The Health Effects of Climate Change in the WHO European Region
Abstract
:1. Introduction
2. Methods
3. Results
- high temperature and heat,
- low temperature and cold,
- floods,
- wildfires, and
- UV radiation.
- Climate sensitive vector-borne infectious diseases (mosquito-, tick- and rodent-borne),
- Food- and water-related health effects;
- those related to air quality, and
- allergic diseases.

| Topic | Identified Studies | |
|---|---|---|
| Direct health effects (48) | High temperature and heat-wave | 23 |
| Low temperature and cold | 10 | |
| Floods | 5 | |
| Wildfires | 6 | |
| Ultraviolet radiation | 4 | |
| Indirect health effects (87) | Vector- (mosquito, tick) and rodent-borne infectious diseases | 37 |
| Food- and water-related diseases | 7 | |
| Air quality | 26 | |
| Allergic diseases | 17 | |
| Total: 135 | ||
3.1. Direct Health Effects
3.1.1. High Temperature and Heatwaves
| Health Effect | Related to Extreme Heat | Related to Higher Temperature |
|---|---|---|
| Observed mortality | Extreme temperatures are associated with increases in mortality [15,16,17,22,32] | Mortality increases are associated with temperatures above a local comfort threshold [13,18] |
| Observed morbidity | Extreme temperature is associated with increases in morbidity [20,23,26] | Morbidity indicators are associated with higher temperatures [24,25,33] |
| Future projection | Future expected extreme temperatures are expected to increase heat-related mortality [14] | Future increases in temperature are estimated to increase health effects of temperatures above a comfort temperature [28,29,31,34] |
| Projected effects of heatwaves and high temperature have economic costs [27,30] | ||
| Other or multiple focus | There is confounding by air pollution for heat and cold effects [35] | |
| Heat and cold, mortality and morbidity, observation and projection are relevant in Europe [19] | ||
| Different population subgroups are at risk [37] | ||
3.1.2. Low Temperature and Cold Spells
3.1.3. Floods
| Health Effect | Low Temperature | Extreme Cold | |
|---|---|---|---|
| Observed mortality | Low temperature is associated with observed mortality in 15 European cities [35,41] | Temperatures lower than the 5th percentile are strongly associated with mortality in Cantabria, Spain [44] | |
| Winter excess mortality rate remains a recurring phenomenon that is quantitatively greater than the isolated summer event in France [42] | |||
| Extreme cold is associated with observed mortality in La Mancha, Spain [45] | |||
| Excess winter mortality of 4597 cases per year for myocardial infarction in Portugal [43] | |||
| Observed morbidity | Low temperature is associated with respiratory tract infections [46] | ||
| Cold weather had an effect on hospital admission in 12 cities in Europe [40] | |||
| Significant negative associations between daily average air temperature and all stroke hospitalizations [39] | |||
| Thermal environment inversely associated with acute myocardial infarction morbidity during winter [38] | |||
| Projections | [30] * | ||
| [19] * |
| Region, Type of Flood or Model | Results | Reference |
|---|---|---|
| Estimation of future fluvial flood risk in Europe with LISFLOOD model | Flood risk (= product of flood probability (or hazard), exposure of capital and population, and vulnerability to the effect of flooding); decrease of flood risk in northeast Europe, increase in northern Europe. | [51] |
| Estimation of future fluvial flood risk in Europe with LISFLOOD model | Robust increase in future flood hazard, mainly due to a pronounced increase in extreme rainfall in western and central Europe, the British Isles, and northern Italy. | [52] |
| A decrease in future flood hazard is projected in eastern Germany, Poland, southern Sweden, and, to a lesser extent, the Baltic countries. | ||
| Estimation of future fluvial flood risk in Europe with LISFLOOD model | Under the no adaptation trajectory current expected annual damages of €5.5 billion/year are projected to reach €98 billion/year by the 2080s due to the combined effects of socioeconomic and climate change. Under the adaptation trajectory the avoided damages (benefits) amount to €53 billion/year by the 2080s. | [54] |
| Assessment of coastal and fluvial flood impacts using the Coastal Fluvial Flood (CFFlood) model in Europe | Approximately 6% of the European population lives in a 100-year event coastal/river flood risk area (2010 estimate) = approx. 28.6 million people. | [61] |
| Observational study about effect of exposure to floodwater in the Netherlands | Mean risk of infection for children who were exposed to floodwater originating from combined sewers was 33%, from storm sewers 23%, and from rainfall-generated surface runoff 3.5%.(For adults—3.9%, 0.58%, and 0.39%, respectively.) | [60] |
3.1.4. Wildfires
| Geographical Area | Study Type | Result | Reference |
|---|---|---|---|
| Greece | Cross-sectional case control study following wildfire August 2007 | Those exposed to disaster have significantly higher rates of symptoms including somatization, depression, anxiety, hostility, phobic anxiety, and paranoia; they are significantly more distressed than controls. | [71] |
| Peloponnesus peninsula, Greece | Cross-sectional case control study | 400 participants (200 fire-affected, 200 controls). Fire victims have a lower quality of life (physical and psychological health, environment)—but only significant impairment in quality of life in the environmental domain after adjustment for confounders. | [72] |
| EU-Mediterranean countries | Assessment of recorded fires (1957 to 2007) and projections of future fire conditions | Under scenarios B2 and A2, seasonal severity rating (SSR) expected to rise from 5.3 to 6.64 (+25%) and 7.34 (+38%) respectively (SSR = seasonal rating of fire danger, dependent on fuel moisture and fire behavior potential, designed to correlate with fire control difficulty). Iberian Peninsula (Spain, Portugal) and Greece most affected by increasing fire danger. | [69] |
| Portugal | Assessment of association between temporal dynamics of fire events in Portugal and several variables—socioeconomic, landscape, climatic | Country-wide increasing trend in numbers of fires from 1980 to mid-1990s, reduction in average area burnt per individual fire (from approx. 20 ha in 1980s to 5 ha in mid-90s). This increase not explained by investigated climatic factors or temperature/precipitation anomalies; not significantly correlated with number of forest fires. | [73] |
| Europe | Impact assessment and assessment of adaptation strategies | In the Mediterranean region the yearly average burned area is projected to increase by 150–200% by 2090 (relative to 2000). Balkan and eastern European countries will have a 150–560% increase in burned areas by 2090 compared with 2000. Central EU and Baltic countries: increase in burned areas of 120–340% by 2090 (compared with 2000). Overall, 65–67% reduction in burned area with prescribed burnings, compared to the “do nothing” scenario. | [70] |
| Portugal | Longitudinal observational and comparative study | Highest levels of PM fractions were observed during July and August of 2010, corresponding to the periods when majority (66%) of forest fires occurred and significantly higher than means for the remainder of the year. This may indicate that forest fires are responsible for increased PM levels. | [66] |
3.1.5. UV Radiation
3.2. Indirect Health Effects
3.2.1. Climate-Sensitive Infectious Diseases
| Geographical Area | Study Type | Result | Reference |
|---|---|---|---|
| Sweden | Spatial comparison of cancer incidence and sunshine | Coastal communities (high sun exposure) have higher incidence of SCC; this correlates with increased UV radiation exposure. | [78] |
| Europe (southern Spain, Paris, Berlin, Stockholm) | Modelling of the UV radiation relevant for health risks (skin cancer) and benefits (vitamin D production) for different scenarios | Estimated reduction of UVR daily doses, but not sufficient to provide a protection from erythema. On the other hand, at higher latitudes, UVR reduction possibly contributes to a relevant increase in the exposure time necessary for the synthesis of vitamin D, mainly during the autumn and spring seasons. | [80] |
| Global | Systematic literature review | UVR exposure is a minor contributor to the world’s disease burden, causing an estimated annual loss of 1.6 million DALYs; i.e., 0.1% of the total global disease burden. A markedly larger annual disease burden, 3.3 billion DALYs, might result from reduction in global UVR exposure to very low levels/ | [81] |
| Croatia | Observational comparative study | The incidence of malignant skin melanoma has risen during the last 10 years. Different distribution in two counties could be related to climate changes or different ways of life. | [79] |
Vector-Borne and Rodent-Borne Diseases
| Observed Effect (7) | Observed and Projected Effects (6) |
|---|---|
| Malaria in Portugal [89] | Malaria in Germany [92] |
| Malaria in Turkey [90] | Aedes albopictus in Europe [98] |
| Malaria in Spain [91] | Recent and future Aedes albopictus suitability [99] |
| WNF in Israel [93] | Dengue in Europe [100] |
| WNF in Hungary and Austria [94] | Dengue in Europe [103] |
| Chikungunya in Italy [96] | Dengue and Chikungunya in Europe [101] |
| Dengue in Madeira 2012 [102] |
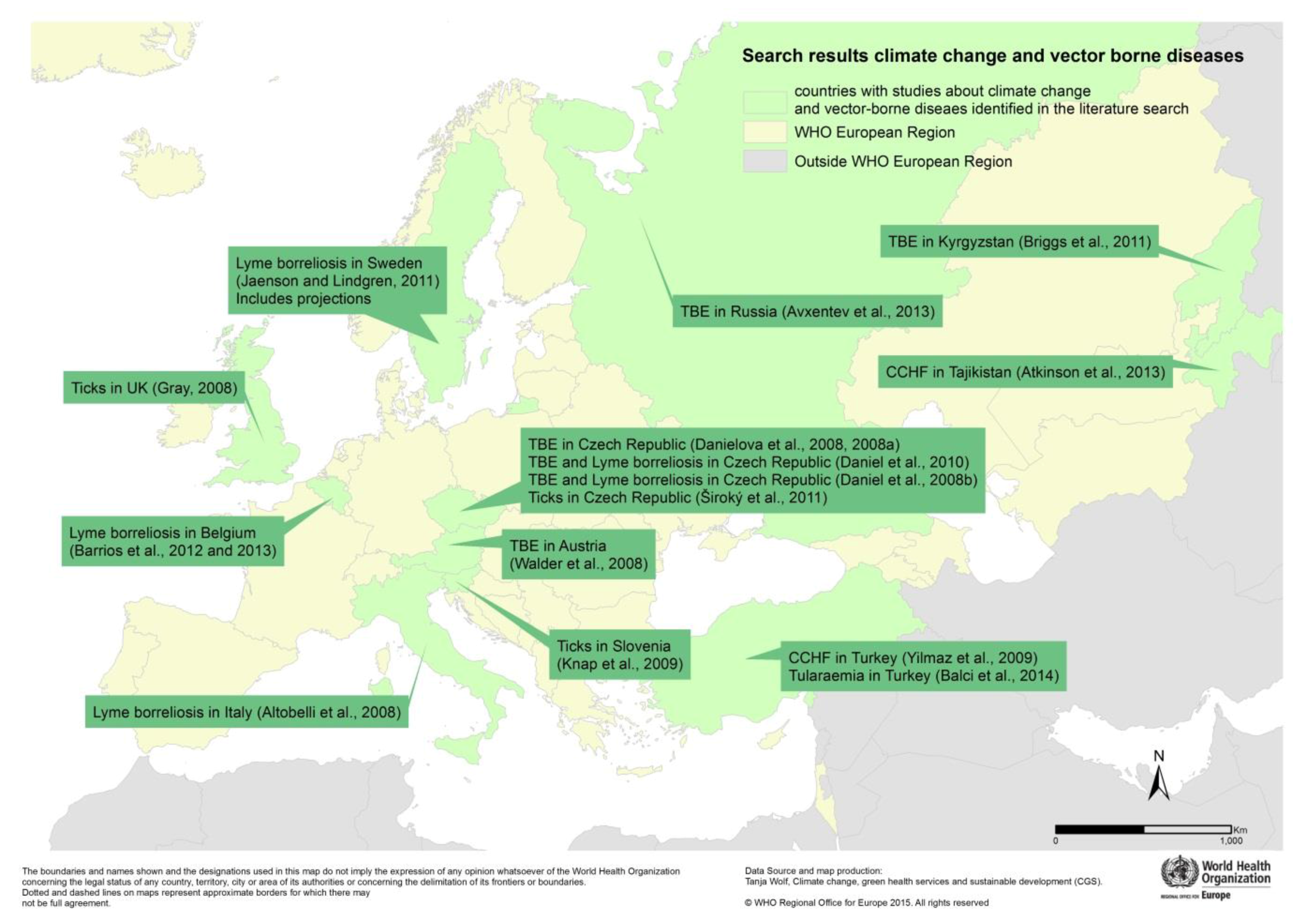
3.2.2. Food- and Water-Related Diseases
| Related to food | Campylobacter in northern Europe [137] |
| Salmonellosis in Kazakhstan [136] | |
| Related to water | Vibrio in Israel [141] |
| Rainfall and outbreaks in the United Kingdom [139] | |
| E. coli in water and mussels in Norway [143] | |
| Toxic cyanobacteria in peri-Alpine lakes [142] | |
| Mycotoxins in maize in the Netherlands [144] |
3.2.3. Air Quality
- (1)
- Those emissions leading to climate change often also pollute the air. Accordingly the air quality benefits from reducing greenhouse gases.
- (2)
- The impacts of climate change on the atmosphere, temperature, precipitation, and extreme events have a variable effect on the level of air pollution.
- (3)
- Warmer temperatures worsen the health effects of some air pollutants.
| Place | Study type | Result | Reference |
|---|---|---|---|
| Oporto, Portugal | Observational study | Ozone and PM 10 have adverse effects on health | [151] |
| Eskisehir, Turkey | Survey; cross-sectional study | Elevated ozone levels were associated with upper respiratory tract complaints in schoolchildren. | [161] |
| 22 cities in the Mediterranean and Europe | Synergistic effects of heat and air pollution (ozone and PM10) on mortality | [148] | |
| Dijon, France | Case-crossover study | Short-term exposure to even low levels of ozone has an effect on ischemic cerebral and cardiac events. | [152] |
| Germany | Descriptive, observational | Exposure to several air pollutants (ozone, PM10, CO, NO2, SO2) significantly affects infant and toddler health. | [162] |
| Northern Italy | Descriptive, observational study | PM2.5, ozone and NO2 are estimated to reduce life expectancy in two towns by 4% (in people aged 30 years) to 20% (in people aged 85 or more years) | [167] |
| Italy (ten cities) | Time-stratified, case-crossover analysis | PM10 exposure is associated with respiratory mortality, especially in summer. More respiratory deaths occur in the cold season. | [156] |
| Helsinki, Finland | Observational | Daily air pollution levels (PM2.5, NO2 and CO) is associated with asthma emergency room visits. There is a 3–5 day lag effect in children, but more immediate effect in the adults and elderly. | [168] |
| Czech Republic | Observational, cross-sectional study | Association between bronchitis and NOx in children increases with child’s age in the under-2 age group | [160] |
| Kotka, Finland | Cohort study | Even low levels of air pollution (PM2.5) are associated with higher inflammatory markers in the blood of elderly people. | [169] |
| Volos, Greece | Time series study | Air pollutants have a significant effect on hospitalization for respiratory and cardiovascular causes. | [153] |
| Israel | Cohort study | When adjusted for socio-demographic factors, cumulative chronic exposure to PM2.5 is positively associated with reoccurrence of cardiovascular events in patients after a first myocardial infarction. | [170] |
| Italy | Descriptive, observational | PM10 has significant impact on COPD hospitalization for children; ozone has significant influence on hospitalization of the elderly. | [150] |
| Romania | Observational, time series | Dry air aggravates the adverse effects of total suspended particles on chronic bronchitis. | [171] |
| Madrid, Spain | Ecological longitudinal time-series study | PM is the primary pollutant that showed a statistically significant association with hospital admission among people over 75 years of age. | [172] |
| Madrid, Spain | Longitudinal ecological time-series | PM2.5 concentrations are an important risk factor for daily circulatory-cause mortality. | [157] |
| England, Belgium, Germany and France | Simulation/climate modelling | Simulation of climate change effect on ozone indicates an increase in ozone concentrations. Higher temperatures are associated with increased biogenic emissions, less precipitation, fewer clouds, and increased photolysis. | [146] |
| Norway | Cohort study | NO2, PM2.5, and PM10 have effects on mortality for cardiovascular causes, lung cancer (threshold effects), and chronic obstructive pulmonary diseases (linear effects). | [173] |
| Vienna, Austria | Time series analysis | Particulate matter and NO2 were associated with mortality from non-trauma causes, even at relatively low levels. | [158] |
| Tuscany, Italy | Time-stratified case-crossover approach | Evidence for an association between air pollutants (PM10, NO2, CO) and hospitalization for acute myocardial infarction was found. | [174] |
| Tuscany, Italy | Time series and case-crossover | Ozone exposure is associated with an increase in out-of-hospital coronary death but not hospitalized acute myocardial infarctions. | [159] |
| 12 European countries | Pooled data from 14 population-based mother-child cohort studies | Exposure to ambient air pollutants and traffic during pregnancy is associated with restricted fetal growth in Europe. | [163] |
| Dresden, Germany | Observational and modelling | PM mass concentrations are higher in winter and estimated to decrease in future due to a decrease in sulfate and soot mass concentrations. | [165] |
| 12 countries in Europe | Cohort study | Long-term exposure to constituents of particles not found to be statistically significantly associated with total cardiovascular mortality. | [175] |
| Emilia-Romagna region, Italy | Time series analysis | In Emilia-Romagna, Italy, there is a positive relationship between PM10 and emergency ambulance dispatches (non-traumatic diseases). | [176] |
| Europe | Modelling | Increase in ground-level ozone and related health impacts is estimated for Belgium, France, Portugal, and Spain (plus 10–14% by 2050), with similar decrease in Nordic and Baltic countries. By 2041–2060, a strong increase in deaths and morbidity is expected in Belgium. | [147] |
3.2.4. Allergic Diseases
| Place | Study Type | Result | Reference |
|---|---|---|---|
| 7 centers in Spain—Asturias, Barcelona, Bilbao, Cartagena, La Coruña, Madrid, Valencia | Semi-individual population-based study based on International Study of Asthma and Allergies in Childhood (ISAAC) cross-sectional study | Higher mean annual concentrations of SO2 and CO are associated with higher prevalence of symptoms of allergic diseases (rhinitis, rhino conjunctivitis, eczema, and asthma). | [198] |
| 29 countries across Europe | Cross-sectional survey | No statistically significant associations between regional air pollution with allergic sensitization among adults in Europe. | [193] |
| Portollano, Spain | Cohort study | Air pollution levels were associated with an increased risk of pollen-allergic asthma symptoms, especially ozone, when exceeding the health threshold. | [194] |
| Szeged region, Southern Hungary | Descriptive, observational, longitudinal | Daily mean concentrations in chemical air pollutants and Ambrosia and other pollen have joint effects on allergic asthma emergency room visits. | [199] |
| Szeged, Hungary | Observational, longitudinal study | In Southern Hungary, Ambrosia, PM10, and ozone are associated with admissions across all age groups; increased wind speed may result in admission reduction by 45%. | [184] |
| Porto, Portugal | Observational, cross-sectional study | Inconsistent correlations between PM10 and pollen, and reduced pollen when ozone increases were observed. | [191] |
| Mainland Portugal—278 municipalities | Retrospective ecological study | In Portugal, positive associations between asthma hospital admission rates and temperature, NO2, and PM10 (summer only); negative associations with vegetation density. | [200] |
| Ankara, Turkey | Observational cross-sectional study | A small sample of people suffering from allergic rhinitis were shown to be sensitive to mainly tree pollens (95%) compared to grasses (3%) and weeds (2%). | [201] |
| Spain | Descriptive, observational | Presence of Amaranthacaea pollen is correlated to maximum spring temperature and rainfall and can change with climate. | [188] |
| Cartagena, Spain | Descriptive, observational | Air pollutants (SO2 and NO2) and Urticaria and Poaeceae pollen increase the risk in asthma hospital emergency room visits; NO2 and SO2 also increase the risk in ER visits due to chronic obstructive pulmonary disease. | [202] |
| Thessaloniki, Greece | Descriptive, observational, time series | Pollen levels, mainly of woody plants, are almost doubling each decade in Thessaloniki, Greece and this coincides with a rise in air temperature. | [190] |
| Hungary | Observational | Total annual pollen counts have increased. The changes in pollen season characteristics were in accordance with risk and expansion potential due to climate change. | [189] |
| Poland | Observational, comparative | The spore count of two species of fungal spores is mainly determined by air temperature. | [186] |
| Hungary | Descriptive, observational | Pollen amount increases with mean temperature and rainfall; little change in duration of pollination season. | [187] |
| Parma area (northern Italy) | Longitudinal descriptive study | In Parma, Italy, a significant increase in the incidence of allergy (to mites, pets, and birch family pollens) and asthma was observed, while allergic reactions to grasses and rhino conjunctivitis decreased and were correlated to a decrease in pollen count, pollen concentration peaks, and pollination period. | [192] |
| Genoa, Italy | Descriptive observational study | In Genoa, Italy, asthma exacerbations have seasonal peaks in spring and autumn and are associated with pollen concentration, wind speed, and rainfall as well as chemical pollutants (SO2, NO, and NO2). | [203] |
| 13 countries across Europe | Time series analysis | Increases in airborne pollen for many taxa in Europe. | [185] |
4. Discussion
- Heat-related mortality is likely to increase, particularly in southern parts of Europe, owing to an increase in the frequency and severity of heatwaves.
- The threat of extreme weather events will grow, with river floods and coastal flooding and associated health effects.
- Wildfire risk is likely to intensify significantly in countries such as Greece, Spain, Portugal, and Italy, with associated threats to life, agriculture, and property.
- The distribution of tick-borne diseases may grow as temperatures increase, and conditions may favor the reintroduction of mosquito-borne diseases including Dengue fever and malaria into parts of Europe.
- Morbidity and mortality caused by air pollution, particularly rising levels of ground-level ozone, may increase, as may allergic diseases due to changes in pollen and aeroallergen production, distribution, and allergenicity.
- The interaction of climate change and changing weather conditions with stratospheric ozone concentration, their joint impacts on UV radiation and human exposure to it is under exploration. The health effects (on the skin, eyes, immunity, etc.) are also determined by lifestyle and behavior.
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IPCC. IPCC summary for policymakers. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1–32. [Google Scholar]
- Kovats, R.S.; Valentini, R.; Bouwer, L.; Georgopoulou, E.; Jacob, D.; Martin, E.; Rounsevell, M.; Soussana, J.F. Part B: Regional aspects. In Climate Change 2014: Impacts, Adaptation, and Vulnerability; Barros, V., Field, C., Dokken, D., Mastrandrea, M., Mach, K., Bilir, T., Chatterjee, M., Ebi, K., Estrada, Y., Genova, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1267–1326. [Google Scholar]
- Smith, K.; Woodward, A.; Campbell-Lendrum, D.; Chadee, D.; Honda, Y.; Liu, Q.; Olwoch, J.; Revich, B.; Sauerborn, R. Human health: Impacts, adaptation, and co-benefits. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 709–754. [Google Scholar]
- World Health Organization. Protecting Health in Europe from Climate Change; Menne, B., Apfel, F., Kovats, S., Racioppi, F., Eds.; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2008. [Google Scholar]
- WHO. Quantitative Risk Assessment of the Effects of Climate Change on Selected Causes of Death, 2030s and 2050s; Hales, S., Kovats, S., Lloyd, S., Campbell-Lendrum, D., Eds.; World Health Organization (WHO): Geneva, Switzerland, 2014; p. 128. [Google Scholar]
- Costello, A.; Abbas, M.; Allen, A.; Ball, S.; Bell, S.; Bellamy, R.; Friel, S.; Groce, N.; Johnson, A.; Kett, M.; et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet 2009, 373, 1693. [Google Scholar] [CrossRef]
- Watts, N.; Adger, W.N.; Agnolucci, P.; Blackstock, J.; Byass, P.; Cai, W.; Chaytor, S.; Colbourn, T.; Collins, M.; Cooper, A.; et al. Health and climate change: Policy responses to protect public health. Lancet 2015, 386, e28–e31. [Google Scholar] [CrossRef]
- Chan, M. Achieving a cleaner, more sustainable, and healthier future. Lancet (Lond. Engl.) 2015, 386, e27–e28. [Google Scholar] [CrossRef]
- Wolf, T.; Martinez, G.S.; Cheong, H.-K.; Williams, E.; Menne, B. Protecting health from climate change in the WHO European region. Int. J. Environ. Res. Public Health 2014, 11, 6265–6280. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Suk, J.E.; Estevez, V.; Ebi, K.L.; Lindgren, E. Mapping climate change vulnerabilities to infectious diseases in Europe. Environ. Health Perspect. 2012, 120, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Health Care Interventions: Explanation and Elaboration. BMJ. 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- European Commission. CORDIS Community Research and Development Information Service. Avaliable online: http://cordis.europa.eu/ (accessed on 10 November 2015).
- Robine, J.M.; Cheung, S.L.K.; le Roy, S.; van Oyen, H.; Griffiths, C.; Michel, J.P.; Herrmann, F.R. Death toll exceeded 70,000 in Europe during the summer of 2003. C.R. Biol. 2008, 331, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Amengual, A.; Homar, V.; Romero, R.; Brooks, H.E.; Ramis, C.; Gordaliza, M.; Alonso, S. Projections of heat waves with high impact on human health in Europe. Glob. Planet. Chang. 2014, 119, 71–84. [Google Scholar] [CrossRef]
- D’Ippoliti, D.; Michelozzi, P.; Marino, C.; de’Donato, F.; Menne, B.; Katsouyanni, K.; Kirchmayer, U.; Analitis, A.; Medina-Ramón, M.; Paldy, A.; et al. The impact of heat waves on mortality in 9 European cities: Results from the EuroHEAT project. Environ. Health 2010. [Google Scholar] [CrossRef]
- Montero, J.C.; Mirón, I.J.; Criado-Álvarez, J.J.; Linares, C.; Díaz, J. Influence of local factors in the relationship between mortality and heat waves: Castile-La Mancha (1975–2003). Sci. Total Environ. 2012, 414, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, K.M.A.; Endlicher, W.R. Urban and rural mortality rates during heat waves in Berlin and Brandenburg, Germany. Environ. Pollut. 2011, 159, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; D’Ippoliti, D.; de Sario, M.; Analitis, A.; Menne, B.; Katsouyanni, K.; de’Donato, F.K.; Basagana, X.; Salah, A.B.; Casimiro, E.; et al. A time series study on the effects of heat on mortality and evaluation of heterogeneity into European and Eastern-Southern Mediterranean cities: Results of EU CIRCE project. Environ. Health Glob. Access. Sci. Source 2013, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Morabito, M.; Crisci, A.; Moriondo, M.; Profili, F.; Francesconi, P.; Trombi, G.; Bindi, M.; Gensini, G.F.; Orlandini, S. Air temperature-related human health outcomes: Current impact and estimations of future risks in Central Italy. Sci. Total Environ. 2012, 441, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Theoharatos, G.; Pantavou, K.; Mavrakis, A.; Spanou, A.; Katavoutas, G.; Efstathiou, P.; Mpekas, P.; Asimakopoulos, D. Heat waves observed in 2007 in Athens, Greece: Synoptic conditions, bioclimatological assessment, air quality levels and health effects. Environ. Res. 2010, 110, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Burkart, K.; Canário, P.; Breitner, S.; Schneider, A.; Scherber, K.; Andrade, H.; Alcoforado, M.J.; Endlicher, W. Interactive short-term effects of equivalent temperature and air pollution on human mortality in Berlin and Lisbon. Environ. Pollut. 2013, 183, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Masocco, M.; Meli, P.; Minelli, G.; Palummeri, E.; Solimini, R.; Toccaceli, V.; Vichi, M. General and specific mortality among the elderly during the 2003 heat wave in Genoa (Italy). Environ. Res. 2007, 103, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Josseran, L.; Caillere, N.; Brun-Ney, D.; Rottner, J.; Filleul, L.; Brucker, G.; Astagneau, P.; Caillère, N.; Brun-Ney, D.; Rottner, J.; et al. Syndromic surveillance and heat wave morbidity: A pilot study based on emergency departments in France. BMC Med. Inform. Decis. Mak. 2009, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Michelozzi, P.; Accetta, G.; de Sario, M.; d’Ippoliti, D.; Marino, C.; Baccini, M.; Biggeri, A.; Anderson, H.R.; Katsouyanni, K.; Ballester, F.; et al. On behalf of the PHEWE collaborative group high temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am. J. Respir. Crit. Care Med. 2009, 179, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, E.; Zauli Sajani, S.; Scotto, F.; Miglio, R.; Marchesi, S.; Lauriola, P. Emergency ambulance dispatches and apparent temperature: A time series analysis in Emilia-Romagna, Italy. Environ. Res. 2011, 111, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Vicedo-Cabrera, A.M.; Iñíguez, C.; Barona, C.; Ballester, F. Exposure to elevated temperatures and risk of preterm birth in Valencia, Spain. Environ. Res. 2014, 134, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Watkiss, P.; Gobiet, A.; Jacob, D.; Kjellstrom, E.; Kotova, L.; Landgren, O.; Lenderink, G.; Mendlik, T.; Nikulin, G.; Sobolowski, S.; Teichmann, C.; Vautard, R. IMPACT2C Policy Update on 2 °C Warming; Climate Service Center: Hamburg, Germany, 2013. [Google Scholar]
- Lung, T.; Lavalle, C.; Hiederer, R.; Dosio, A.; Bouwer, L.M. A multi-hazard regional level impact assessment for Europe combining indicators of climatic and non-climatic change. Glob. Environ. Chang. 2013, 23, 522–536. [Google Scholar] [CrossRef]
- Åström, C.; Orru, H.; Rocklöv, J.; Strandberg, G.; Ebi, K.L.; Forsberg, B. Heat-related respiratory hospital admissions in Europe in a changing climate: A health impact assessment. BMJ Open 2013, 3, e001842. [Google Scholar] [CrossRef] [PubMed]
- Ciscar, J.C.C.; Iglesias, A.; Feyen, L.; Goodess, C.M.; Szabo, L.; Christensen, O.B.; Nicholls, R.; Amelung, B.; Watkiss, P.; Bosello, F.; et al. Climate Change Impacts in Europe; Final Report of the PESETA Research Project; European Commission Joint Research Centre: Seville, Spain, 2009. [Google Scholar]
- Kovats, S.; Lloyd, S.; Hunt, A.; Watkiss, P. Technical policy briefing Note 5: The impacts and economic costs on health in Europe, and the costs and benefits of adaptation, results of the EC RTD ClimateCost Project. In The ClimateCost Project. Final Report. Volume 1: Europe; Watkiss, P., Ed.; Stockholm Environment Institute: Stockholm, Sweden, 2011; pp. 1–31. [Google Scholar]
- Schifano, P.; Leone, M.; de Sario, M.; de’Donato, F.; Bargagli, A.M.; d’Ippoliti, D.; Marino, C.; Michelozzi, P. Changes in the effects of heat on mortality among the elderly from 1998–2010: Results from a multicenter time series study in Italy. Environ. Health Glob. Access. Sci. Source 2012, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Kriszbacher, I.; Bódis, J.; Csoboth, I.; Boncz, I. The occurrence of acute myocardial infarction in relation to weather conditions. Int. J. Cardiol. 2009, 135, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Robine, J.M.M.; Michel, J.P.P.; Herrmann, F.R.R. Excess male mortality and age-specific mortality trajectories under different mortality conditions: A lesson from the heat wave of summer 2003. Mech. Ageing Dev. 2012, 133, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Analitis, A.; Katsouyanni, K.; Biggeri, A.; Baccini, M.; Forsberg, B.; Bisanti, L.; Kirchmayer, U.; Ballester, F.; Cadum, E.; Goodman, P.G.; et al. Effects of cold weather on mortality: Results from 15 European cities within the PHEWE project. Am. J. Epidemiol. 2008, 168, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Hajat, S.; Kovats, R.S.S.; Lachowycz, K. Heat-related and cold-related deaths in England and Wales: Who is at risk? Occup. Env. Med. 2007, 64, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Bliziotis, I.A.; Kosmidis, J.; Daikos, G.K. Unusual climatic conditions and infectious diseases: Observations made by Hippocrates. Enferm. Infect. Microbiol. Clin. 2010, 28, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, J.; Freire, E.; Almendra, R.; Silva, G.L.; Santana, P. The impact of winter cold weather on acute myocardial infarctions in Portugal. Environ. Pollut. 2013, 183, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Morabito, M.; Crisci, A.; Vallorani, R.; Modesti, P.A.; Gensini, G.F.; Orlandini, S. Innovative approaches helpful to enhance knowledge on weather-related stroke events over a wide geographical area and a large population. Stroke 2011, 42, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Marino, C.; de’Donato, F.; Michelozzi, P.; d’Ippoliti, D.; Katsouyanni, K.; Analitis, A.; Biggeri, A.; Baccini, M.; Accetta, G.; Perucci, C. A Effects of cold weather on hospital admissions: Results from 12 European cities within the PHEWE project. Epidemiology 2009, 20, S67–S68. [Google Scholar] [CrossRef]
- Gómez-Acebo, I.; Llorca, J.; Dierssen, T. Cold-related mortality due to cardiovascular diseases, respiratory diseases and cancer: A case-crossover study. Public Health 2013, 127, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Phu Pin, S.; Golmard, J.L.; Cotto, E.; Rothan-Tondeur, M.; Chami, K.; Piette, F. Excess winter mortality in France: Influence of temperature, influenza like illness, and residential care status. J. Am. Med. Dir. Assoc. 2012, 13, e309. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, J.; Freire, E.; Morais, J.; Machado, J.R.; Santana, P. The health impacts of poor housing conditions and thermal discomfort. Procedia Environ. Sci. 2011, 4, 158–164. [Google Scholar] [CrossRef]
- Gómez-Acebo, I.; Dierssen-Sotos, T.; Llorca, J. Effect of cold temperatures on mortality in Cantabria (Northern Spain): A case-crossover study. Public Health 2010, 124, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.C.; Mirón, I.J.; Criado-Álvarez, J.J.; Linares, C.; Díaz, J. Mortality from cold waves in Castile—La Mancha, Spain. Sci. Total Environ. 2010, 408, 5768–5774. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Theocharis, G.; Spanos, A.; Vlara, L.A.; Issaris, E.A.; Panos, G.; Peppas, G. Effect of meteorological variables on the incidence of respiratory tract infections. Respir. Med. 2008, 102, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Guha-Sapir, D.; Below, R.; Hoyois, P. EM-DAT The International Disaster Datablase, Centre for Research in Epidemiology of Disasters—CRED. Available online: http://www.emdat.be (accessed on 6 October 2011).
- EEA. Climate Change, Impacts and Vulnerability in Europe 2012: An Indicator-Based Report; Rosendahls-Schultz Grafisk: Copenhagen, Denmark, 2012. [Google Scholar]
- Bindi, M.; Olesen, J.E. The responses of agriculture in Europe to climate change. Reg. Environ. Chang. 2010, 11, 151–158. [Google Scholar] [CrossRef]
- Borga, M.; Anagnostou, E.; Blöschl, G.; Creutin, J.D. Flash flood forecasting, warning and risk management: The HYDRATE project. Environ. Sci. Policy 2011, 14, 834–844. [Google Scholar] [CrossRef]
- Feyen, L.; Dankers, R.; Bódis, K.; Salamon, P.; Barredo, J.I. Fluvial flood risk in Europe in present and future climates. Clim. Chang. 2011, 112, 47–62. [Google Scholar] [CrossRef]
- Rojas, R.; Feyen, L.; Bianchi, A.; Dosio, A. Assessment of future flood hazard in Europe using a large ensemble of bias corrected regional climate simulations. J. Geophys. Res. 2012, 117, D17109. [Google Scholar]
- Ciscar, J.C.; Feyen, L.; Soria, A.; Lavalle, C.; Raes, F.; Perry, M.; Nemry, F.; Demirel, H.; Rozsai, M.; Dosio, A.; et al. Climate Impacts in Europe: The JRC PESETA II Project; European Commission Joint Research Centre: Seville, Spain, 2014. [Google Scholar]
- Rojas, R.; Feyen, L.; Watkiss, P. Climate change and river floods in the European Union: Socio-economic consequences and the costs and benefits of adaptation. Glob. Environ. Chang. 2013, 23, 1737–1751. [Google Scholar] [CrossRef]
- Nakicenovic, N.; Alcamo, J.; Davis, G.; de Vries, B.; Fenhann, J.; Gaffin, S.; Gregory, K.; Grübler, A.; Jung, T.Y.; Kram, T.; et al. IPCC Special Report: Emissions Scenarios. Summary for Policymakers. Summary for Policymakers Emissions Scenarios A Special Report of IPCC Working Group III; Nakicenovic, N., Swart, R., Eds.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Brown, S.; Nicholls, R.J.R.; Vafeidis, A.; Hinkel, J.; Watkiss, P.; Watkiss, P. The Impacts and Economic Costs of Sea-Level Rise in Europe and the Costs and Benefits of Adaptation. Summary of Results from the EC RTD ClimateCost Project; Technical Policy Briefing Note 2; Stockholm Environment Institute: Stockholm, Sweden, 2011. [Google Scholar]
- Jakubicka, T.; Vos, F.; Phalkey, R.; Marx, M.; Guha-Sapir, D. Health Impacts of Floods in Europe. Data Gaps and Information Needs from a Spatial Perspective; MICRODIS: Heidelberg, Germany, 2010. [Google Scholar]
- Vasconcelos, P. Flooding in Europe: A brief review of the health risks. Eurosurveillance 2006, 11, 2947. [Google Scholar]
- WHO. Floods in the WHO European Region: Health Effects and Their Prevention; WHO: Copenhagen, Denmark, 2013. [Google Scholar]
- De Man, H.; van den Berg, H.H.J.L.; Leenen, E.J.T.M.; Schijven, J.F.; Schets, F.M.; van der Vliet, J.C.; van Knapen, F.; de Roda Husman, A.M. Quantitative assessment of infection risk from exposure to waterborne pathogens in urban floodwater. Water Res. 2014, 48, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Mokrech, M.; Kebede, A.; Nicholls, R.; Wimmer, F. An integrated approach for assessing flood impacts due to future climate and socio-economic conditions and the scope of adaptation in Europe. In Climatic Change: Regional Integrated Assessment of Cross-Sectoral Climate Change Impacts, Adaptation, and Vulnerability; Harrison, P., Berry, P., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 245–260. [Google Scholar]
- EEA Indicator Assessment: CLIM 035: Forest Fires. Available online: http://www.eea.europa.eu/data-and-maps/indicators/forest-fire-danger-1/assessment (accessed on 18 January 2015).
- EEA. Mapping the Impacts of Natural Hazards and Technological Accidents in Europe: An Overview of the Last Decade; European Environment Agency: Copenhagen, Denmark, 2010. [Google Scholar]
- Finlay, S.; Moffat, A.; Gazzard, R.; Baker, D.; Murray, V. Health impacts of wildfires. PLoS Curr. Disasters 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Adamis, D.; Papanikolaou, V.; Mellon, R.C.; Prodromitis, G.; Tyrovola, K.; Kyriopoulos, J. Long-term psychological effects of a wildfire disaster in Greece. Eur. Psychiatry 2012, 27, 1. [Google Scholar]
- Slezakova, K.; Morais, S.; Pereira, M.C. Forest fires in Northern region of Portugal: Impact on PM levels. Atmos. Res. 2013, 127, 148–153. [Google Scholar] [CrossRef]
- Youssouf, H.; Liousse, C.; Roblou, L.; Assamoi, E.M.; Salonen, R.O.; Maesano, C. Quantifying wildfires exposure for investigating health-related effects. Atmos. Environ. 2014, 97, 239–251. [Google Scholar] [CrossRef]
- Shaposhnikov, D.; Revich, B.; Bellander, T.; Bedada, G.B.; Bottai, M.; Kharkova, T.; Kvasha, E.; Lezina, E.; Lind, T.; Semutnikova, E.; et al. Mortality related to air pollution with the Moscow heat wave and wildfire of 2010. Epidemiology 2014, 25, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Amatulli, G.; Camia, A.; San-Miguel-Ayanz, J. Estimating future burned areas under changing climate in the EU-Mediterranean countries. Sci. Total Environ. 2013, 450–451, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Khabarov, N.; Krasovskii, A.; Obersteiner, M.; Swart, R.; Dosio, A.; San-Miguel-Ayanz, J.; Durrant, T.; Camia, A.; Migliavacca, M. Forest fires and adaptation options in Europe. Reg. Environ. Chang. 2014. [Google Scholar] [CrossRef]
- Adamis, D.; Papanikolaou, V.; Mellon, R.C.; Prodromitis, G. The impact of wildfires on mental health of residents in a rural area of Greece. A case control population based study. Eur. Psychiatry 2011, 26, 1188. [Google Scholar]
- Papanikolaou, V.; Adamis, D.; Kyriopoulos, J. Long term quality of life after a wildfire disaster in a rural part of Greece. Open J. Psychiatry 2012, 2, 164–170. [Google Scholar] [CrossRef]
- Costa, L.; Thonicke, K.; Poulter, B.; Badeck, F.W. Sensitivity of Portuguese forest fires to climatic, human, and landscape variables: Subnational differences between fire drivers in extreme fire years and decadal averages. Reg. Environ. Chang. 2010, 11, 543–551. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar]
- Bais, A.F.; McKenzie, R.L.; Bernhard, G.; Aucamp, P.J.; Ilyas, M.; Madronich, S.; Tourpali, K.; Bais, A.F.; Björn, L.O.; Ilyas, M.; et al. Ozone depletion and climate change: Impacts on UV radiation. Photochem. Photobiol. Sci. 2014, 14, 19–52. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Lucas, R.M.; Cullen, A.P.; de Gruijl, F.R.; Longstreth, J.; Takizawa, Y.; van der Leun, J.C. The human health effects of ozone depletion and interactions with climate change. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2011, 10, 199–225. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.M.; Norval, M.; Neale, R.E.; Young, A.R.; de Gruijl, F.R.; Takizawa, Y.; van der Leun, J.C. The Consequences for Human Health of Stratospheric Ozone Depletion in Association with Other Environmental Factors—c4pp90033b. Available online: http://pubs.rsc.org/en/content/articlepdf/2015/pp/c4pp90033b (accessed on 15 January 2015).
- Andersson, E.M.; Paoli, J.; Wastensson, G. Incidence of cutaneous squamous cell carcinoma in coastal and inland areas of Western Sweden. Cancer Epidemiol. 2011, 35, e69–e74. [Google Scholar] [CrossRef] [PubMed]
- Materljan, E.; Zamolo, G.; Petkovic, M.; Ivosevic, D.; Popovic, B.; Materljan, M.; Katunaric, M.; Jurisic, D. Malignant skin melanoma in Croatia. Coll. Antropol. 2009, 33, 1363–1368. [Google Scholar] [PubMed]
- Corrêa, M.D.P.; Godin-Beekmann, S.; Haeffelin, M.; Bekki, S.; Saiag, P.; Badosa, J.; Jégou, F.; Pazmiño, A.; Mahé, E. Projected changes in clear-sky erythemal and vitamin D effective UV doses for Europe over the period 2006 to 2100. Photochem. Photobiol. Sci. 2013, 12, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.M.; McMichael, A.J.; Armstrong, B.K.; Smith, W.T. Estimating the global disease burden due to ultraviolet radiation exposure. Int. J. Epidemiol. 2008, 37, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.E.; Ebi, K.L.; Vose, D.; Wint, W.; Alexander, N.; Mintiens, K.; Semenza, J.C. Indicators for tracking European vulnerabilities to the risks of infectious disease transmission due to climate change. Int. J. Environ. Res. Public Health 2014, 11, 2218–2235. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Owers, K.A.; Waret-Szkuta, A.; McIntyre, K.M.; Baylis, M. Climate variability and outbreaks of infectious diseases in Europe. Sci. Rep. 2013, 3, 1774. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Menne, B. Climate change and infectious diseases in Europe. Lancet Infect. Dis. 2009, 9, 365–375. [Google Scholar] [CrossRef]
- Lindgren, E.; Andersson, Y.; Suk, J.E.; Sudre, B.; Semenza, J.C. Monitoring EU emerging infectious disease risk due to climate change. Science 2012, 336, 418–419. [Google Scholar] [CrossRef] [PubMed]
- WHO. Regional Office for Europe Information leaflet: Malaria in the WHO European Region (English); WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Arends, J.E.; Oosterheert, J.J.; Kraaij-Dirkzwager, M.M.; Kaan, J.A.; Fanoy, E.B.; Haas, P.J.; Scholte, E.J.; Kortbeek, L.M.; Sankatsing, S.U.C. Two cases of Plasmodium falciparum malaria in the Netherlands without recent travel to a malaria-endemic country. Am. J. Trop. Med. Hyg. 2013, 89, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Gallien, S.; Taieb, F.; Hamane, S.; De Castro, N.; Molina, J.M. Autochthonous falciparum malaria possibly transmitted by luggage-carried vector in Paris, France, February 2013. Eurosurveillance 2013, 18. [Google Scholar] [CrossRef]
- Benali, A.; Nunes, J.P.; Freitas, F.B.; Sousa, C.A.; Novo, M.T.; Lourenço, P.M.; Lima, J.C.; Seixas, J.; Almeida, A.P.G. Satellite-derived estimation of environmental suitability for malaria vector development in Portugal. Remote Sens. Environ. 2014, 145, 116–130. [Google Scholar] [CrossRef]
- Dogan, H.M.; Cetin, I.; Egri, M. Spatiotemporal change and ecological modelling of malaria in Turkey by means of geographic information systems. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Elipe, S.; Latorre, J.M.; Escosa, R.; Masià, M.; Fuentes, M.V.; Mas-Coma, S.; Bargues, M.D. Malaria resurgence risk in southern Europe: Climate assessment in an historically endemic area of rice fields at the Mediterranean shore of Spain. Malar. J. 2010, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Schröder, W.; Schmidt, G. Mapping the potential temperature-dependent tertian malaria transmission within the ecoregions of Lower Saxony (Germany). Int. J. Med. Microbiol. 2008, 298, 38–49. [Google Scholar] [CrossRef]
- Anis, E.; Grotto, I.; Mendelson, E.; Bin, H.; Orshan, L.; Gandacu, D.; Warshavsky, B.; Shinar, E.; Slater, P.E.; Lev, B. West Nile fever in Israel: The reemergence of an endemic disease. J. Infect. 2014, 68, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Bakonyi, T.; Ferenczi, E.; Erdélyi, K.; Kutasi, O.; Csörgő, T.; Seidel, B.; Weissenböck, H.; Brugger, K.; Bán, E.; Nowotny, N. Explosive spread of a neuroinvasive lineage 2 West Nile virus in Central Europe, 2008/2009. Vet. Microbiol. 2013, 165, 61–70. [Google Scholar] [CrossRef] [PubMed]
- WHO. Regional Office for Europe Information Leaflet: West Nile Virus in the WHO European Region (English); WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Rezza, G.; Nicoletti, L.; Angelini, R.; Romi, R.; Finarelli, A.C.; Panning, M.; Cordioli, P.; Fortuna, C.; Boros, S.; Magurano, F.; et al. Infection with chikungunya virus in Italy: An outbreak in a temperate region. Lancet 2007, 370, 1840–1846. [Google Scholar] [CrossRef]
- WHO. Regional Office for Europe Information Leaflet: Chikungunya in the WHO European Region (English); WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Fischer, D.; Moeller, P.; Thomas, S.M.; Naucke, T.J.; Beierkuhnlein, C. Combining climatic projections and dispersal ability: A method for estimating the responses of sandfly vector species to climate change. PLoS Negl. Trop. Dis. 2011, 5, e1407. [Google Scholar] [CrossRef] [PubMed]
- Caminade, C.; Medlock, J.; Ducheyne, E.; McIntyre, K.; Leach, S.; Baylis, M.; Morse, A. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: Recent trends and future scenarios. J. R. Soc. Interface 2012, 9, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.J.; Suk, J.E.; Semenza, J.C. Using global maps to predict the risk of dengue in Europe. Acta Trop. 2014, 129, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Seyler, T.; Grandesso, F.; Strat, Y.L.; Tarantola, A.; Depoortere, E. Assessing the risk of importing dengue and chikungunya viruses to the European Union. Epidemics 2009, 1, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, J.; Recker, M. The 2012 Madeira dengue dutbreak: Epidemiological determinants and future epidemic potential. PLoS Negl. Trop. Dis. 2014, 8, e3083. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, M.; Colón-González, F.; Lung, T.; Lake, I.; Hunter, P. Climate change and the emergence of vector-borne diseases in Europe: Case study of dengue fever. BMC Public Health 2014, 14, 781. [Google Scholar] [CrossRef] [PubMed]
- Sudre, B.; Rakotoarivony, L.M.; Vas, J. Mission Report: Dengue outbreak in Madeira, Portugal; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2012. [Google Scholar]
- Marchand, E.; Prat, C.; Jeannin, C.; Lafont, E.; Bergmann, T.; Flusin, O.; Rizzi, J.; Roux, N.; Busso, V.; Deniau, J.; et al. Autochthonous case of dengue in France, October 2013. Eurosurveillance 2013, 18. [Google Scholar] [CrossRef]
- WHO. Regional Office for Europe Information Leaflet: Dengue in the WHO European Region (English); WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Fischer, D.; Thomas, S.M.; Niemitz, F.; Reineking, B.; Beierkuhnlein, C. Projection of climatic suitability for Aedes albopictus Skuse (Culicidae) in Europe under climate change conditions. Glob. Planet. Chang. 2011, 78, 54–64. [Google Scholar] [CrossRef]
- Yilmaz, G.R.; Buzgan, T.; Irmak, H.; Safran, A.; Uzun, R.; Cevik, M.A.; Torunoglu, M.A. The epidemiology of Crimean-Congo hemorrhagic fever in Turkey, 2002–2007. Int. J. Infect. Dis. 2009, 13, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.; Chamberlain, J.; Jameson, L.J.; Logue, C.H.; Lewis, J.; Belobrova, E.A.; Valikhodzhaeva, M.; Mullojonova, M.; Tishkova, F.H.; Hewson, R. Identification and analysis of Crimean-Congo hemorrhagic fever virus from human sera in Tajikistan. Int. J. Infect. Dis. 2013, 17, e1031–e1037. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, A.; Boemo, B.; Mignozzi, K.; Bandi, M.; Floris, R.; Menardi, G.; Cinco, M. Spatial Lyme borreliosis risk assessment in north-eastern Italy. Int. J. Med. Microbiol. 2008, 298, 125–128. [Google Scholar] [CrossRef]
- Barrios, J.M.; Verstraeten, W.W.; Maes, P.; Clement, J.; Aerts, J.M.; Farifteh, J.; Lagrou, K.; van Ranst, M.; Coppin, P. Remotely sensed vegetation moisture as explanatory variable of Lyme borreliosis incidence. Int. J. Appl. Earth Obs. Geoinf. 2012, 18, 1–12. [Google Scholar] [CrossRef]
- Jaenson, T.G.T.; Jaenson, D.G.E.; Eisen, L.; Petersson, E.; Lindgren, E. Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Walder, G.; Falkensammer, B.; Heinz, F.X.; Holzmann, H.; Dierich, M.P.; Würzner, R. Tick-borne encephalitis in the Tyrol (Austria): Changes in incidence and endemicity 2000–2006. Int. J. Med. Microbiol. 2008, 298, 88–93. [Google Scholar] [CrossRef]
- Avxentev, N.; Avxentyeva, M.; Platonov, A.; Kolyasnikova, N.; Gridneva, K.; Dolgin, V.; Titkov, A.; Derkach, E.V. Evaluating economic burden of tick-borne encephalitis. Evidence from Russia. Value Health 2013, 16, A347–A348. [Google Scholar] [CrossRef]
- Briggs, B.J.; Atkinson, B.; Czechowski, D.M.; Larsen, P.A.; Meeks, H.N.; Carrera, J.P.; Duplechin, R.M.; Hewson, R.; Junushov, A.T.; Gavrilova, O.N.; et al. Tick-borne encephalitis virus, Kyrgyzstan. Emerg. Infect. Dis. 2011, 17, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Brinkley, C.; Nolskog, P.; Golovljova, I.; Lundkvist, Å.; Bergström, T. Tick-borne encephalitis virus natural foci emerge in western Sweden. Int. J. Med. Microbiol. 2008, 298, 73–80. [Google Scholar] [CrossRef]
- Daniel, M.; Danielova, V.; Kriz, B.; Jirsa, A.; Nozicka, J.; Nozicka, J. Shift of the tick ixodes ricinus and tick-borne encephalitis to higher altitudes in Central Europe. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 327–328. [Google Scholar] [PubMed]
- Daniel, M.; Kříž, B.; Danielová, V.; Beneš, Č. Sudden increase in tick-borne encephalitis cases in the Czech Republic, 2006. Int. J. Med. Microbiol. 2008, 298, 81–87. [Google Scholar] [CrossRef]
- Daniel, M.; Schwarzova, L.; Materna, J.; Rudenko, N.; Golovchenko, M.; Holubova, J.; Grubhoffer, L.; Kilian, P. Integration of a tick-borne encephalitis virus and borrelia burgdorferi sensu lato into mountain ecosystems, following a shift in the altitudinal limit of distribution of their vector, Ixodes ricinus (Krkonose Mountains, Czech Republic). Vector Borne Zoonotic Dis. 2010, 10, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J. Climate change and Norman Daniels’ theory of just health: An essay on basic needs. Med. Health Care Philos. 2012, 15, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Balci, E.; Borlu, A.; Kilic, A.U.; Demiraslan, H.; Oksuzkaya, A.; Doganay, M. Tularemia outbreaks in Kayseri, Turkey: An evaluation of the effect of climate change and climate variability on tularemia outbreaks. J. Infect. Public Health 2014, 7, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S. Ixodes ricinus seasonal activity: Implications of global warming indicated by revisiting tick and weather data. Int. J. Med. Microbiol. 2008, 298, 19–24. [Google Scholar] [CrossRef]
- Knap, N.; Durmiši, E.; Saksida, A.; Korva, M.; Petrovec, M.; Avšič-Županc, T. Influence of climatic factors on dynamics of questing Ixodes ricinus ticks in Slovenia. Vet. Parasitol. 2009, 164, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Široký, P.; Kubelová, M.; Bednář, M.; Modrý, D.; Hubálek, Z.; Tkadlec, E.; Siroký, P. The distribution and spreading pattern of Dermacentor reticulatus over its threshold area in the Czech Republic—How much is range of this vector expanding? Vet. Parasitol. 2011, 183, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.T.T.; Lindgren, E. The range of Ixodes ricinus and the risk of contracting Lyme borreliosis will increase northwards when the vegetation period becomes longer. Ticks Tick Borne Dis. 2011, 2, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Barrios, J.M.; Verstraeten, W.W.; Maes, P.; Aerts, J.M.; Farifteh, J.; Coppin, P. Seasonal vegetation variables and their impact on the spatio-temporal patterns of nephropathia epidemica and Lyme borreliosis in Belgium. Appl. Geogr. 2013, 45, 230–240. [Google Scholar] [CrossRef]
- Danielová, V.; Schwarzová, L.; Materna, J.; Daniel, M.; Metelka, L.; Holubová, J.; Kříž, B. Tick-borne encephalitis virus expansion to higher altitudes correlated with climate warming. Int. J. Med. Microbiol. 2008, 298, 68–72. [Google Scholar] [CrossRef]
- Daniel, M.; Kříz˘, B.; Valter, J.; Kott, I.; Danielová, V. The influence of meteorological conditions of the preceding winter on the incidences of tick-borne encephalitis and Lyme borreliosis in the Czech Republic. Int. J. Med. Microbiol. 2008, 298, 60–67. [Google Scholar] [CrossRef]
- Kausrud, K.L.; Viljugrein, H.; Frigessi, A.; Begon, M.; Davis, S.; Leirs, H.; Dubyanskiy, V.; Stenseth, N. Climatically driven synchrony of gerbil populations allows large-scale plague outbreaks. Proc. R. Soc. 2007, 274, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Kausrud, K.L.; Begon, M.; Ari, T.B.; Viljugrein, H.; Esper, J.; Büntgen, U.; Leirs, H.; Junge, C.; Yang, B.; Yang, M.; et al. Modeling the epidemiological history of plague in Central Asia: Palaeoclimatic forcing on a disease system over the past millennium. BMC Biol. 2010, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- ECDC “Leptospirosis.” European Centre for Disease Prevention and Control, 2015. Available online: http://www.ecdc.europa.eu/en/healthtopics/leptospirosis/pages/index.aspx (accessed on 11 November 2015).
- Lau, C.L.; Smythe, L.D.; Craig, S.B.; Weinstein, P. Climate change, flooding, urbanisation and leptospirosis: Fuelling the fire? Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Marrama-Rakotoarivony, L.; Sudre, B.; van Bortel, W.; Warns-Petit, E.; Zeller, H. Annual Epidemiological Report 2014: Emerging and Vector-Borne Diseases; ECDC: Stockholm, Sweden, 2014. [Google Scholar]
- Heyman, P.; Ceianu, C.; Christova, I.; Tordo, N.; Beersma, M.; Joao Alves, M.; Lundkvist, A.; Hukic, M.; Papa, A.; Tenorio, A.; et al. A five-year perspective on the situation of haemorrhagic fever with renal syndrome and status of the hantavirus reservoirs in Europe, 2005–2010. Eurosurveillance 2011, 16, 19961. [Google Scholar] [PubMed]
- Kovats, R.S.; Edwards, S.J.; Hajat, S.; Armstrong, B.G.; Ebi, K.L.; Menne, B. The effect of temperature on food poisoning: A time-series analysis of salmonellosis in ten European countries. Epidemiol. Infect. 2004, 132, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Grjibovski, A.; Kosbayeva, A.; Menne, B. The effect of ambient air temperature and precipitation on monthly counts of salmonellosis in four regions of Kazakhstan, Central Asia, in 2000–2010. Epidemiol. Infect. 2014, 142, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Jore, S.; Viljugrein, H.; Brun, E.; Heier, B.T.; Borck, B.; Ethelberg, S.; Hakkinen, M.; Kuusi, M.; Reiersen, J.; Hansson, I.; et al. Trends in Campylobacter incidence in broilers and humans in six European countries, 1997–2007. Prev. Vet. Med. 2010, 93, 33–41. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Assessing the Potential Impacts of Climate Change on Food- and Waterborne Diseases in Europe; ECDC: Stockholm, Sweden, 2012; p. 19. [Google Scholar]
- Nichols, G.; Lane, C.; Asgari, N.; Verlander, N.; Charlett, A.; Charlette, A. Rainfall and outbreaks of drinking water related disease and in England and Wales. J. Water Health 2009, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Beauté, J.; Zucs, P.; de Jong, B. Legionnaires disease in Europe, 2009–2010. Eurosurveillance 2013, 18, 20417. [Google Scholar] [PubMed]
- Paz, S.; Bisharat, N.; Paz, E.; Kidar, O.; Cohen, D. Climate change and the emergence of Vibrio vulnificus disease in Israel. Env. Res 2007, 103, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Gallina, N.; Anneville, O.; Beniston, M. Impacts of extreme air temperatures on cyanobacteria in five deep peri-Alpine lakes. J. Limnol. 2011, 70, 186–196. [Google Scholar] [CrossRef]
- Tryland, I.; Myrmel, M.; Østensvik, Ø.; Wennberg, A.C.; Robertson, L.J. Impact of rainfall on the hygienic quality of blue mussels and water in urban areas in the Inner Oslofjord, Norway. Mar. Pollut. Bull. 2014, 85, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Van Asselt, E.D.; Azambuja, W.; Moretti, A.; Kastelein, P.; de Rijk, T.C.; Stratakou, I.; van der Fels-Klerx, H.J. A Dutch field survey on fungal infection and mycotoxin concentrations in maize. Food Addit. Contam. Part A Chem. Anal. Control Exp. Risk Assess. 2012, 29, 1556–1565. [Google Scholar] [CrossRef] [PubMed]
- WHO. Economic Cost of the Health Impact of Air Pollution in Europe: Clean Air, Health and Wealth; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Meleux, F.; Solmon, F.; Giorgi, F. Increase in summer European ozone amounts due to climate change. Atmos. Environ. 2007, 41, 7577–7587. [Google Scholar] [CrossRef]
- Orru, H.; Andersson, C.; Ebi, K.L.; Langner, J.; Aström, C.; Forsberg, B. Impact of climate change on ozone-related mortality and morbidity in Europe. Eur. Respir. J. 2013, 41, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Katsouyanni, K.; Analitis, A. Investigating the synergistic effects between meteorological variables and air pollutants: Results from the European PHEWE, EUROHEAT and CIRCE projects. Epidemiology 2009, 20, S264–S264. [Google Scholar] [CrossRef]
- Analitis, A.; Katsouyanni, K.; Biggeri, A.; Baccini, M.; McGregor, G.; Michelozzi, P. Temperature effects on mortality: Potential confounding by air pollution and possible interactions within the PHEWE project. Epidemiology 2008, 19, S214. [Google Scholar]
- Lagravinese, R.; Moscone, F.; Tosetti, E.; Lee, H. The impact of air pollution on hospital admissions: Evidence from Italy. Reg. Sci. Urban Econ. 2014, 49, 278–285. [Google Scholar] [CrossRef]
- Almeida, S.P. de; Casimiro, E.; Calheiros, J.; de Almeida, S.P.; Casimiro, E.; Calheiros, J. Short-term association between exposure to ozone and mortality in Oporto, Portugal. Environ. Res. 2011, 111, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Bejot, Y.; Henrotin, J.B.; Osseby, G.V.; Zeller, M.; Cottin, Y.; Giroud, M. Impact of ozone air pollution on ischemic cerebral and cardiac events in Dijon, France. Arch. Cardiovasc. Dis. Suppl. 2011, 3, 93. [Google Scholar] [CrossRef]
- Kalantzi, E.G.; Makris, D.; Duquenne, M.N.; Kaklamani, S.; Stapountzis, H.; Gourgoulianis, K.I. Air pollutants and morbidity of cardiopulmonary diseases in a semi-urban Greek peninsula. Atmos. Environ. 2011, 45, 7121–7126. [Google Scholar] [CrossRef]
- De Sario, M.; Katsouyanni, K.; Michelozzi, P. Climate change, extreme weather events, air pollution and respiratory health in Europe. Eur. Respir. J. 2013, 42, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, S.; Denic, L.M.; Milic, N.; Spiric, V.T.; Gledovic, Z.; Marinkovic, J. The effects of air pollution on the respiratory and cardiovascular system: A systematic review. Med. Data 2011, 3, 57–62. [Google Scholar]
- Faustini, A.; Stafoggia, M.; Berti, G.; Bisanti, L.; Chiusolo, M.; Cernigliaro, A.; Mallone, S.; Primerano, R.; Scarnato, C.; Simonato, L.; et al. The relationship between ambient particulate matter and respiratory mortality: A multi-city study in Italy. Eur. Respir. J. 2011, 38, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Maté, T.; Guaita, R.; Pichiule, M.; Linares, C.; Díaz, J. Short-term effect of fine particulate matter (PM2.5) on daily mortality due to diseases of the circulatory system in Madrid (Spain). Sci. Total Environ. 2010, 408, 5750–5757. [Google Scholar]
- Neuberger, M.; Rabczenko, D.; Moshammer, H. Extended effects of air pollution on cardiopulmonary mortality in Vienna. Atmos. Environ. 2007, 41, 8549–8556. [Google Scholar] [CrossRef]
- Nuvolone, D.; Balzi, D.; Pepe, P.; Chini, M.; Scala, D.; Giovannini, F.; Cipriani, F.; Barchielli, A. Ozone short-term exposure and acute coronary events: A multicities study in Tuscany (Italy). Environ. Res. 2013, 126, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Joad, J.; Benes, I.; Dostal, M.; Sram, R.J.; Hertz-Picciotto, I. Ambient nitrogen oxides exposure and early childhood respiratory illnesses. Environ. Int. 2012, 39, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Altuğ, H.; Gaga, E.O.; Döğeroğlu, T.; Brunekreef, B.; Hoek, G.; van Doorn, W. Effects of ambient air pollution on respiratory tract complaints and airway inflammation in primary school children. Sci. Total Environ. 2014, 479–480, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Coneus, K.; Spiess, C.K. Pollution exposure and child health: Evidence for infants and toddlers in Germany. J. Health Econ. 2012, 31, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Giorgis-Allemand, L.; Bernard, C.; Aguilera, I.; Andersen, A.M.; Ballester, F.; Beelen, R.M.; Chatzi, L.; Cirach, M.; Danileviciute, A.; et al. Ambient air pollution and low birthweight: A European cohort study (ESCAPE). Lancet Respir. Med. 2013, 1, 695–704. [Google Scholar] [CrossRef]
- Amann, M.; Klimont, Z.; Wagner, F. Regional and global emissions of air pollutants: Recent trends and future scenarios. Annu. Rev. Environ. Resour. 2013, 38, 31–55. [Google Scholar] [CrossRef]
- Scheinhardt, S.; Spindler, G.; Leise, S.; Müller, K.; Iinuma, Y.; Zimmermann, F.; Matschullat, J.; Herrmann, H. Comprehensive chemical characterisation of size-segregated PM10 in Dresden and estimation of changes due to global warming. Atmos. Environ. 2013, 75, 365–373. [Google Scholar] [CrossRef]
- Makkonen, U.; Hellén, H.; Anttila, P.; Ferm, M. Size distribution and chemical composition of airborne particles in south-eastern Finland during different seasons and wildfire episodes in 2006. Sci. Total Environ. 2010, 408, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Fattore, E.; Paiano, V.; Borgini, A.; Tittarelli, A.; Bertoldi, M.; Crosignani, P.; Fanelli, R. Human health risk in relation to air quality in two municipalities in an industrialized area of Northern Italy. Environ. Res. 2011, 111, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Halonen, J.I.; Lanki, T.; Yli-Tuomi, T.; Kulmala, M.; Tiittanen, P.; Pekkanen, J. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax 2008, 63, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, K.; Siponen, T.; Salonen, I.; Yli-Tuomi, T.; Aurela, M.; Dufva, H.; Hillamo, R.; Linkola, E.; Pekkanen, J.; Pennanen, A.; et al. Low-level exposure to ambient particulate matter is associated with systemic inflammation in ischemic heart disease patients. Environ. Res. 2012, 116, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Koton, S.; Molshatzki, N.; Yuval; Myers, V.; Broday, D.M.; Drory, Y.; Steinberg, D.M.; Gerber, Y. Cumulative exposure to particulate matter air pollution and long-term post-myocardial infarction outcomes. Prev. Med. (Baltim.) 2013, 57, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Leitte, A.M.; Petrescu, C.; Franck, U.; Richter, M.; Suciu, O.; Ionovici, R.; Herbarth, O.; Schlink, U. Respiratory health, effects of ambient air pollution and its modification by air humidity in Drobeta-Turnu Severin, Romania. Sci. Total Environ. 2009, 407, 4004–4011. [Google Scholar] [CrossRef] [PubMed]
- Linares, C.; Díaz, J. Short-term effect of concentrations of fine particulate matter on hospital admissions due to cardiovascular and respiratory causes among the over-75 age group in Madrid, Spain. Public Health 2010, 124, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Naess, O.; Nafstad, P.; Aamodt, G.; Claussen, B.; Rosland, P. Relation between concentration of air pollution and cause-specific mortality: Four-year exposures to nitrogen dioxide and particulate matter pollutants in 470 neighborhoods in Oslo, Norway. Am. J. Epidemiol. 2007, 165, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Nuvolone, D.; Balzi, D.; Chini, M.; Scala, D.; Giovannini, F.; Barchielli, A. Short-term association between ambient air pollution and risk of hospitalization for acute myocardial infarction: Results of the cardiovascular risk and air pollution in Tuscany (RISCAT) study. Am. J. Epidemiol. 2011, 174, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Beelen, R.; Stafoggia, M.; Raaschou-Nielsen, O.; Andersen, Z.J.; Hoffmann, B.; Fischer, P.; Houthuijs, D.; Nieuwenhuijsen, M.; Weinmayr, G.; et al. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: Results from the ESCAPE and TRANSPHORM projects. Environ. Int. 2014, 66, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Zauli Sajani, S.; Alessandrini, E.; Marchesi, S.; Lauriola, P. Are day-to-day variations of airborne particles associated with emergency ambulance dispatches? Int. J. Occup. Environ. Health 2014, 20, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.; Truckner, R.; Weber, R.; Peden, D. Climate change and allergic disease. Clin. Rev. Allergy Immunol. 2008, 122, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Bielory, L.; Lyons, K.; Goldberg, R. Climate change and allergic disease. Curr. Allergy Asthma Rep. 2012, 12, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Weber, R. Impact of climate change on aeroallergens. Ann. Allergy Asthma Immunol. 2012, 108, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; D’Amato, G.; Ayres, J.G.; Galan, C.; Forastiere, F.; Forsberg, B.; Gerritsen, J.; Nunes, C.; Behrendt, H.; Akdis, C.; et al. Projections of the effects of climate change on allergic asthma: The contribution of aerobiology. Allergy 2010, 65, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Cecchi, L.; D’Amato, M.; Annesi-Maesano, I. Climate change and respiratory diseases. Eur. Respir. Rev. 2014, 23, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Lotvall, J.; Simoens, S.; Subramanian, S.; Church, M. Economic burden of inadequate management of allergic diseases in the European Union: A GA2LEN review. Eur. J. Allergy Clin. Immunol. 2014, 69, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Tosca, M.A.; Ruffoni, S.; Canonica, G.W.; Ciprandi, G. Asthma exacerbation in children: Relationship among pollens, weather, and air pollution. Allergol. Immunopathol. 2014, 42, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Matyasovszky, I.; Makra, L.; Bálint, B.; Guba, Z.; Sümeghy, Z. Multivariate analysis of respiratory problems and their connection with meteorological parameters and the main biological and chemical air pollutants. Atmos. Environ. 2011, 45, 4152–4159. [Google Scholar] [CrossRef]
- Ziello, C.; Sparks, T.H.; Estrella, N.; Belmonte, J.; Bergmann, K.C.; Bucher, E.; Brighetti, M.A.; Damialis, A.; Detandt, M.; Galán, C.; et al. Changes to Airborne Pollen Counts across Europe. PLoS ONE 2012, 7, e34076. [Google Scholar] [CrossRef] [PubMed]
- Grinn-Gofroń, A.; Rapiejko, P. Occurrence of Cladosporium spp. and Alternaria spp. spores in Western, Northern and Central-Eastern Poland in 2004–2006 and relation to some meteorological factors. Atmos. Res. 2009, 93, 747–758. [Google Scholar]
- Makra, L.; Matyasovszky, I.; Deák, Á.J. Trends in the characteristics of allergenic pollen circulation in central Europe based on the example of Szeged, Hungary. Atmos. Environ. 2011, 45, 6010–6018. [Google Scholar] [CrossRef]
- Cariñanos, P.; Alcázar, P.; Galán, C.; Domínguez, E. Environmental behaviour of airborne Amaranthaceae pollen in the southern part of the Iberian Peninsula, and its role in future climate scenarios. Sci. Total Environ. 2014, 470–471, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Deák, Á.J.; Makra, L.; Matyasovszky, I.; Csépe, Z.; Muladi, B. Climate sensitivity of allergenic taxa in Central Europe associated with new climate change related forces. Sci. Total Environ. 2013, 442, 36–47. [Google Scholar]
- Damialis, A.; Halley, J.M.; Gioulekas, D.; Vokou, D. Long-term trends in atmospheric pollen levels in the city of Thessaloniki, Greece. Atmos. Environ. 2007, 41, 7011–7021. [Google Scholar] [CrossRef]
- Sousa, S.I.V.; Martins, F.G.; Pereira, M.C.; Alvim-Ferraz, M.C.M.; Ribeiro, H.; Oliveira, M.; Abreu, I. Influence of atmospheric ozone, PM10 and meteorological factors on the concentration of airborne pollen and fungal spores. Atmos. Environ. 2008, 42, 7452–7464. [Google Scholar] [CrossRef]
- Ridolo, E.; Albertini, R.; Giordano, D.; Soliani, L.; Usberti, I.; Dall’Aglio, P. Respiratory Allergy and Airborne Pollen Concentrations in Italy from 1992 to 2004. J. Allergy Clin. Immunol. 2007, 119, S100. [Google Scholar] [CrossRef]
- Bedada, G.B.; Heinrich, J.; Götschi, T.; Downs, S.H.; Forsberg, B.; Jarvis, D.; Luczynska, C.; Soon, A.; Sunyer, J.; Toren, K.; et al. Urban background particulate matter and allergic sensitization in adults of ECRHS II. Int. J. Hyg. Environ. Health 2007, 210, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Feo Brito, F.; Mur Gimeno, P.; Martinez, C.; Tobias, A.; Suarez, L.; Guerra, F.; Borja, J.M.; Alonso, A.M. Air pollution and seasonal asthma during the pollen season. A cohort study in Puertollano and Ciudad Real (Spain). Allergy 2007, 62, 1152–1157. [Google Scholar] [PubMed]
- Albertine, J.M.; Manning, W.J.; Dacosta, M.; Stinson, K.A.; Muilenberg, M.L.; Rogers, C.A. Projected carbon dioxide to increase grass pollen and allergen exposure despite higher ozone levels. PLoS ONE 2014, 9, e111712. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.; Ziska, L.; Frenz, D.; Gebhard, D.; Straka, J. Increasing Amb a 1 content in common ragweed (Ambrosia artemisiifolia) pollen as a function of rising atmospheric CO2 concentration. Funct. Plant Biol. 2005, 32, 667–670. [Google Scholar] [CrossRef]
- El Kelish, A.; Zhao, F.; Heller, W.; Durner, J.; Winkler, J.B.; Behrendt, H.; Traidl-Hoffmann, C.; Horres, R.; Pfeifer, M.; Frank, U.; et al. Ragweed (Ambrosia artemisiifolia) pollen allergenicity: SuperSAGE transcriptomic analysis upon elevated CO2 and drought stress. BMC Plant Biol. 2014, 14, 176. [Google Scholar] [CrossRef] [PubMed]
- Arnedo-Pena, A.; García-Marcos, L.; Urueña, I.C.; Monge, R.B.; Suárez-Varela, M.M.; Canflanca, I.M.; Garrido, J.B.; Quirós, A.B.; López-Silvarrey Varela, Á.; Hernández, G.G.; et al. Air pollution and recent symptoms of asthma, allergic rhinitis, and atopic eczema in schoolchildren aged between 6 and 7 Years. Arch. Bronconeumol. (Engl. Ed.) 2009, 45, 224–229. [Google Scholar] [CrossRef]
- Makra, L.; Matyasovszky, I.; Bálint, B. Association of allergic asthma emergency room visits with the main biological and chemical air pollutants. Sci. Total Environ. 2012, 432, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Ayres-Sampaio, D.; Teodoro, A.C.; Sillero, N.; Santos, C.; Fonseca, J.; Freitas, A. An investigation of the environmental determinants of asthma hospitalizations: An applied spatial approach. Appl. Geogr. 2014, 47, 10–19. [Google Scholar] [CrossRef]
- Berna Dursun, A.; Çelik, G.E.; Alan, S.; Münevver Pinar, N.; Mungan, D.; Misirligil, Z. Regional pollen load: Effect on sensitisation and clinical presentation of seasonal allergic rhinitis in patients living in Ankara, Turkey. Allergol. Immunopathol. (Madr.) 2008, 36, 371–378. [Google Scholar] [CrossRef]
- Cirera, L.; García-Marcos, L.; Giménez, J.; Moreno-Grau, S.; Tobías, A.; Pérez-Fernández, V.; Elvira-Rendeles, B.; Guillén, J.J.; Navarro, C. Daily effects of air pollutants and pollen types on asthma and COPD hospital emergency visits in the industrial and Mediterranean Spanish city of Cartagena. Allergol. Immunopathol. (Madr.) 2012, 40, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ruffoni, S.; Passalacqua, G.; Ricciardolo, F.; Furgani, A.; Negrini, A.C.; De Amici, M.; Ciprandi, G. A 10-year survey on asthma exacerbations: Relationships among emergency medicine calls, pollens, weather, and air pollution. Rev. Fr. Allergol. 2013, 53, 569–575. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolf, T.; Lyne, K.; Martinez, G.S.; Kendrovski, V. The Health Effects of Climate Change in the WHO European Region. Climate 2015, 3, 901-936. https://doi.org/10.3390/cli3040901
Wolf T, Lyne K, Martinez GS, Kendrovski V. The Health Effects of Climate Change in the WHO European Region. Climate. 2015; 3(4):901-936. https://doi.org/10.3390/cli3040901
Chicago/Turabian StyleWolf, Tanja, Katrina Lyne, Gerardo Sanchez Martinez, and Vladimir Kendrovski. 2015. "The Health Effects of Climate Change in the WHO European Region" Climate 3, no. 4: 901-936. https://doi.org/10.3390/cli3040901
APA StyleWolf, T., Lyne, K., Martinez, G. S., & Kendrovski, V. (2015). The Health Effects of Climate Change in the WHO European Region. Climate, 3(4), 901-936. https://doi.org/10.3390/cli3040901





