Abstract
There is substantial global concern over the potential impacts of plant invasions on native biodiversity in protected areas (PAs). Protected areas in tropical island countries that host rich biodiversity face an imminent risk from the potential spread of invasive alien plant species. Thus, the aim of this study was to gain a general understanding of the potential risks of multiple plant invasions in PAs located in the tropical island of Sri Lanka under projected climate change. We conducted a further analysis of a multi-species climate suitability assessment, based on a previous study using the Maximum Entropy (MaxEnt) modeling approach, and tested how species invasion may change in protected areas under climate change. We evaluated how the climate suitability of 14 nationally recognized invasive alien plant species (IAPS) will vary within PAs and outside PAs by 2050 under two climate change scenarios, representative concentration pathways (RCP) 4.5 and 8.5. Our findings suggest that there will be increased risks from multiple IAPS inside PAs and outside PAs in Sri Lanka in the future; however, the potential risk is comparatively less in PAs. We provide an overview of the species richness of selected threatened vertebrate groups, which can be potentially impacted by IAPS in PAs. The findings of this study highlight important implications for the strategic management of plant invasions in PAs in order to safeguard native biodiversity, with special reference to vertebrates.
1. Introduction
Biodiversity is diminishing at an unprecedented rate, despite increasing conservation initiatives worldwide [1]. The over-exploitation of natural resources, habitat degradation and loss of habitats, environmental pollution, climate change, and invasive alien species (IAS) are key drivers that have led to the depletion of global biodiversity [2]. Global climate is changing due to anthropogenic activities [3]; thus, climate-induced impacts on the earth’s biota are undeniable, particularly in tropical island countries [4]. The Earth’s temperature has increased substantially (approximately 0.6 °C) over the last 10 decades [5]. According to predictions, the temperature of South Asia will increase in the next few decades, and thus, many countries in this region, especially small islands, are at the frontline of a climate crisis [6,7]. Many developing island nations in tropical regions are highly vulnerable to climate change and its associated impacts (i.e., impacts from IAS), due to their small size and fragile ecosystems [8,9,10,11]. Climate change is expected to influence the introduction and spread of IAS in the future [12,13,14]. Furthermore, IAS are considered to be the second leading factor (i.e., after habitat destruction) that endangers biodiversity [15,16]. The potential impacts of IAS are enormous and mostly irreversible under global climate change [5]. How the potential risks of IAS in protected areas (PAs) vary in response to climate change remains understudied, and such quantitative data are limited [14,17]. However, such information is fundamental in developing management strategies in the face of increasing plant invasions [18].
Protected areas are widely considered to be a key strategy against biodiversity loss by the commitment to the protection of threatened species and their habitats, and safeguarding the sustenance of ecosystem services [19,20]. The important contribution of PAs, as a vital approach to biodiversity conservation, has been acknowledged by the global environmental commitments, such as the strategic plan for biodiversity, for example, the Aichi Biodiversity Targets and Sustainable Development Goals (SDGs) [21]. These obligations have set agreed targets that are to be achieved during a specific time period in order to halt biodiversity loss. The introduction and spread of invasive alien plant species (IAPS) is one of the leading causes of biodiversity loss in PAs, and thus, it is a growing concern as IAPS significantly change ecosystem processes (i.e., nutrient cycling) and cause a decline in native species abundance and richness [22,23]. Such consequences can be harmful to the ecological integrity and ecosystem services of PAs [24]. Therefore, the control and management of IAPS is one of the key activities that PA managers are confronted with in terms of the long-term management of biodiversity and as to how to ensure the sustainability of the system [25].
Vertebrates represent less than 1% of all species and are important for ecosystem sustainability, since their presence ensures greater species diversity [26]. Generally, vertebrate species in Sri Lanka are at a high risk of extinction due to climate change and several other anthropogenic factors, i.e., one in every six inland indigenous vertebrate species are considered to be critically endangered [27]. The national red list comprises 748 vertebrate species (i.e., amphibians, freshwater fish, reptiles, birds, and mammals) in Sri Lanka. Of these vertebrate species, 345 (46%) are considered to be nationally threatened, of which 233 (68%) are endemic to Sri Lanka [27]. Threatened endemic species are particularly vulnerable and therefore represent the main challenge for conservation [28]. Table 1 shows the national conservation status of the vertebrate fauna of Sri Lanka, based on taxonomic groups.

Table 1.
The species richness of selected vertebrate (i.e., amphibians, freshwater fish, reptiles, birds, and mammals) faunal groups in Sri Lanka and their national threat status. CR: Critically endangered; EN: Endangered; VU: Vulnerable. The number of endemic species is given within brackets (Source: Weerakoon [27]).
This study is an extension of a previous work by Kariyawasam et al. [29] that used the MaxEnt niche model to define potential overlapping areas of 14 IAPS of national significance in Sri Lanka (Table S1). MaxEnt is underpinned on the maximum entropy principle [30]. It is a robust modeling technique, and at times, it outperforms other techniques in predicting the potential area of species using small sample sizes of presence-only data [31,32]. The study modeled 14 out of the 16 terrestrial plant species included in the National List of Invasive Plants of Sri Lanka prepared by the Government of Sri Lanka in 2015 (two species were disregarded due to an inadequacy of occurrence data). These IAPS have invaded many PAs in the country through various mechanisms, resulting in negative influences on biodiversity. The modeling study used 1460 geo-referenced and spatially-filtered (i.e., in order to correct sampling bias) species occurrence records that were obtained from published or gray literature.
The study used downscaled data from the fifth version of the atmosphere–ocean general circulation model (GCM), which is the Model for Interdisciplinary Research on Climate (MIROC) for future projections. The performance of this GCM is better in terms of climate change simulations, especially for South Asia [33,34]. The study used Worldclim 19 variables at a resolution of 30 arc seconds (approximately 1 km2) for current and future events, and RCPs (representative concentration pathways) 4.5 and 8.5 scenarios for 2050 and 2070 [35] (http://www.worldclim.org). The study undertaken only considered bioclimatic data, as most of the non-climate data are not available at the required resolution and spatial extent. Moreover, several studies have successfully used and proven the good performance of bioclimatic data in modeling invasive species [36,37]. Highly correlated variables were excluded with Pearson Correlation Coefficients (r) ≥ 0.7, using the ‘removeCollinearity’ function of the R Package “virtualspecies” (version 1.4–4) [38]. Thus, seven non-correlated variables were selected for model development (Table S2). Logistic output was selected to improve model calibration [39]. In addition, cross-validation was undertaken with 10 replicates and 1000 maximum iterations. MaxEnt feature classes; linear, quadratic, and hinge features, derived from continuous environmental variables, were selected to produce smooth models [39,40]. MaxEnt models built under the current climate scenario for IAPS were projected to future scenarios. Model performance was tested using two well-known measures—the area under the receiver operating characteristic curve (AUC) and the true skill statistic (TSS) [41,42] (Table S3). These measures are popularly used in predictive modeling literature to test model performance [43,44]. Maximum training sensitivity plus the specificity logistic threshold was used to classify potentially suitable areas (presence–absence) for species, as was recommended for presence-only models [45,46]. All 14 of the classified layers were combined to develop combined maps of climate suitability (i.e., “heat maps” of 14 IAPS). In this study, the combined maps developed under current and future climate scenarios were classified into five classes—very low (0 IAPS), low (1–2 IAPS), moderate (3–4 IAPS), high (5–6 IAPS), and very high (7–8 IAPS)—using manual classification. Further details that are relevant to model building and model results were provided by Kariyawasam et al. [29].
The climate suitability for IAPS is an important factor to understand the risks of biological invasions in a particular geographic area [47]. The main aim of this study was to examine the potential risks of multiple plant invasions in the PAs of Sri Lanka and to identify the potential risks to threatened vertebrates under climate change. The specific objectives of this study were to (i) assess the climatic suitability for multiple IAPS inside and outside PAs under climate change, (ii) identify individual IAPS that have the highest potential to spread further in PAs, and (iii) identify the richness of threatened vertebrates in PAs that can be potentially influenced by IAPS. We used multi-species climate suitability maps, based on a previous study by Kariyawasam et al. [29]. This is the first study in Sri Lanka that considers the potential risks of multiple invasive plants, as well as assesses the likely influences on threatened vertebrates under projected climate change.
2. Methods
2.1. Study Area
Located at the southern tip of the Indian sub-continent, between 5°54′–9°52′ N and 79°39′–81°53′ E, Sri Lanka is home to rich biodiversity, despite its small size of 65,610 km2 [48]. Sri Lanka, along with the Western Ghats of India, is considered to be one of the 25 global biodiversity hotspots, based on two critical criteria—high levels of endemicity and threat [20]. In Sri Lanka, 25.6% of the total land area is under the protection of PAs, which is central to conserving the country’s native biodiversity [48,49].
2.2. Risk Assessments of Multiple IAPS into Protected Areas
We used the combined climate suitability maps of 14 IAPS under current and future climate scenarios, RCP 4.5 and 8.5 for 2050, for the analysis. In the PAs we considered, ‘very high’ climate suitability implied potentially high risks from IAPS, whereas ‘very low’ climate suitability implied potentially very low risks from IAPS. In this study, terrestrial PAs, administered by the Department of Wildlife Conservation (hereafter wildlife PAs) and the Department of Forests (hereafter forest PAs), were considered. In ArcMap (Version 10.4.1), wildlife PAs and forest PAs were individually overlain with potential climate suitability maps to recognize the likelihood of multiple IAPS introductions. We calculated the area of suitability classes (i.e., very high to very low) inside PAs. The same procedure was applied to the areas outside the PA systems.
2.3. Risk Assessments of Individual IAPS into Protected Areas
We used individual maps of climate suitability for the 14 priority IAPS to assess their potential risks in PAs under climate change. In this analysis, both wildlife and forest PAs were considered together as Sri Lanka’s PAs. Using ArcMap, we calculated the area of suitability for each species in the PAs under current and future climate scenarios of RCP 4.5 and 8.5 for 2050 in order to identify the species that could potentially be challenging in PA systems.
2.4. Potential Risks of IAPS on Threatened Vertebrates
In order to understand the potential impacts of IAPS on threatened animals, we limited our analysis to threatened vertebrates, as detailed data on the threatened status of invertebrates at a PA level is mostly incomplete and not commonly available, particularly in developing tropical island countries. We only considered four vertebrate groups in our analysis, i.e., amphibians, reptiles, birds, and mammals. Freshwater fish were excluded because our climate suitability maps are based on terrestrial IAPS. We used 5528 occurrence records of threatened vertebrate species that were defined as ‘Critically Endangered’ by the national red list [50] and available at Global Biodiversity Information Facility [51] (accessed on September 21, 2019). These records represent three amphibian (n = 12), 16 reptile (n = 91), 11 bird (n = 5307), and 5 mammal (n = 15) species, respectively (Table S4). In ArcMap, species’ occurrences were overlain on climate suitability maps of the current climate and future scenarios of RCP 4.5 and 8.5 for 2050. Suitability changes in these occurrences were visually observed under climate change.
In order to identify the PAs that are climatically suitable for multiple IAPS invasions, we examined the combined climate suitability maps of 14 IAPS overlaid on the PAs of Sri Lanka. We reviewed the literature to broadly understand the species richness of vertebrates (i.e., amphibians, reptiles, birds, and mammals) in the PAs that could be potentially influenced by IAPS (Figure S1).
3. Results
3.1. Risk Assessments of Multiple IAPS into Protected Areas
Figure 1 represents the wildlife and forest PAs overlaid on defined climate suitability maps of current and future climate scenarios for 14 nationally prioritized IAPS. The maps show how PAs potentially undergo changes under future climate scenarios, i.e., RCP 4.5 and 8.5 for 2050. The calculated area of the suitability of five classes (i.e., very high to very low) in wildlife and forest PAs are represented in Figure 2 and Figure 3. In both PA types, the area that potentially supports the establishment of more than five IAPS (represented by very high and high suitability classes) was relatively low under current and future climate scenarios, while the area favorable to establish less than five IAPS (represented by moderate, low, and very low suitability classes) was comparatively high (Table 2). The area of suitability in PAs is predicted to vary noticeably in the future as a response to climate change. In the current climate, the relative contribution of the very low climate suitability was much greater (approximately 50%) than the other four suitability classes in both types of PAs; however, this area is predicted to decrease substantially (approximately 20%) under future climate conditions. In contrast, the relative contributions of ‘moderate’ and ‘low’ suitability classes are predicted to increase. As the contribution of ‘very high’ and ‘high classes are minimal, these two findings projected a decreasing pattern of ‘very low’ suitability and an increasing pattern of ‘moderate’ and ‘low’ suitability, which suggests the likely increasing and potentially negative influences of IAPS in PAs in Sri Lanka in the future under climate change. The findings also indicate a generally close pattern of suitable area changes in the five climate suitability classes in wildlife and forest PAs. The area outside the PAs is also predicted to have potentially increasing negative influences from IAPS under climate change. This is evident by the increased moderate suitability, which is approximately 18% in the current climate and is elevated to around 50% under future scenarios, and the very low suitability is drastically decreased from 36% in the current climate to around 10% in the future scenarios. However, in comparison, we have observed a difference in the suitability distribution pattern in the area outside the PAs, which perhaps implies that the priority invasive species are likely not to have the same effects outside PAs as they do in PAs (Figure 4). We observed comparatively high invasion risks outside PAs, as compared to the wildlife and forest PAs under all climate scenarios, due to the better representation of very high, high, and moderate classes, and the poor representation of low and very low classes (Table 2). Thus, our results suggest that the invasion risk in PAs is relatively less intensive as compared to the area outside PAs. However, one should keep in mind that the negative consequences of IAPS may not always be related to the IAPS numbers. Additionally, climate responses to invasive plants can be different under climate change [33]; therefore, we need to be cautious in using these results to make conservation decisions.
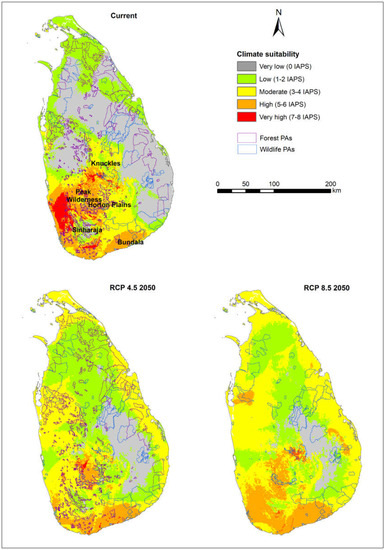
Figure 1.
Protected areas overlaid on projected climate suitability for 14 nationally prioritized invasive alien plant species in Sri Lanka under the current climate and Model for Interdisciplinary Research on Climate (MIROC5) representative concentration pathway (RCP) 4.5 and 8.5 for 2050.
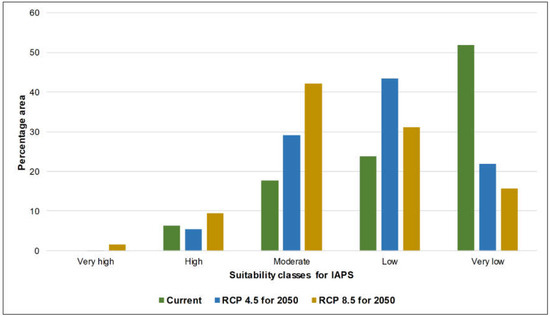
Figure 2.
Representation of five climate suitability classes in the wildlife protected areas of Sri Lanka under the current climate and MIROC5 RCP 4.5 and 8.5 for 2050.
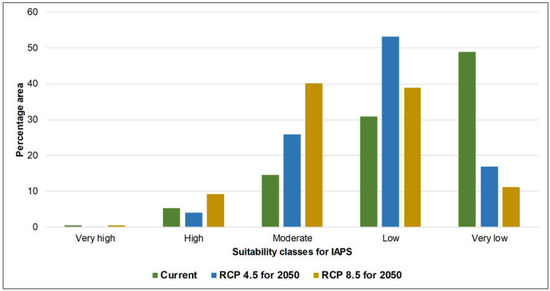
Figure 3.
Representation of five climate suitability classes in the forest protected areas of Sri Lanka under the current climate and MIROC5 RCP 4.5 and 8.5 for 2050.

Table 2.
Climate suitability classification for wildlife, forest protected areas, and outside protected areas under the current climate and MIROC5 RCP 4.5 and 8.5 for 2050.
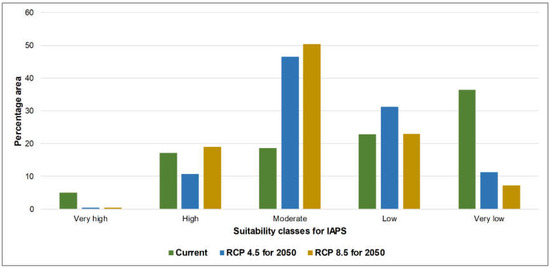
Figure 4.
Representation of five climate suitability classes outside protected areas of Sri Lanka under the current climate and MIROC5 RCP 4.5 and 8.5 for 2050.
3.2. Risk Assessments of Individual IAPS into Protected Areas
We investigated the projected area of suitability of 14 IAPS in the PA system under the current climate and future scenarios for 2050. Accordingly, Panicum maximum shows the largest potential distribution under the current climate, followed by Lantana camara and Leucaena leucocephala (Table 3). Opuntia dillenii, followed by Parthenium hysterophorus and Mimosa pigra, has the maximum potential suitable area under both future scenarios, RCP 4.5 and 8.5 for 2050. By 2050, six IAPS, namely A. macrophylla, A. glabra, D. suffruticosa, M. pigra, O. dillenii, and P. histerophorus are projected to increase in their climate suitability in the PA system, while the other eight IAPS, namely A. inulifolium, C. hirta, L. camara, L. leucocephala, P. maximum, P. juliflora, S. trilobata, and U. europaeus are projected to decrease.

Table 3.
Projected area of climate suitability of 14 individual invasive alien plant species (IAPS) in the protected area system under the current climate and MIROC5 RCP 4.5 and 8.5 for 2050.
3.3. Potential Risks of IAPS on Threatened Vertebrates
We examined the distribution of occurrences of critically endangered vertebrates, amphibian, reptile, bird, and mammal species on climate suitability maps under current and future climate scenarios. Almost all occurrences of mammals, amphibians, and reptiles were found around the Central Highlands and Sinharaja PAs (Figure 5), whereas the selected bird occurrences were mostly distributed around the mid-country and south-west wet zone (Figure 6). According to the figures, the occurrences of critically endangered vertebrates are concentrated in climatically suitable areas for IAPS under current and future climate scenarios. Further, we found that several PAs containing relatively high vertebrate diversity are located in climatically suitable areas for IAPS (Figure 1).
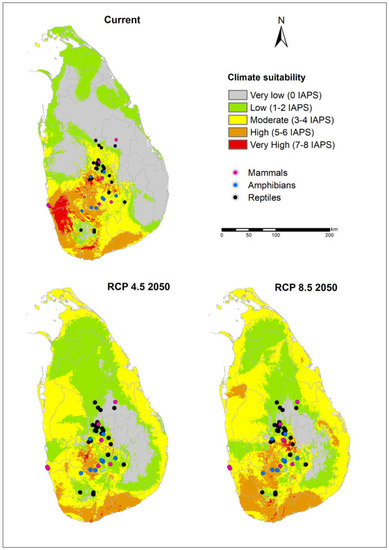
Figure 5.
Occurrences of critically endangered amphibians, reptiles, and mammals (available at GBIF on September 21, 2019) overlaid on climate suitability for invasive alien plant species under the current climate and MIROC5 RCP 4.5 and 8.5 for 2050.
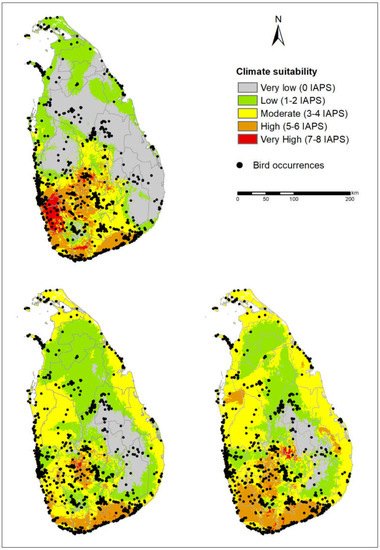
Figure 6.
Occurrences of critically endangered birds (available at GBIF on September 21, 2019) overlaid on climate suitability for invasive alien plant species establishment under the current climate and MIROC5 RCP 4.5 and 8.5 for 2050.
4. Discussion
Sri Lanka, being an island country located in the tropics, faces potential risks of IAPS invasion on native biodiversity, which can be challenging. In particular, south-west Sri Lanka is likely to face a high risk from multiple IAPS invasion under projected climate change [29]. Island species are generally highly susceptible to climate change-associated negative influences [4]. Thus, the potential impacts of IAPS on island biodiversity can be significant. This increased vulnerability can be due to the less competitive ability of island species, lack of predators, or high propagule pressure [52]. Island species have less of a chance to spread out and to avoid risk conditions, and this has aggravated the vulnerability of island species [4].
4.1. Risk Assessments of Multiple IAPS into Protected Areas
PAs located in the south-west Sri Lanka, Sinharaja, Knuckles, Horton Plains, and Peak Wilderness contain relatively high species diversity, especially vertebrate fauna [53,54]. Therefore, the projected increased pressure from IAPS in PAs in Sri Lanka may result in detrimental consequences to rich vertebrate diversity, particularly threatened vertebrates that are in need of protection.
According to our findings, the potentially suitable areas for IAPS are increasing in both PAs and outside PAs under climate change (Figure 2, Figure 3 and Figure 4, Table 2). Thus, this may suggest there to be a likely increased susceptibility of the areas to IAPS in the future. Though poorly understood, climate change has the capacity to change the distribution of invasive species in geographic areas, as many invasive species have broad climatic tolerances [14]. Additionally, the frequency of extreme climatic events (i.e., floods and droughts) will likely increase under climate change, and this may influence the increased invasion through several mechanisms, such as transporting propagules and reducing the resistance of native species to invasions [14,55]. The results also suggested that the intensity of invasion risk is lower in PAs as compared to outside PAs. Gallardo et al. [56] reported that the richness of invasive species is expected to increase comparatively less within protected areas than outside them. The authors suggest that this could turn such protected areas into vital and viable refuges against invasion. Further, invasive species establishment is relatively difficult in the well-conserved PAs located in pristine environments where human impacts are less and management is rigorous (i.e., Sinharaja forest) [56]. Potential invaders easily fill the gaps in disturbed environments, which is common outside PAs [57,58]. Therefore, the richness of invasive species can differ significantly inside and outside PAs, suggesting that the potential impacts may not be the same [56]. However, more studies are needed to ascertain this conception.
4.2. Risk Assessments of Individual IAPS into Protected Areas
The analysis of the potentially suitable climate of individual IAPS in the PA system reveals that the high-risk species (in terms of suitable area) in the current climate are not the high-risk species in the future. The three most potentially high-risk species in the current climate (Panicum maximum, Lantana camara, and Leucaena leucocephala) are projected to decrease in overall suitability in the PA system under climate change. Generally, different species of terrestrial plants can have different range size changes [59]. Out of the 14 evaluated IAPS, six species are projected to expand the range in the PA system under climate change, while the other eight species are projected to decline. Invasive species are range-shifting species that generally do not acquire the entire niche of the species; this issue may potentially influence the performance of predictive models and could interfere with conservation decisions. However, the evaluated IAPS were introduced to Sri Lanka a long time ago, and thus, they are likely to have spread to all suitable habitats in the country by now. Further, our findings suggest that the potential area changes of range-expanding IAPS were much higher than the range contracting IAPS (Table 3). Shrestha and Shrestha [47] have already demonstrated that the niches of range-expanding species are larger than the range-contracting species. Thus, our findings corroborate these results. The potential risks of these IAPS on native biodiversity in PAs are different, complex, and poorly studied. However, the available literature confirms that the potential negative consequences of these 14 IAPS can lead to pronounced changes in biodiversity and ecosystems in PAs (Table S1).
4.3. Potential Risks of IAPS on Threatened Vertebrates
The potential impacts of IAPS on vertebrate fauna are not comprehensively studied; however, this rich vertebrate diversity can be potentially at risk due to plant invasions, as the direct and indirect impacts of biological invasions are significant to global terrestrial biodiversity [11,60,61]. Generally, native fauna, particularly threatened endemics, are more susceptible to habitat changes [53]. Studies have already highlighted the high native species richness around the mid-country and south-west Sri Lanka, particularly for some vertebrate groups [54]. Many such threatened endemic species have restricted ranges or localized habitats, e.g., Cophotis ceylanica (EN) and Ceratophora tennenti (CR) [48]; thus, such areas are important refuge areas for the conservation of reptiles. Our models suggested that the climate in the mid-country and south-west Sri Lanka is more vulnerable to IAPS invasion, as compared to the other areas of the country under current and projected climate change. This may potentially influence threatened vertebrate fauna in this area through direct and indirect means. Thus, the likely negative influences should be considered in future conservation management programs, with particular attention paid to these areas. Plant invaders can change the characteristics of the entire ecosystem, such as the productivity, nutrient cycling, and disturbance regimes, leading to drastic changes in ecosystem structures and functioning [62]. The deterioration of habitat quality may result in negative effects for the long-term survival of vertebrate fauna, which are unique and have adapted well to the ecology of their habitat [63]. In Udawalawe national park, L. camara has created vigorous and persistent alterations in the grassland vegetation, converting it to scrublands [64]. Invasive species may impact native fauna, particularly mammals, by destroying habitats and restricting access to food resources, i.e., limiting foraging grounds or fodder contents [65]. Habitat deterioration and loss are serious concerns for the conservation of herpetofauna in Sri Lanka, as they are mostly restricted to specialized niches in the ecosystem [66]. Further, this can have severe consequences, particularly for herbivores such as elephants (Elephas maximus, EN), as elephants never feed on L. camara [67]. Elephants prefer to graze in open grassland habitats that are easily acquired by invasive plants, such as L. camara, resulting in devastating effects on habitat quality, which in turn influences the viable populations of large herbivores [68,69]. When the habitat quality degrades and food resources become limited, large mammals come out of the reserves closer to human settlements in search of food, leading to devastating human–wildlife conflict [63]. Currently, human–elephant conflict has become a serious environmental problem in Sri Lanka. This likely disturbs the first trophic level (primary producers) of the food chain, which can affect the energy flow and collapse higher trophic levels, herbivores, and then carnivores at the end [70]. Though these negative effects are mostly on native plants, such consequences can indirectly increase pressure (i.e., reduce food and foraging habitats, interfere with behavior and habitat use) on the continued existence of native fauna. However, how the consequences of climate change mediate biological invasions and influence threatened vertebrates in PAs is poorly studied. Such quantitative data are not available in developing countries, but they are relevant to many animal groups. A study by Schirmel et al. [71] reported that invasive plants reduce the abundance, diversity, and fitness of native animals. Many scientific studies confirm that biological invasions threaten native vertebrate species on Earth, particularly on island countries [16,72,73]. However, the mechanism of impact, overall extent, and magnitude are poorly understood, and this is mainly due to several research gaps. Many native fauna, particularly large mammals, do not have enough strategies (i.e., range shift or adjust to new climate conditions) to face the potentially harmful effects associated with climate-mediated changes [74]. Another study showed that amphibians and reptiles in South Asia have poor adaptation capacity, which is relatively slower than the anticipated level [75]. Therefore, the potential consequences of biological invasions, as drivers of global change, can be important to threatened vertebrates, but remain understudied.
4.4. Vertebrate-Rich Protected Areas Vulnerable to Multiple IAPS Invasion
This study found that several vertebrate-rich PAs are located in climatically suitable areas for multiple IAPS invasions, and thus, such PAs can be potentially influenced by IAPS. Sinharaja forest (UNESCO Man and Biosphere Reserve, World Heritage Site, Strict Nature Reserve, National Heritage Wilderness Area), which is the only remaining, intact lowland rain forest in the country, contains rich faunal diversity [48,76]. Sinharaja has about 359 species of vertebrates, including 52 amphibians, 95 species of terrestrial reptiles, 125 bird species, and 41 species of mammals, with exceptionally high endemism [77]. As cited by Surasinghe [54], Sinharaja is rich in herpetofauna, i.e., out of the amphibians and reptiles fauna, 70% and 50% are endemic to the country, respectively. Another study revealed that approximately 42% of small mammals and 32% of bird species found in this forest are endemic [53]. The Central Highlands (UNESCO World Heritage Site), which comprises three highly important reserves—Knuckles Conservation Forest (KCF), the Peak Wilderness PA (PWPA), and the Horton Plains National Park (HPNP)—provides important habitats to 48% of the country’s endemic vertebrate species, including some point endemics that are found in no other place in the world [77]. The faunal biodiversity of HPNP shows high species richness with outstanding threatened and endemic species, which is particularly relevant to herpetofauna [78]. It is a key habitat for the endemic Sri Lanka Purple-faced langur (Semnopithecus vetulus; EN) and the Sri Lankan red slender loris (Loris tardigradus; VU). Endemic lizard species, including Ceratophora tennentii (Agamidae; CR), are restricted to the upper altitudes of KCF [66]. As a refuge for a number of rare endemic amphibians, such as Adenomus dasi (Bufonidae; CR), PWPA is considered to be the most important amphibian hotspot in Sri Lanka, and it is currently at a high threat due to habitat degradation [79]. Hence, this high level of vertebrate diversity can be influenced by the potential expansion of IAPS. Located in Southern Sri Lanka, Bundala National Park (International Ramsar Site; UNESCO Biosphere Reserve) is home to a variety of species and is at a high risk of multiple IAPS invasions under climate change. Forty-five percent of migratory birds coming to Sri Lanka have been reported in Bundala [80]. Thus, the degradation of many of the habitats by the fast-spreading IAPS (i.e., P. juliflora and O. dillenii) may result in negative influences, particularly on avifauna diversity. Table S5 provides the vertebrate species richness recorded by the biodiversity baseline surveys in six of the wildlife PAs in Sri Lanka, which are relevant to the vertebrate groups we studied. Such information is not available for many PAs, as comprehensive and systematic biodiversity assessments have not been undertaken across PAs in the country.
4.5. Limitations of the Study
Even though species distribution models are frequently used and vital tools, there are some common limitations, and due to the level of uncertainty associated with modeling techniques, the general circulation models (GCMs) and representative concentration pathways (RCPs) used may result in serious implications for model performance [7,14,81]. We have discussed the general limitations of MaxEnt models and the approaches used to overcome them by Kariyawasam et al. [29], with particular attention paid to the uncertainty associated with the model building, spatial scales, and selection of data. Thus, some limitations that are relevant to the present study are discussed here. MaxEnt uses presence–background data as the input for modeling species distribution and provides estimates of the relative suitability [82]. Therefore, we did not model the probability of the occurrence of the target species, but rather an index of their habitat suitability, due to the lack of absence data (or species non-detection). Invasive species are generally highly adaptable to a wide range of climatic variability, and thus, they can tolerate environmental changes. As a result, these plants are likely to persist, even if climatic conditions become unfavorable [83]. In addition, climate change models may be conservative for invasive plant species, and thus, they can potentially underestimate the distribution in novel climates where temperature and precipitation can be rather different from the current climate [84]. Thus, modeling species distribution in a novel climate can be challenging, and such results need to be interpreted with caution. Conversely, invasive plants may not occupy all potentially suitable areas (niches), as invasion may be obstructed by several fundamental factors, such as the absence of vectors, physical barriers, biotic interactions (i.e., competition, predation), and accessibility for resources [85,86,87]. Additionally, the vectors of the spread of these 14 IAPS and their relative importance can be different. Different IAPS can perhaps have different vectors of spread, and frequently, some IAPS have several vectors of spread [88]. IAPS having several vectors can acquire an extensive area of suitability [89]. Therefore, individual IAPS may not have the potential to invade all likely suitable areas in the protected areas and fill their climatic niche in the same way. Pyšek et al. [90] show that the post-invasion success (i.e., distribution and habitat range) of alien plants depends on their pathways of introduction. The potential pathways are strongly influenced by climate change and may result in differences in the invasion dynamics of IAPS [91]. Additionally, modeling studies underpin the uncertainty that is relevant to the responses of invasive species to the climate changes [92], the consequences of climate change on native species [57], the resistance and resilience capacity of native species toward invasion [93], and the impacts of invasive species on native species [57]. In view of that, the potential geographic distribution of IAPS under climate change is a rather complex process that can be influenced by several factors; however, it is an established and widely used technique in many conservation science applications today [94].
4.6. Recommendations for Future Studies
Predictive modeling provides useful information for conservation planning and management. Our results suggest that vertebrates in PAs may experience potentially increased pressure from IAPS under climate change, although PAs may serve as refugees for animals. Vertebrates are of great importance in the structure and functioning of ecosystems [95,96]. Potential harmful consequences of IAPS to vertebrate fauna can vary from destroying habitats and restricting access to food resources [65] and to the most extreme challenge of species extinctions [16]. These impacts on vertebrate fauna may result in a number of important implications for their conservation in the future. This highlights the requirements of climate change adaptation strategies and focused research that can minimize the vulnerability of these species to climate change [97,98]. Given the importance of IAPS as an agent of global environmental change, the role they play in degrading the habitat quality of native vertebrates in PAs remains poorly understood. Such findings provide insights for the early detection of risks and for the application of successful habitat enrichment programs. Empirical studies that focus on how IAPS could alter the behavior pattern and population dynamics of large mammals are important to broaden our understanding [67,99]. Studying the impacts of toxic IAPS is also important, i.e., animal poisoning, as there is a significant knowledge gap that is relevant to this subject. Such studies provide the basis for conservation decision-making, which is relevant to IAPS management in PAs [100]. Further, systematic biodiversity surveys are important in unsurveyed PAs, especially for the least studied taxonomic groups. The ecosystem approach helps to conserve the whole landscape, ensuring the protection of many more living beings [26]. Thus, the strategic management of IAPS within the context of ecosystems is recommended.
5. Conclusions
We assessed the potential risks of multiple IAPS within the context of PAs and outside PAs in the tropical island country of Sri Lanka under the projected climate. Our findings provide quantitative evidence of the likely increasing risks of IAPS invasion in PAs and outside PAs under climate change; however, invasion risk is expected to increase comparatively less within PAs than outside them. We defined the species that can potentially pose a high risk to the PA system under current and future climate scenarios. The increased pressure from plant invasions may result in negative consequences, e.g., threats to vertebrate–fauna, which are susceptible to changes in their habitat. The majority of threatened vertebrates are concentrated in the central and south-west wet zone of the country, which is relatively more vulnerable to multiple plant invaders. Thus, this can have serious potential consequences for the survival of threatened vertebrates. Therefore, this study highlights the need for more rigorous management and conservation efforts that focus on the montane and wet zone PAs in order to safeguard threatened vertebrates from the likely and impending harmful effects of plant invasions.
Supplementary Materials
The following are available online at https://www.mdpi.com/2225-1154/8/4/51/s1, Table S1: Fourteen priority IAPS used for MaxEnt model run, Table S2: The environmental variables used in the MaxEnt models, Table S3: Model performances of 14 priority IAPS in Sri Lanka, Table S4: List of ‘Critically Endangered’ amphibian, reptile, bird and mammal species for which occurrences were downloaded from GBIF, Figure S1: Wildlife and forest protected areas considered by the study, Table S5: Number of vertebrate species recorded in six wildlife protected areas with endemicity and national conservation status.
Author Contributions
Conceptualization, C.S.K., L.K. and S.S.R.; Methodology, C.S.K., L.K. and S.S.R.; Validation, C.S.K., L.K. and S.S.R.; Formal Analysis, C.S.K., L.K. and S.S.R.; Investigation, C.S.K., L.K. and S.S.R.; Writing—Original Draft Preparation C.S.K. and S.S.R.; Writing—Review and Editing, C.S.K., L.K. and S.S.R.: supervision, L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Catherine Macgregor of the University of New England is acknowledged for her valuable assistance throughout the study. The authors also acknowledge the reviewers of the Climate journal for their valuable comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rands, M.R.; Adams, W.M.; Bennun, L.; Butchart, S.H.; Clements, A.; Coomes, D.; Entwistle, A.; Hodge, I.; Kapos, V.; Scharlemann, J.P. Biodiversity conservation: Challenges beyond 2010. Science 2010, 329, 1298–1303. [Google Scholar] [CrossRef]
- Secretariat of the Convention on Biological Diversity. Global Biodiversity Outlook 4; Secretariat of the Convention on Biological Diversity: Montréal, QC, Canada, 2014; p. 155.
- IPCC. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; p. 582. [Google Scholar]
- Kumar, L.; Tehrany, M.S. Climate change impacts on the threatened terrestrial vertebrates of the Pacific Islands. Sci. Rep. 2017, 7, 5030. [Google Scholar] [CrossRef] [PubMed]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, S.S. Invasive Plants Distribution Modeling: A Tool for Tropical Biodiversity Conservation with Special Reference to Sri Lanka. Trop. Conserv. Sci. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Achard, F.; Eva, H.D.; Stibig, H.-J.; Mayaux, P.; Gallego, J.; Richards, T.; Malingreau, J.-P. Determination of deforestation rates of the world’s humid tropical forests. Science 2002, 297, 999–1002. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Paini, D.R.; Sheppard, A.W.; Cook, D.C.; De Barro, P.J.; Worner, S.P.; Thomas, M.B. Global threat to agriculture from invasive species. Proc. Natl. Acad. Sci. USA 2016, 113, 7575–7579. [Google Scholar] [CrossRef]
- Leadley, P.; Pereira, H.M.; Alkemade, R.; Fernandez-Manjarrés, J.F.; Proença, V.; Scharlemann, J.P.W.; Walpole, M.J. Biodiversity Scenarios: Projections of 21st Century Change in Biodiversity and Associated Ecosystem Services; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2010; p. 132.
- Fandohan, A.B.; Oduor, A.M.; Sodé, A.I.; Wu, L.; Cuni-Sanchez, A.; Assédé, E.; Gouwakinnou, G.N. Modeling vulnerability of protected areas to invasion by Chromolaena odorata under current and future climates. Ecosyst. Health Sustain. 2015, 1, 1–12. [Google Scholar] [CrossRef]
- Taylor, S.; Kumar, L. Potential distribution of an invasive species under climate change scenarios using CLIMEX and soil drainage: A case study of Lantana camara L. in Queensland, Australia. J. Environ. Manag. 2013, 114, 414–422. [Google Scholar] [CrossRef]
- Bellard, C.; Jeschke, J.M.; Leroy, B.; Mace, G.M. Insights from modeling studies on how climate change affects invasive alien species geography. Ecol. Evol. 2018, 8, 5688–5700. [Google Scholar] [CrossRef]
- McNeely, J.A. Strangers in our midst: The problem of invasive alien species. Environment 2004, 46, 16. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef] [PubMed]
- Foxcroft, L.C.; Pyšek, P.; Richardson, D.M.; Pergl, J.; Hulme, P.E. The bottom line: Impacts of alien plant invasions in protected areas. In Plant Invasions in Protected Areas; Springer: Dordrecht, The Netherlands, 2013; pp. 19–41. [Google Scholar]
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Dudley, N. Guidelines for Applying Protected Area Management Categories; IUCN: Gland, Switzerland, 2008. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853. [Google Scholar] [CrossRef]
- UNEP-WCMC IUCN and NGS. Protected Planet Report 2018. 2018. Available online: https://livereport.protectedplanet.net/pdf/Protected_Planet_Report_2018.pdf (accessed on 10 August 2019).
- Pauchard, A.; Alaback, P.B. Influence of elevation, land use, and landscape context on patterns of alien plant invasions along roadsides in protected areas of South-Central Chile. Conserv. Biol. 2004, 18, 238–248. [Google Scholar] [CrossRef]
- Mack, R.N.; Simberloff, D.; Mark Lonsdale, W.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Foxcroft, L.C.; Pyšek, P.; Richardson, D.M.; Genovesi, P. Plant Invasions in Protected Areas: Patterns, Problems and Challenges; Springer: Berlin, Germany, 2013; Volume 7. [Google Scholar]
- Wilson, K.A.; Underwood, E.C.; Morrison, S.A.; Klausmeyer, K.R.; Murdoch, W.W.; Reyers, B.; Wardell-Johnson, G.; Marquet, P.A.; Rundel, P.W.; McBride, M.F. Conserving biodiversity efficiently: What to do, where, and when. PLoS Biol. 2007, 5, e223. [Google Scholar] [CrossRef]
- Franklin, J.F. Preserving biodiversity: Species, ecosystems, or landscapes? Ecol. Appl. 1993, 3, 202–205. [Google Scholar] [CrossRef]
- Weerakoon, D. Analysis of Faunal Groups. In The National Red List 2012 of Sri Lanka: Conservation Status of the Fauna and Flora; Weerakoon, D.K., Wijesundara, S., Eds.; Ministry of Environment: Colombo, Sri Lanka, 2012; pp. 145–147. [Google Scholar]
- Cumberlidge, N.; Ng, P.K.; Yeo, D.C.; Magalhães, C.; Campos, M.R.; Alvarez, F.; Naruse, T.; Daniels, S.R.; Esser, L.J.; Attipoe, F.Y. Freshwater crabs and the biodiversity crisis: Importance, threats, status, and conservation challenges. Biol. Conserv. 2009, 142, 1665–1673. [Google Scholar] [CrossRef]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, R.S.S. Invasive Plant Species Establishment and Range Dynamics in Sri Lanka under Climate Change. Entropy 2019, 21, 571. [Google Scholar] [CrossRef]
- Phillips, S.; Anderson, R.P.; Schapire, R.E. Maximum entropy modelling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Thibaud, E.; Petitpierre, B.; Broennimann, O.; Davison, A.C.; Guisan, A. Measuring the relative effect of factors affecting species distribution model predictions. Methods Ecol. Evol. 2014, 5, 947–955. [Google Scholar] [CrossRef]
- Lamsal, P.; Kumar, L.; Aryal, A.; Atreya, K. Invasive alien plant species dynamics in the Himalayan region under climate change. Ambio 2018, 47, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Suzuki, T.; O’ishi, R.; Komuro, Y.; Watanabe, S.; Emori, S.; Takemura, T.; Chikira, M.; Ogura, T.; Sekiguchi, M. Improved climate simulation by MIROC5: Mean states, variability, and climate sensitivity. J. Clim. 2010, 23, 6312–6335. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; PYŠEK, P.; Midgley, G.F.; Hughes, G.O.; Rouget, M. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob. Chang. Biol. 2005, 11, 2234–2250. [Google Scholar] [CrossRef]
- Adhikari, D.; Tiwary, R.; Barik, S.K. Modelling Hotspots for Invasive Alien Plants in India. PLoS ONE 2015, 10, e0134665. [Google Scholar] [CrossRef]
- Leroy, B.; Delsol, R.; Hugueny, B.; Meynard, C.N.; Barhoumi, C.; Barbet-Massin, M.; Bellard, C. Without quality presence–absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 2018, 45, 1994–2002. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Shabani, F.; Ahmadi, M.; Peters, K.J.; Haberle, S.; Champreux, A.; Saltré, F.; Bradshaw, C.J. Climate-driven shifts in the distribution of koala-browse species from the Last Interglacial to the near future. Ecography 2019, 42, 1587–1599. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A comparison of absolute performance of different correlative and mechanistic species distribution models in an independent area. Ecol. Evol. 2016, 6, 5973–5986. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Shrestha, B.B. Climate change amplifies plant invasion hotspots in Nepal. Divers. Distrib. 2019, 25, 1599–1612. [Google Scholar] [CrossRef]
- MoFE. Biodiversity Conservation in Sri Lanka: A Framework for Action; Ministry of Forestry and Environment: Battaramulla, Sri Lanka, 1999.
- Gunawardene, N.R.; Daniels, D.A.; Gunatilleke, I.; Gunatilleke, C.; Karunakaran, P.; Nayak, G.K.; Prasad, S.; Puyravaud, P.; Ramesh, B.; Subramanian, K. A brief overview of the Western Ghats–Sri Lanka biodiversity hotspot. Curr. Sci. 2007, 93, 1567–1572. [Google Scholar]
- MOE. The National Red List 2012 of Sri Lanka; Conservation Status of the Fauna and Flora; Ministry of Environment: Colombo, Sri Lanka, 2012; p. 476.
- GBIF. GBIF Home Page. Available online: https://www.gbif.org (accessed on 21 September 2019).
- Allison, S.D.; Vitousek, P.M. Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 2004, 141, 612–619. [Google Scholar] [CrossRef]
- Wijesinghe, M.R.; Brooke, M.d.L. Impact of habitat disturbance on the distribution of endemic species of small mammals and birds in a tropical rain forest in Sri Lanka. J. Trop. Ecol. 2005, 21, 661–668. [Google Scholar] [CrossRef]
- Surasinghe, T.D. Conservation overview of herpetofauna of Sinharaja man and biosphere reserve of Sri Lanka. Zoos’Print J. 2007, 22, 2535–2538. [Google Scholar] [CrossRef]
- Diez, J.M.; D’Antonio, C.M.; Dukes, J.S.; Grosholz, E.D.; Olden, J.D.; Sorte, C.J.; Blumenthal, D.M.; Bradley, B.A.; Early, R.; Ibáñez, I. Will extreme climatic events facilitate biological invasions? Front. Ecol. Environ. 2012, 10, 249–257. [Google Scholar] [CrossRef]
- Gallardo, B.; Aldridge, D.C.; González-Moreno, P.; Pergl, J.; Pizarro, M.; Pyšek, P.; Thuiller, W.; Yesson, C.; Vilà, M. Protected areas offer refuge from invasive species spreading under climate change. Glob. Chang. Biol. 2017, 23, 5331–5343. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Hodgson, J.G.; Rich, T.C. Native and alien invasive plants: More of the same? Ecography 1995, 18, 390–402. [Google Scholar] [CrossRef]
- Daehler, C.C. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 183–211. [Google Scholar] [CrossRef]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Chang. Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef]
- Waser, A.M.; Splinter, W.; Van der Meer, J. Indirect effects of invasive species affecting the population structure of an ecosystem engineer. Ecosphere 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Taylor, S.; Kumar, L. Global climate change impacts on pacific islands terrestrial biodiversity: A review. Trop. Conserv. Sci. 2016, 9, 203–223. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Walker, L.R. Biological invasion by Myrica faya in Hawaii: Plant demography, nitrogen fixation, ecosystem effects. Ecol. Monogr. 1989, 59, 247–265. [Google Scholar] [CrossRef]
- Buckingham, L. Threats to wild cats across international borders. Biodiversity 2019, 20, 41–42. [Google Scholar] [CrossRef]
- De Silva, S.; Webber, C.E.; Weerathunga, U.; Pushpakumara, T.; Weerakoon, D.K.; Wittemyer, G. Demographic variables for wild Asian elephants using longitudinal observations. PLoS ONE 2013, 8, e82788. [Google Scholar] [CrossRef] [PubMed]
- Bachen, D.A.; Litt, A.R.; Gower, C.N. Simulating cheatgrass (Bromus tectorum) invasion decreases access to food resources for small mammals in sagebrush steppe. Biol. Invasions 2018, 20, 2301–2311. [Google Scholar] [CrossRef]
- Bahir, M.M.; Surasinghe, T.D. A conservation assessment of the Sri Lankan Agamidae (Reptilia: Sauria). Raffles Bull. Zool. 2005, 12, 407–412. [Google Scholar]
- Wilson, G.; Gruber, M.A.; Lester, P.J. Foraging relationships between elephants and Lantana camara invasion in Mudumalai Tiger Reserve, India. Biotropica 2014, 46, 194–201. [Google Scholar] [CrossRef]
- Sampson, C.; Leimgruber, P.; Tonkyn, D.; Pastorini, J.; Janaka, H.; Sotherden, E.; Fernando, P. Effects of illegal grazing and invasive Lantana camara on Asian elephant habitat use. Biol. Conserv. 2018, 220, 50–59. [Google Scholar] [CrossRef]
- Regmi, S.; Chalise, M.K. Food Habit and Conservation Threats of Wild Water Buffalo. Nature Khabar. 2019. Available online: http://naturekhabar.com/en/archives/12250 (accessed on 28 August 2019).
- Ullah, H.; Nagelkerken, I.; Goldenberg, S.U.; Fordham, D.A. Climate change could drive marine food web collapse through altered trophic flows and cyanobacterial proliferation. PLoS Biol. 2018, 16, e2003446. [Google Scholar] [CrossRef] [PubMed]
- Schirmel, J.; Bundschuh, M.; Entling, M.H.; Kowarik, I.; Buchholz, S. Impacts of invasive plants on resident animals across ecosystems, taxa, and feeding types: A global assessment. Glob. Chang. Biol. 2016, 22, 594–603. [Google Scholar] [CrossRef]
- Spatz, D.R.; Zilliacus, K.M.; Holmes, N.D.; Butchart, S.H.; Genovesi, P.; Ceballos, G.; Tershy, B.R.; Croll, D.A. Globally threatened vertebrates on islands with invasive species. Sci. Adv. 2017, 3, e1603080. [Google Scholar] [CrossRef]
- Bellard, C.; Rysman, J.-F.; Leroy, B.; Claud, C.; Mace, G.M. A global picture of biological invasion threat on islands. Nat. Ecol. Evol. 2017, 1, 1862–1869. [Google Scholar] [CrossRef]
- Hetem, R.S.; Fuller, A.; Maloney, S.K.; Mitchell, D. Responses of large mammals to climate change. Temperature 2014, 1, 115–127. [Google Scholar] [CrossRef]
- Bickford, D.; Howard, S.D.; Ng, D.J.; Sheridan, J.A. Impacts of climate change on the amphibians and reptiles of Southeast Asia. Biodivers. Conserv. 2010, 19, 1043–1062. [Google Scholar] [CrossRef]
- Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N.; Ethugala, A.U.K.; Esufali, S. Ecology of Sinharaja Rain Forest and the Forest Dynamics Plot in Sri Lanka’s Natural World Heritage Site; WHT Publications: Colombo, Sri Lanka, 2004. [Google Scholar]
- ME&RE. Sri Lanka’s Fifth National Report to the Convention on Biological Diversity; Ministry of Environment & Renewable Energy: Colombo, Sri Lanka, 2014; p. 128.
- DWC. Biodiversity Baseline Survey: Horton Plains National Park; Sri Lanka Protected Areas Management and Wildlife Conservation Project (PAM&WCP/CONSULT/02/BDBS); Department of Wildlife Conservation: Colombo, Sri Lanka, 2007; p. 40.
- Karunarathna, D.M.S.S.; Peabotuwage, P.I.K.; Perera, B.N.H.; Karunatilaka, H.M.A. Second known locality of the Critically Endangered Adenomus dasi Manamendra-Arachchi & Pethiyagoda, 1998 (Bufonidae) from Samanala Nature Reserve, Sri Lanka. Frog Leg 2012, 18, 21. [Google Scholar]
- DWC. Biodiversity Baseline Survey: Bundala National Park; Sri Lanka Protected Areas Management and Wildlife Conservation Project (PAM&WCP/CONSULT/02/BDBS); Department of Wildlife Conservation, Ministry of Environment and Natural Resources: Colombo, Sri Lanka, 2008; p. 46.
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [PubMed]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J. Maxent is not a presence–absence method: A comment on Thibaud et al. Methods Ecol. Evol. 2014, 5, 1192–1197. [Google Scholar] [CrossRef]
- Masters, G.; Norgrove, L. Climate Change and Invasive Alien Species; CABI Working Paper 1; CABI: Wallingford, UK, 2010; p. 30. [Google Scholar]
- Webber, B.L.; Yates, C.J.; Le Maitre, D.C.; Scott, J.K.; Kriticos, D.J.; Ota, N.; McNeill, A.; Le Roux, J.J.; Midgley, G.F. Modelling horses for novel climate courses: Insights from projecting potential distributions of native and alien Australian acacias with correlative and mechanistic models. Divers. Distrib. 2011, 17, 978–1000. [Google Scholar] [CrossRef]
- Gallardo, B.; Aldridge, D.C.; Frid, C. The ‘dirty dozen’: Socio-economic factors amplify the invasion potential of 12 high-risk aquatic invasive species in Great Britain and Ireland. J. Appl. Ecol. 2013, 50, 757–766. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Peterson, A.T.; Soberón, J.; Overton, J.; Aragón, P.; Lobo, J.M. Use of niche models in invasive species risk assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef]
- Brancatelli, G.I.; Zalba, S.M. Vector analysis: A tool for preventing the introduction of invasive alien species into protected areas. Nat. Conserv. 2018, 24, 43. [Google Scholar] [CrossRef]
- Foxcroft, L.C.; Spear, D.; Van Wilgen, N.J.; McGeoch, M.A. Assessing the association between pathways of alien plant invaders and their impacts in protected areas. NeoBiota 2019, 43, 1. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Pergl, J. Alien plants introduced by different pathways differ in invasion success: Unintentional introductions as a threat to natural areas. PLoS ONE 2011, 6, e24890. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Wan, J.-Z.; Qu, H.; Zhang, Z.-X. Modelling plant invasion pathways in protected areas under climate change: Implication for invasion management. Web Ecol. 2017, 17, 69–77. [Google Scholar] [CrossRef]
- Bradley, B.A.; Blumenthal, D.M.; Wilcove, D.S.; Ziska, L.H. Predicting plant invasions in an era of global change. Trends Ecol. Evol. 2010, 25, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Standish, R.J.; Robertson, A.W.; Williams, P.A. The impact of an invasive weed Tradescantia fluminensis on native forest regeneration. J. Appl. Ecol. 2001, 38, 1253–1263. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J. Predicting species distributions from museum and herbarium records using multiresponse models fitted with multivariate adaptive regression splines. Divers. Distrib. 2007, 13, 265–275. [Google Scholar] [CrossRef]
- Sekercioglu, C.H. Increasing awareness of avian ecological function. Trends Ecol. Evol. 2006, 21, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Stoner, K.E.; Riba-Hernández, P.; Vulinec, K.; Lambert, J.E. The role of mammals in creating and modifying seedshadows in tropical forests and some possible consequences of their elimination. Biotropica 2007, 39, 316–327. [Google Scholar] [CrossRef]
- Ratnayake, R.S.S.; Kumar, L.; Kariyawasam, C.S. Neglected and Underutilized Fruit Species in Sri Lanka: Prioritisation and Understanding the Potential Distribution under Climate Change. Agronomy 2020, 10, 34. [Google Scholar] [CrossRef]
- Kogo, B.K.; Kumar, L.; Koech, R.; Kariyawasam, C.S. Modelling climate suitability for rainfed Maize cultivation in Kenya using a Maximum Entropy (MaxENT) approach. Agronomy 2019, 9, 727. [Google Scholar] [CrossRef]
- Lavoie, C. The impact of invasive knotweed species (Reynoutria spp.) on the environment: Review and research perspectives. Biol. Invasions 2017, 19, 2319–2337. [Google Scholar] [CrossRef]
- Hulme, P.E.; Pyšek, P.; Pergl, J.; Jarošík, V.; Schaffner, U.; Vilà, M. Greater focus needed on alien plant impacts in protected areas. Conserv. Lett. 2014, 7, 459–466. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).