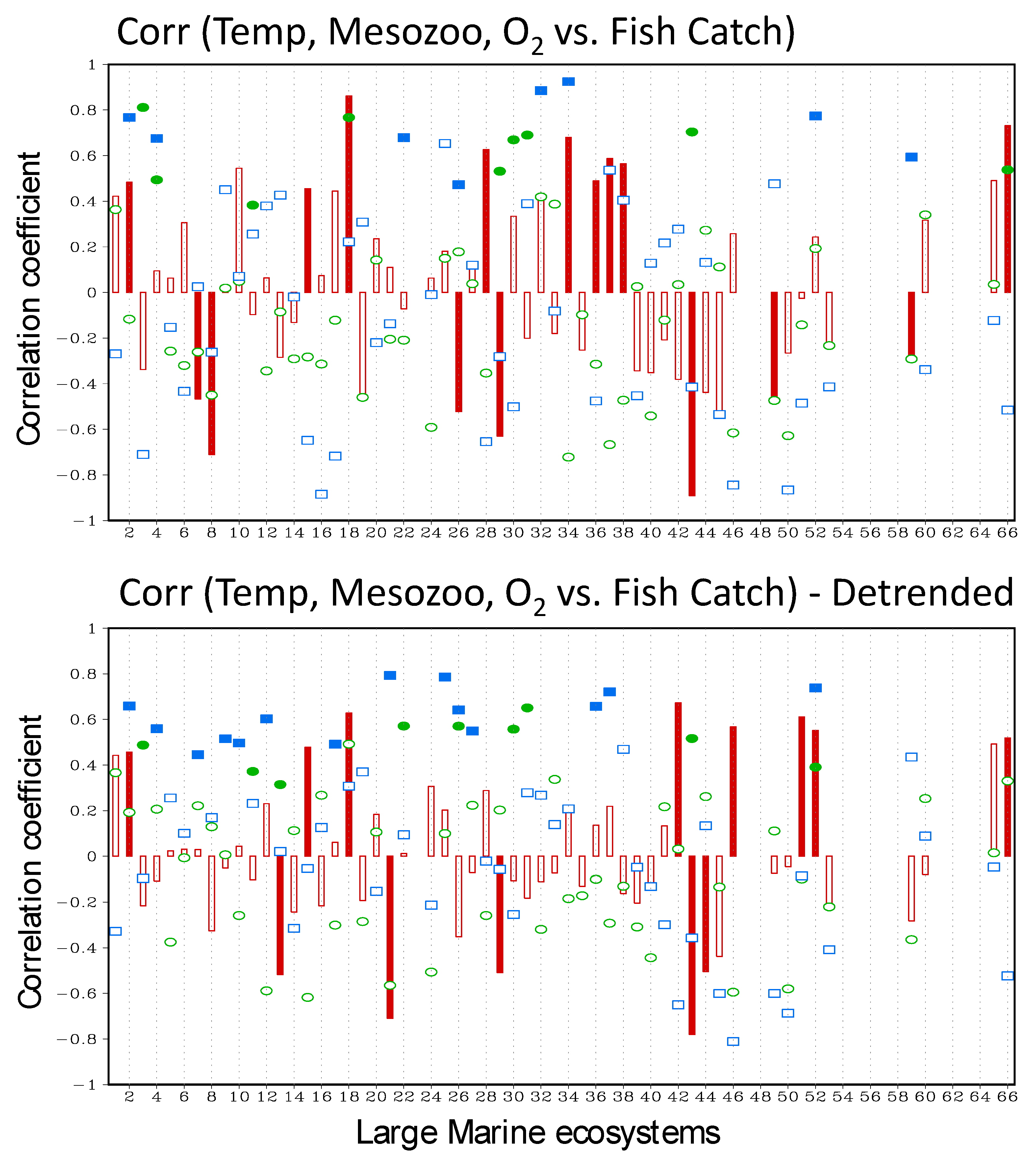
3.1. LME-Scale Relationship between Environmental Drivers and Annual Fish Catch
3.2. Relative Importance of Environmental Drivers
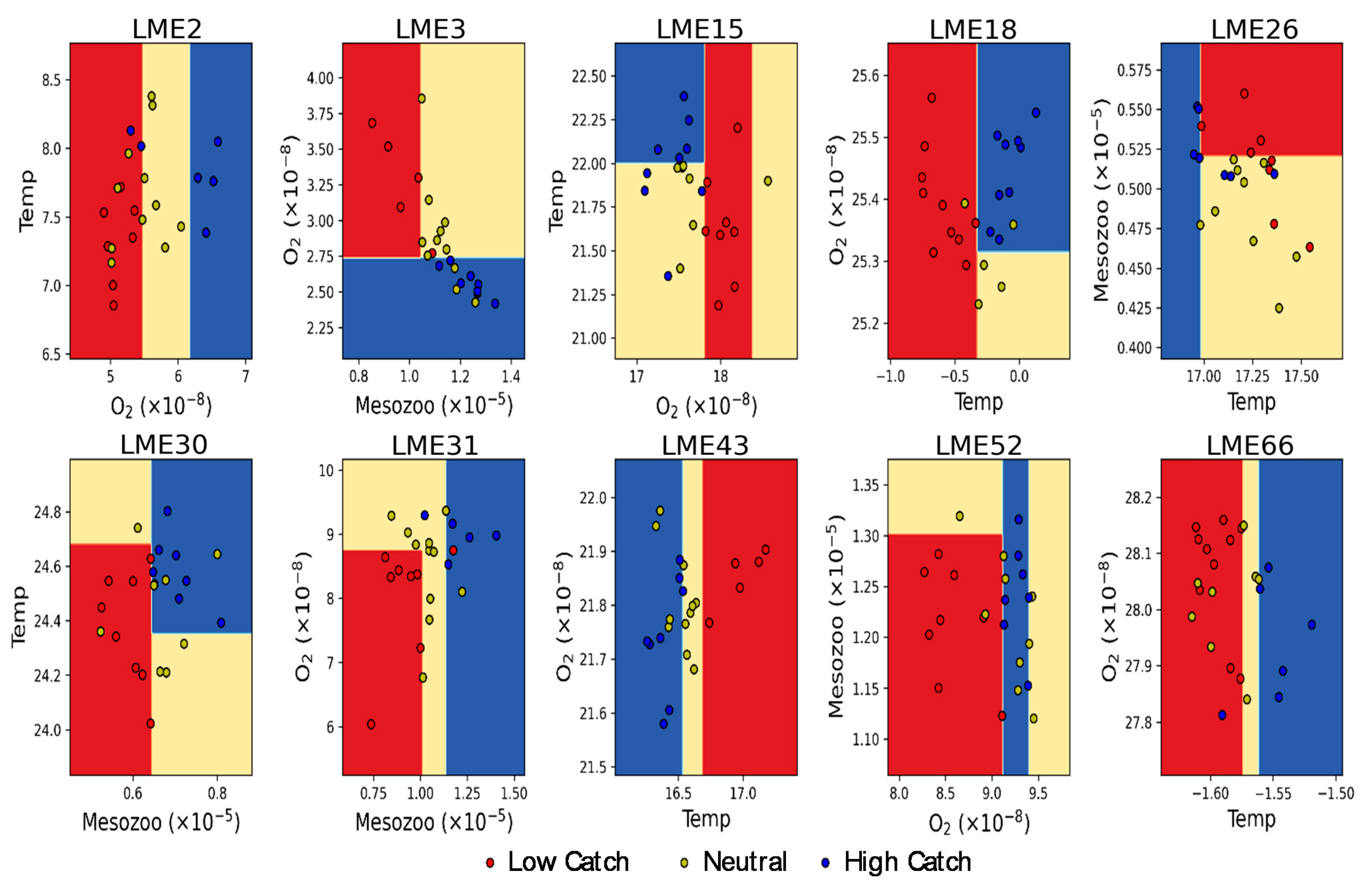
3.3. Thresholds of Multiple Environmental Forcing for High and Low Fish Catch
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ottersen, G.; Kim, S.; Huse, G.; Polovina, J.J.; Stenseth, N.C. Major Pathways by which Climate may Force Marine Fish Populations. J. Mar. Syst. 2010, 79, 343–360. [Google Scholar] [CrossRef]
- Finney, B.P.; Alheit, J.; Emeis, K.-C.; Field, D.B.; Gutiérrez, D.; Struck, U. Paleoecological Studies on Variability in Marine Fish Populations: A Long-Term Perspective on the Impacts of Climatic Change on Marine Ecosystems. J. Mar. Syst. 2010, 79, 316–326. [Google Scholar] [CrossRef]
- Deutsch, C.; Ferrel, A.; Seibel, B.; Pörtner, H.-O.; Huey, R.B. Climate Change Tightens a Metabolic Constraint on Marine Habitats. Science 2015, 348, 1132–1135. [Google Scholar] [CrossRef]
- Bopp, L.; Resplandy, L.; Orr, J.C.; Doney, S.C.; Dunne, J.P.; Gehlen, M.; Halloran, P.R.; Heinze, C.; Ilyina, T.; Seferian, R.; et al. Multiple Stressors of Ocean Ecosystems in the 21st Century: Projections with CMIP5 Models. Biogeosciences 2013, 10, 6225–6245. [Google Scholar] [CrossRef]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate Change and Distribution Shifts in Marine Fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Duffy, J.E.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change Impacts on Marine Ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Sarmiento, J.L.; Dunne, J.P.; Frölicher, T.L.; Lam, V.W.Y.; Palomares, M.L.D.; Watson, R.; Pauly, D. Shrinking of Fishes Exacerbates Impacts of Global Ocean Changes on Marine Ecosystems. Nat. Clim. Chang. 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Hare, J.A.; Alexander, M.A.; Fogarty, M.J.; Williams, E.H.; Scott, J.D. Forecasting the Dynamics of a Coastal Fishery Species using a Coupled Climate–Population Model. Ecol. Appl. 2010, 20, 452–464. [Google Scholar] [CrossRef]
- Essington, T.E.; Moriarty, P.E.; Froehlich, H.E.; Hodgson, E.E.; Koehn, L.E.; Oken, K.L.; Siple, M.C.; Stawitz, C.C. Fishing Amplifies Forage Fish Population Collapses. Proc. Natl. Acad. Sci. USA 2015, 112, 6648–6652. [Google Scholar] [CrossRef] [PubMed]
- Sharp, G.D. Climate and Fisheries: Cause and Effect or Managing the Long and Short of it All. S. Afr. J. Mar. Sci. 1987, 5, 811–838. [Google Scholar] [CrossRef]
- McOwen, C.J.; Cheung, W.W.L.; Rykaczewski, R.R.; Watson, A.R.; Wood, L.J. Is Fisheries Production within Large Marine Ecosystems Determined by Bottom-Up or Top-Down Forcing? Fish Fish. 2014, 16, 623–632. [Google Scholar] [CrossRef]
- Stock, C.A.; John, J.G.; Rykaczewski, R.R.; Asch, R.G.; Cheung, W.W.L.; Dunne, J.P.; Friedland, K.D.; Lam, V.W.Y.; Sarmiento, J.L.; Watson, R.A. Reconciling Fisheries Catch and Ocean Productivity. Proc. Natl. Acad. Sci. USA 2017, 114, E1441–E1449. [Google Scholar] [CrossRef]
- Nieto, K.; McClatchie, S.; Weber, E.D.; Lennert-Cody, C.E. Effect of Mesoscale Eddies and Streamers on Sardine Spawning Habitat and Recruitment Success off Southern and Central California. J. Geophys. Res. Oceans 2014, 119, 6330–6339. [Google Scholar] [CrossRef]
- Tommasi, D.; Stock, C.A.; Pegion, K.; Vecchi, G.A.; Methot, R.D.; Alexander, M.A.; Checkley, D.M. Improved Management of Small Pelagic Fisheries through Seasonal Climate Prediction. Ecol. Appl. 2017, 27, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Lindegren, M.; Checkley, D.M. Temperature Dependence of Pacific Sardine (Sardinops Sagax) Recruitment in the California Current Ecosystem Revisited and Revised. Can. J. Fish. Aquat. Sci. 2013, 70, 245–252. [Google Scholar] [CrossRef]
- Fu, C.; Gaichas, S.; Link, J.S.; Bundy, A.; Boldt, J.L.; Cook, A.M.; Gamble, R.; Utne, K.R.; Liu, H.; Friedland, K.D. Relative Importance of Fisheries, Trophodynamic and Environmental Drivers in a Series of Marine Ecosystems. Mar. Ecol. Prog. Ser. 2012, 459, 169–184. [Google Scholar] [CrossRef]
- Park, J.-Y.; Stock, C.A.; Dunne, J.P.; Yang, X.; Rosati, A. Seasonal to Multiannual Marine Ecosystem Prediction with a Global Earth System Model. Science 2019, 365, 284–288. [Google Scholar] [CrossRef]
- Sachoemar, S. Variability of Sea Surface Chlorophyll-A, Temperature and Fish Catch within Indonesian Region Revealed by Satellite Data. Mar. Res. Indones. 2012, 37, 75–87. [Google Scholar] [CrossRef]
- Solanki, H.U.; Dwivedi, R.M.; Nayak, S.R. Synergistic Analysis of SeaWiFS Chlorophyll Concentration and NOAA-AVHRR SST Features for Exploring Marine Living Resources. Int. J. Remote Sens. 2001, 22, 3877–3882. [Google Scholar] [CrossRef]
- Antoine, D.; Morel, A.; Gordon, H.R.; Banzon, V.F.; Evans, R.H. Bridging Ocean Color Observations of the 1980s and 2000s in Search of Long-Term Trends. J. Geophys. Res. Space Phys. 2005, 110, 110. [Google Scholar] [CrossRef]
- Friedland, K.D.; Stock, C.; Drinkwater, K.F.; Link, J.S.; Leaf, R.T.; Shank, B.V.; Rose, J.M.; Pilskaln, C.H.; Fogarty, M.J. Pathways between Primary Production and Fisheries Yields of Large Marine Ecosystems. PLoS ONE 2012, 7, e28945. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Stock, C.A.; Yang, X.; Dunne, J.P.; Rosati, A.; John, J.; Zhang, S. Modeling Global Ocean Biogeochemistry With Physical Data Assimilation: A Pragmatic Solution to the Equatorial Instability. J. Adv. Model. Earth Syst. 2018, 10, 891–906. [Google Scholar] [CrossRef]
- Zhang, S.; Harrison, M.J.; Rosati, A.; Wittenberg, A. System Design and Evaluation of Coupled Ensemble Data Assimilation for Global Oceanic Climate Studies. Mon. Weather Rev. 2007, 135, 3541–3564. [Google Scholar] [CrossRef]
- Kanamitsu, M.; Ebisuzaki, W.; Woollen, J.; Yang, S.-K.; Hnilo, J.J.; Fiorino, M.; Potter, G.L. NCEP–DOE AMIP-II Reanalysis (R-2). Bull. Am. Meteorol. Soc. 2002, 83, 1631–1644. [Google Scholar] [CrossRef]
- Levitus, S.; Antonov, J.I.; Baranova, O.K.; Boyer, T.P.; Coleman, C.L.; Garcia, H.E.; Grodsky, A.I.; Johnson, D.R.; Locarnini, R.A.; Mishonov, A.V.; et al. The World Ocean Database. Data Sci. J. 2013, 12, WDS229–WDS234. [Google Scholar] [CrossRef]
- Reynolds, R.W.; Smith, T.M.; Liu, C.Y.; Chelton, D.B.; Casey, K.S.; Schlax, M.G. Daily High-Resolution-Blended Analyses for Sea Surface Temperature. J. Clim. 2007, 20, 5473–5496. [Google Scholar] [CrossRef]
- Roemmich, D.; Riser, S.; Davis, R.; Desaubies, Y. Autonomous Profiling Floats: Workhorse for Broad-Scale Ocean Observations. Mar. Technol. Soc. J. 2004, 38, 21–29. [Google Scholar] [CrossRef]
- Stock, C.A.; Dunne, J.P.; John, J.G. Global-Scale Carbon and Energy Flows through the Marine Planktonic Food Web: An Analysis with a Coupled Physical–Biological Model. Prog. Oceanogr. 2014, 120, 1–28. [Google Scholar] [CrossRef]
- Laufkötter, C.; Vogt, M.; Gruber, N.; Aita-Noguchi, M.; Aumont, O.; Bopp, L.; Buitenhuis, E.; Doney, S.C.; Dunne, J.; Hashioka, T.; et al. Drivers and Uncertainties of Future Global Marine Primary Production in Marine Ecosystem Models. Biogeosciences 2015, 12, 6955–6984. [Google Scholar] [CrossRef]
- Dunne, J.P.; John, J.G.; Shevliakova, E.; Stouffer, R.J.; Krasting, J.P.; Malyshev, S.L.; Milly, P.C.D.; Sentman, L.T.; Adcroft, A.J.; Cooke, W.; et al. GFDL’s ESM2 Global Coupled Climate–Carbon Earth System Models. Part II: Carbon System Formulation and Baseline Simulation Characteristics. J. Clim. 2013, 26, 2247–2267. [Google Scholar] [CrossRef]
- Esaias, W.; Abbott, M.; Barton, I.; Brown, O.; Campbell, J.; Carder, K.; Clark, D.; Evans, R.; Hoge, F.; Gordon, H.; et al. An Overview of MODIS Capabilities for Ocean Science Observations. IEEE Trans. Geosci. Remote Sens. 1998, 36, 1250–1265. [Google Scholar] [CrossRef]
- McClain, C.R.; Cleave, M.L.; Feldman, G.C.; Gregg, W.W.; Hooker, S.B.; Kuring, N. Science Quality SeaWiFS Data for Global Biosphere Research. Sea Technol. 1998, 39, 10–16. [Google Scholar]
- Pauly, D.; Zeller, D. Catch Reconstructions Reveal that Global Marine Fisheries Catches are Higher than Reported and Declining. Nat. Commun. 2016, 7, 10244. [Google Scholar] [CrossRef]
- Sherman, K. The Large Marine Ecosystem Approach for Assessment and Management of Ocean Coastal Waters. In Sustaining Large Marine Ecosystems: The Human Dimension; Hennessey, T.M., Sutinen, J.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 13, pp. 3–16. [Google Scholar]
- Chassot, E.; Bonhommeau, S.; Dulvy, N.K.; Mélin, F.; Watson, R.; Gascuel, D.; Le Pape, O. Global Marine Primary Production Constrains Fisheries Catches. Ecol. Lett. 2010, 13, 495–505. [Google Scholar] [CrossRef]
- Bretherton, C.S.; Widmann, M.; Dymnikov, V.P.; Wallace, J.M.; Blade, I. The Effective Number of Spatial Degrees of Freedom of a Time-Varying Field. J. Clim. 1999, 12, 1990–2009. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification and Regression Trees; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- McGovern, A.; Elmore, K.L.; Gagne, D.J.; Haupt, S.E.; Karstens, C.D.; Lagerquist, R.; Smith, T.; Williams, J.K. Using Artificial Intelligence to Improve Real-Time Decision-Making for High-Impact Weather. Bull. Am. Meteorol. Soc. 2017, 98, 2073–2090. [Google Scholar] [CrossRef]
- Chavez, F.P.; Ryan, J.; Lluch-Cota, S.E.; Ñiquen, M. From Anchovies to Sardines and Back: Multidecadal Change in the Pacific Ocean. Science 2003, 299, 217–221. [Google Scholar] [CrossRef]
- Chassot, E.; Melin, F.; Le Pape, O.; Gascuel, D. Bottom-Up Control Regulates Fisheries Production at the Scale of Eco-Regions in European Seas. Mar. Ecol. Prog. Ser. 2007, 343, 45–55. [Google Scholar] [CrossRef]
- Field, J.C.; MacCall, A.D.; Bradley, R.W.; Sydeman, W.J. Estimating the Impacts of Fishing on Dependent Predators: A Case Study in the California Current. Ecol. Appl. 2010, 20, 2223–2236. [Google Scholar] [CrossRef]
- Stramma, L.; Prince, E.D.; Schmidtko, S.; Luo, J.; Hoolihan, J.P.; Visbeck, M.; Wallace, D.W.R.; Brandt, P.; Körtzinger, A. Expansion of Oxygen Minimum Zones may Reduce Available Habitat for Tropical Pelagic Fishes. Nat. Clim. Chang. 2011, 2, 33–37. [Google Scholar] [CrossRef]
- Grønbæk, L.; Lindroos, M.; Munro, G.; Pintassilgo, P. Game Theory and Fisheries. Fish. Res. 2018, 203, 1–5. [Google Scholar] [CrossRef]
- Memarzadeh, M.; Britten, G.L.; Worm, B.; Boettiger, C. Rebuilding Global Fisheries under Uncertainty. Proc. Natl. Acad. Sci. USA 2019, 116, 15985–15990. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, E.; Ohman, M.D. A Double-Integration Hypothesis to Explain Ocean Ecosystem Response to Climate Forcing. Proc. Natl. Acad. Sci. USA 2013, 110, 2496–2499. [Google Scholar] [CrossRef] [PubMed]
- Le Mézo, P.; Lefort, S.; Séférian, R.; Aumont, O.; Maury, O.; Murtugudde, R.; Bopp, L. Natural Variability of Marine Ecosystems Inferred from a Coupled Climate to Ecosystem Simulation. J. Mar. Syst. 2016, 153, 55–66. [Google Scholar] [CrossRef]


| LME Number | LME Name | LME Number | LME Name | LME Number | LME Name | LME Number | LME Name |
|---|---|---|---|---|---|---|---|
| 1 | East Bering Sea | 18 | Canadian Eastern Arctic | 35 | Gulf of Thailand | 52 | Sea of Okhotsk |
| 2 | Gulf of Alaska | 19 | East Greenland Shelf | 36 | South China Sea | 53 | West Bering Sea |
| 3 | California Current | 20 | Barents Sea | 37 | Sulu-Celebes Sea | 54 | Chukchi Sea |
| 4 | Gulf of California | 21 | Norwegian Shelf | 38 | Indonesian Sea | 55 | Beaufort Sea |
| 5 | Gulf of Mexico | 22 | North Sea | 39 | North Australian Shelf | 56 | East Siberian Sea |
| 6 | Southeast U.S. Continental Shelf | 23 | Baltic Sea | 40 | Northeast Australian Shelf | 57 | Laptev Sea |
| 7 | Northeast U.S. Continental Shelf | 24 | Celtic-Biscay Shelf | 41 | East-Central Australian Shelf | 58 | Kara Sea |
| 8 | Scotian Shelf | 25 | Iberian Coastal | 42 | Southeast Australian Shelf | 59 | Iceland Shelf |
| 9 | Newfoundland-Labrador Shelf | 26 | Mediterranean Sea | 43 | Southwest Australian Shelf | 60 | Faroe Plateau |
| 10 | Insular Pacific-Hawaiian | 27 | Canary Current | 44 | West-Central Australian Shelf | 61 | Antarctica |
| 11 | Pacific Central-American | 28 | Guinea Current | 45 | Northwest Australian Shelf | 62 | Black Sea |
| 12 | Caribbean Sea | 29 | Benguela Current | 46 | New Zealand Shelf | 63 | Hudson Bay |
| 13 | Humboldt Current | 30 | Agulhas Current | 47 | East China Sea | 64 | Arctic Ocean |
| 14 | Patagonian Shelf | 31 | Somali Coastal Current | 48 | Yellow Sea | 65 | Aleutian Islands |
| 15 | South Brazil Shelf | 32 | Arabian Sea | 49 | Kuroshio Current | 66 | Canadian High Arctic |
| 16 | East Brazil Shelf | 33 | Red Sea | 50 | East Sea | ||
| 17 | North Brazil Shelf | 34 | Bay of Bengal | 51 | Oyashio Current |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.-J.; Park, J.-Y. Bottom-Up Drivers for Global Fish Catch Assessed with Reconstructed Ocean Biogeochemistry from an Earth System Model. Climate 2021, 9, 83. https://doi.org/10.3390/cli9050083
Song H-J, Park J-Y. Bottom-Up Drivers for Global Fish Catch Assessed with Reconstructed Ocean Biogeochemistry from an Earth System Model. Climate. 2021; 9(5):83. https://doi.org/10.3390/cli9050083
Chicago/Turabian StyleSong, Hyo-Jong, and Jong-Yeon Park. 2021. "Bottom-Up Drivers for Global Fish Catch Assessed with Reconstructed Ocean Biogeochemistry from an Earth System Model" Climate 9, no. 5: 83. https://doi.org/10.3390/cli9050083
APA StyleSong, H.-J., & Park, J.-Y. (2021). Bottom-Up Drivers for Global Fish Catch Assessed with Reconstructed Ocean Biogeochemistry from an Earth System Model. Climate, 9(5), 83. https://doi.org/10.3390/cli9050083






