Recent Advances in Understanding the Impact of Environmental Heat Stress on Sheep Production and Reproductive Performance: A Subtropical Climate Perspective
Abstract
1. Introduction
2. Methodology
Literature Search
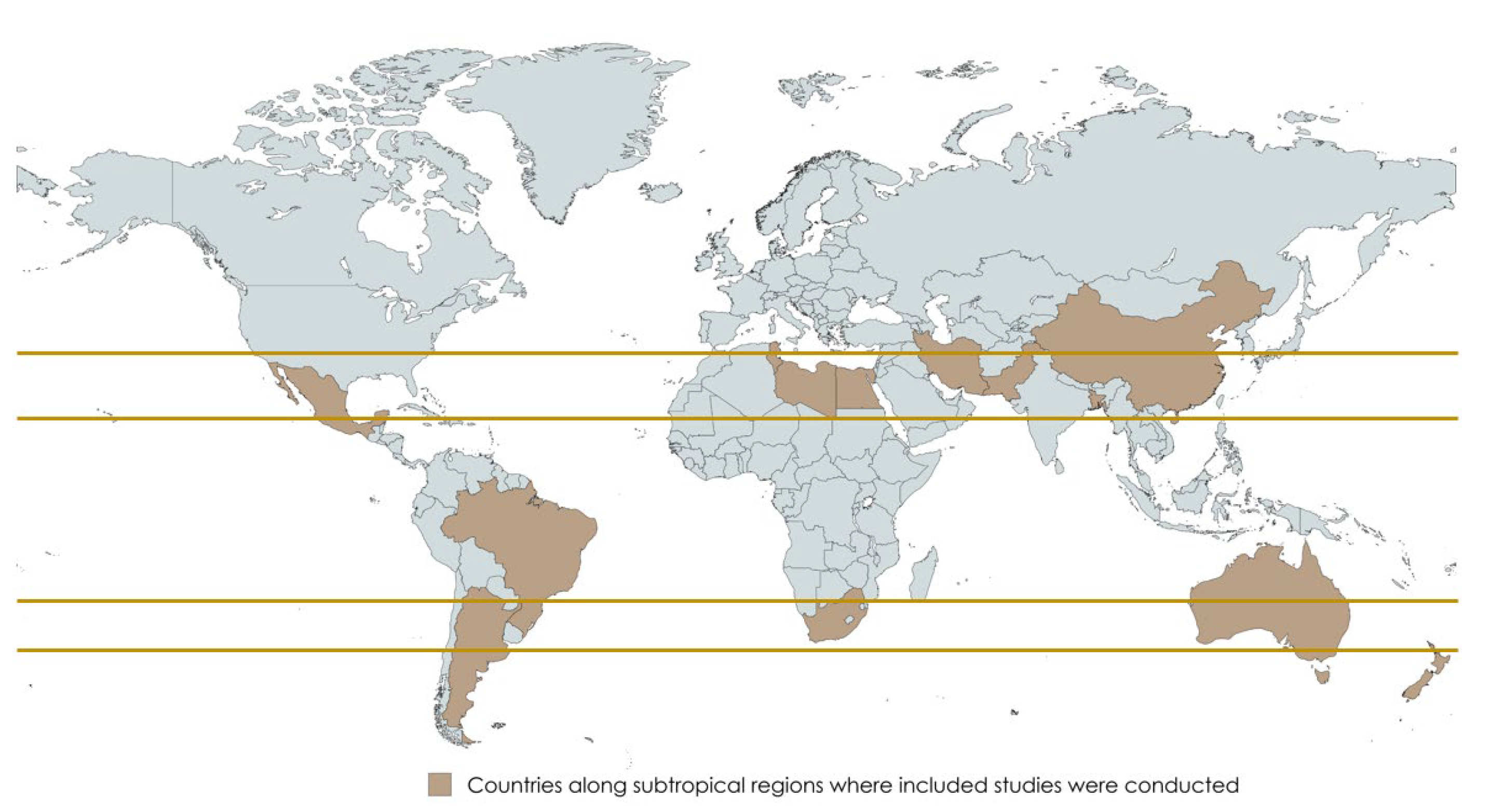
3. The Geographical Region of the Included Studies: The Subtropics
4. Overview of Sustainable Development Goals 1, 2, and 13
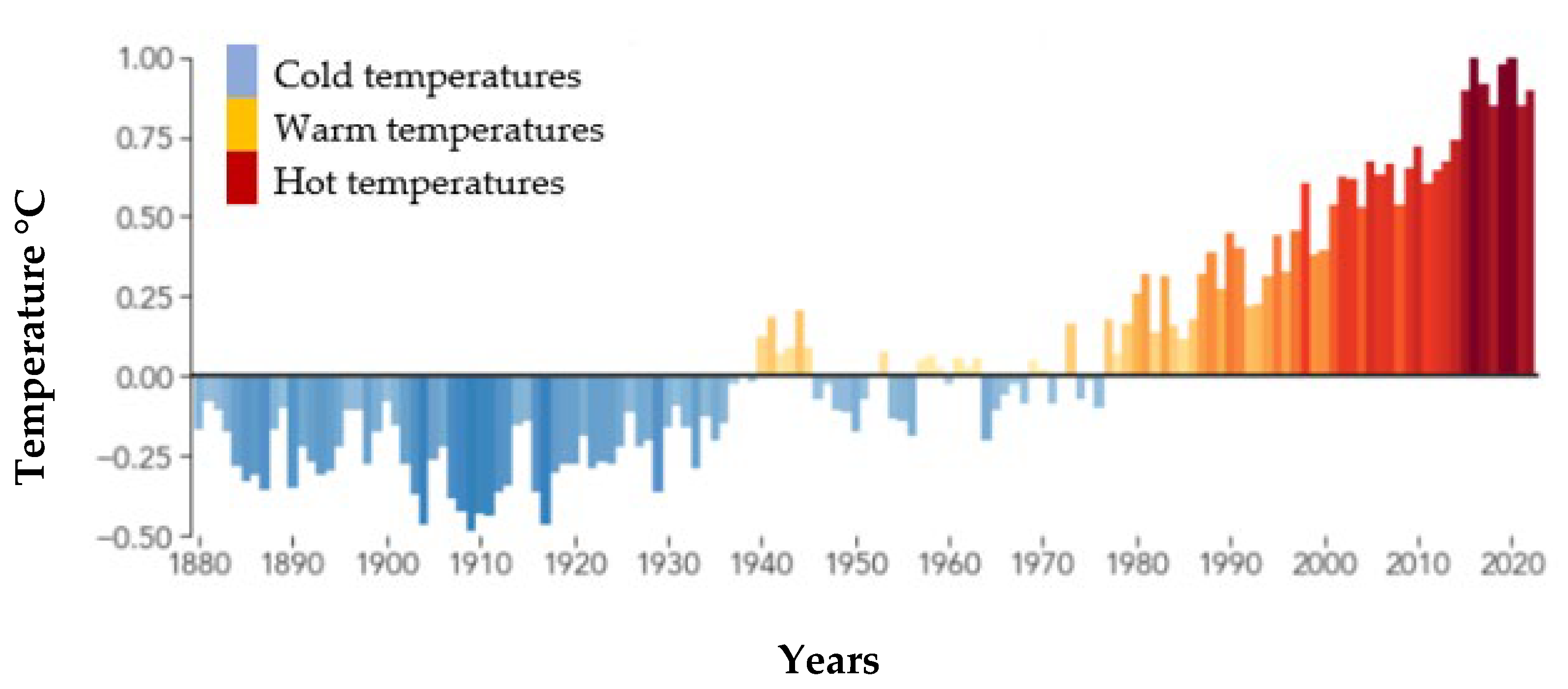
5. A Synopsis of Heat Stress, with an Emphasis on Temperature and Humidity
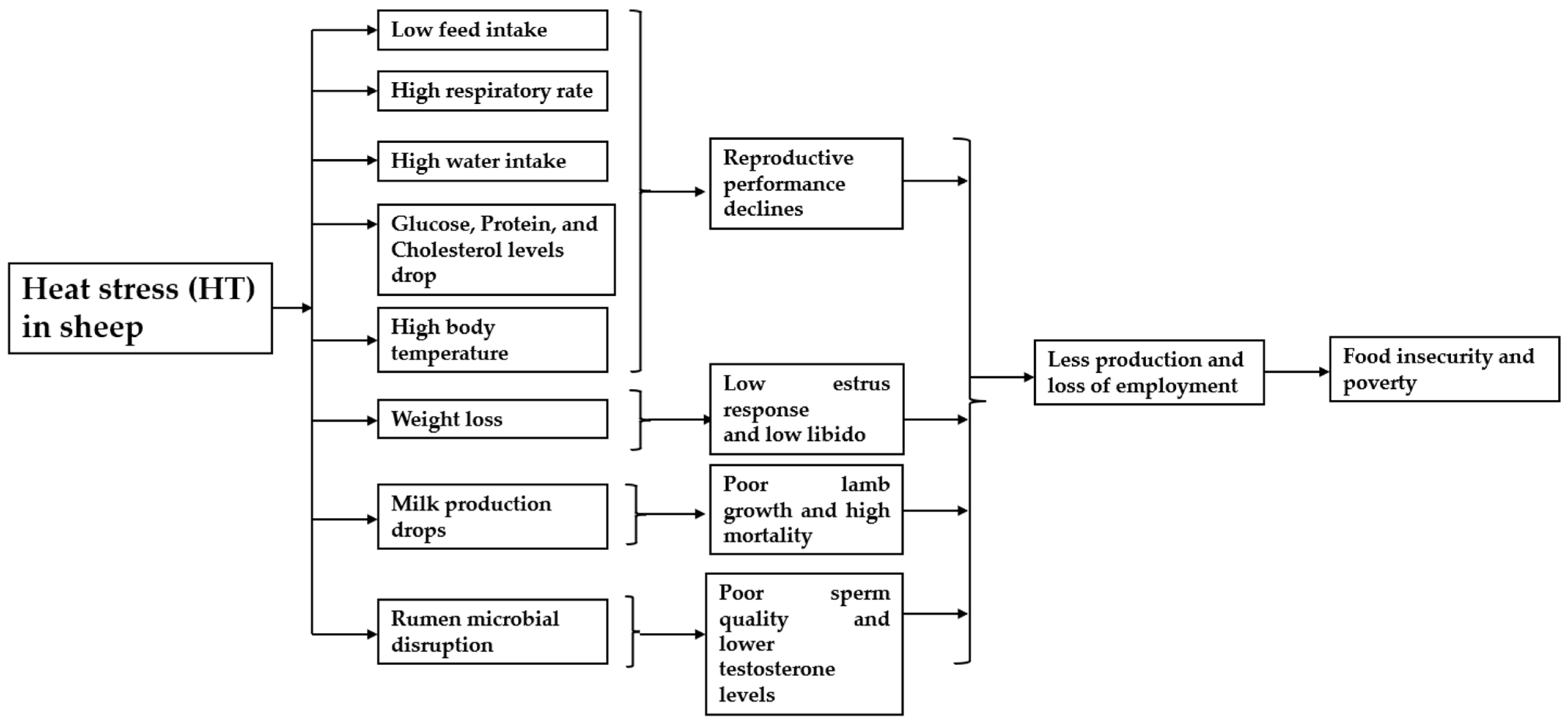
6. The Threat Heat Stress Poses to Food Security and Poverty Alleviation
7. Effect of Heat Stress on Sheep Growth Performance
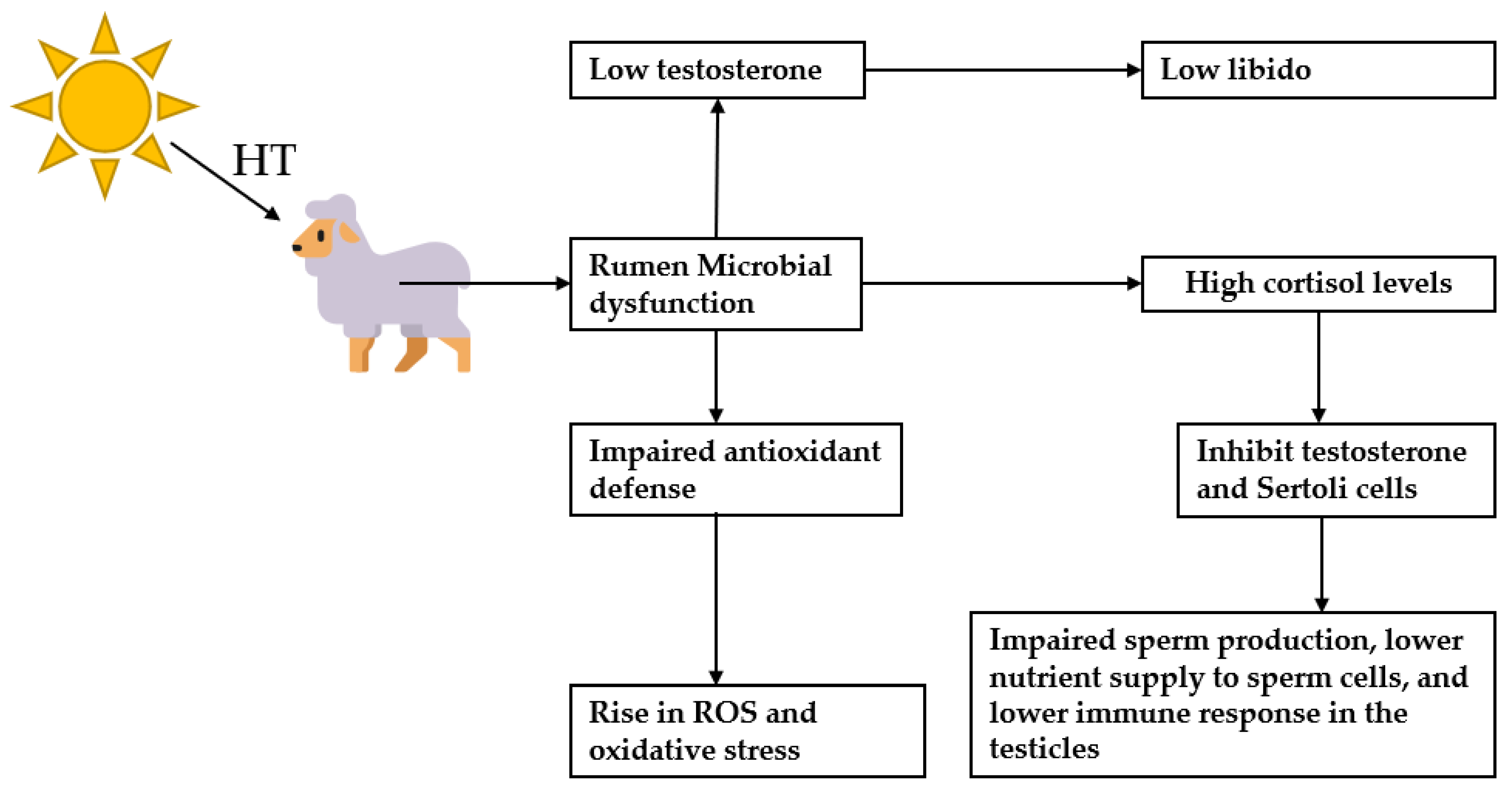
8. The Influence of the Microbiota–Testis Interaction and Environmental Heat Stress on Sheep Fertility
| Breed | Effect | Reference |
|---|---|---|
| Pelibuey ewes | Reduced corpus luteum functionality | [42] |
| Australian Merino ewes | Oocyte and embryo quality were compromised | [11] |
| Unidentified sheep | Higher embryo mortality | [10] |
| Unidentified sheep | Reduced placental and fetal weight | [10] |
| Unidentified sheep | A 1.13 °C increase in body temperature in pregnant ewes | [10] |
| Unidentified sheep | Increased scrotal temperature, reduced sperm motility, decreased testicular weight and seminiferous tubule diameter, and reduced sperm count | [64] |
| Hu and Wugu crosses | Sperm motility and transcriptome were downregulated and greatly influenced | [66] |
| Australian Merino | Increased sperm abnormalities, including tailless sperm and proximal droplets | [67] |
9. Physiological Mechanism for Adaptation to Heat Stress
| THI Spectrum and Image | Humidity (%) | Temperature (°C) | Breaths/Minute | Panting Score | Stress Level | Description |
|---|---|---|---|---|---|---|
 | 20–29 | <23 | 40–60 | 0 | No stress | No panting, normal respiratory rate |
| 30–40 | 24–25.5 | 60–80 | 1 | Mild stress | Slight panting, mouth closed, and rapid chest movement | |
| 30–40 | 24–25.5 | 60–80 | 1.5 | Mild stress | Mouth still closed, with fast chest movement | |
| 41–59 | 26–28 | Between >120 | 2 | Moderate | Rapid panting, with the mouth opened slightly | |
| 60–70 | >30 | >120–200 | 3 | Severe stress | Mouth opened, neck extended, head held up, tongue extended, and rapid panting rate | |
| >70 | >35 | >200 | 4 | Extreme stress | Open mouth with tongue fully extended for a longer period, head lowered, and deeper breathing may occur, with a reduced panting rate for a shorter period |
10. Challenges Associated with Rural Farming and Heat Stress
11. Prospective Solutions
11.1. Shelter, Shades, and Afforestation
11.2. The Use of Antioxidants to Ameliorate Heat Stress in Sheep
| Breed | Antioxidant Used | Type of Supplementation | Effect | Reference |
|---|---|---|---|---|
| Ossimi rams | L-carnitine | - | Ameliorates testicular hemodynamic disruption | [81] |
| Ossimi rams | Zinc sulphate | Dietary supplementation | Increases testicular volume, testosterone levels, and semen quality | [82] |
| Merino × Poll Dorset | VitE + Se; 100 IU vitamin E and 1.20 mg Se/kg DM | Dietary supplementation | Significantly decreases respiration rate and rectal temperature | [83] |
| Farafra | Astaxanthin (Keto antioxidants) | Oral administration (0.25 mg) | Improves estrus response, conception, and twinning rate | [84] |
| Barbarine | 100 µL/day/animal of thyme essential oil | Oral supplementation | Does not increase fertility | [85] |
11.3. Studying Candidate Genes Associated with Heat Stress
| Breed | Candidate Genes | Function | Reference |
|---|---|---|---|
| Iranian sheep | SIK2, FER, ATP1A1, CDK5RAP3, and TLR4 | Associated with heat stress tolerance | [87] |
| CD109, CR2, EOMES, and MARCHF1 | Promote immune response under arid and warm conditions | ||
| ZEP1, PLCB1, and PDGFD | Induce response to drought stress and adaptation | ||
| HTR4, TRHDE, and ALDH1A3 | Induce a response to heat stress by controlling digestive metabolism | ||
| Barki and Aboudeleik | CAST, LEP, MYLK4, MEF2B, STAT5A, TRPV1, HSP90AB1, HSPB6, HSF1, ST1P1, and ATP1A1 | Correlated with growth and heat tolerance | [88] |
| Sarda sheep | FCGR1A, MDH1, UGP2, MYO1G, and HSPB3 | Associated with heat tolerance | [89] |
| Turban Black sheep | SYCP2, TDRD9, BRDT, CEP120, and BRCA1 | Protect spermatogenesis for normal production of sperm after heat stress | [90] |
11.4. Adopting Climate-Smart Agricultural Practices
| Adaptative Response to Heat Stress | Indigenous Sheep | Exotic Sheep | Reference |
|---|---|---|---|
| Morphological response | |||
| Body size | Small body (±39.1 kg) (e.g., Zulu sheep) and slow growth | Large bodies (±50 kg) (e.g., Dohne Merino) and high growth performance | [96] |
| Body shape | Long legged | Short legs | |
| Coat and skin color | Multicolored | Pure color (e.g., white Dorper) | |
| Hair or wool | Hair (e.g., Zulu sheep) | Fine wool | [96] |
| Mobility | Nomadic | Stationary, sometimes transhuman | [96] |
| Fat storage | Fat-tailed | Thin tail | |
| Behavioral response | |||
| Food intake | Lower feed intake, consumed in small portions more frequently | High feed intake, in large portions | [97] |
| Quality of feed intake | Able to utilize feed with low nutritive value | Require balanced nutritional value | |
| Water intake | Lower water intake and can walk long distances in search of water | High water intake and inability to walk long distances in search of water sources | |
| Physiological response | |||
| Heart rate | - | >107.79 beats/min, Merino sheep | [98] |
| Respiratory rate | Increased by only 84% from a cool morning (18.9 °C) to a warm afternoon (30.2 °C) (e.g., Namaqua Afrikaner) | Spikes by 181% from cool morning (18.9 °C, 203%, and 278%) to warm afternoon (30.2 °C) (e.g., Dohne Merino, Dormer, and Merino) | [99] |
| Rectal temperature | <38 °C in the morning and ±39 °C afternoon (e.g., Namaqua Afrikaner) | ±38.8 °C in the morning and ±39.3 °C in the afternoon (e.g., Dohne Merino, Dormer, and Merino) | [99] |
| Blood biochemical response | |||
| Red blood cells (million/cubic mm) | 10.28 | - | [100] |
| Hemoglobin (g/percent) | 9.80 | - | [100] |
| White blood cells (thousand/cubic mm) | 9.03 | 9.12 | [100] |
| Packed cell volume (%) | 31.47 | - | [100] |
| Heat stress implications | Tolerate heat stress | Need more prevention measures to mitigate heat stress | - |
12. Research Gaps
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Ed.) Building Climate Resilience for Food Security and Nutrition; The State of Food Security and Nutrition in the world; FAO: Rome, Italy, 2018; ISBN 978-92-5-130571-3. [Google Scholar]
- THE 17 GOALS|Sustainable Development. Available online: https://sdgs.un.org/goals (accessed on 31 May 2025).
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 1st ed.; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Hyder, I.; Ravi Kanth Reddy, P.; Raju, J.; Manjari, P.; Srinivasa Prasad, C.; Aswani Kumar, K.; Sejian, V. Alteration in Rumen Functions and Diet Digestibility During Heat Stress in Sheep. In Sheep Production Adapting to Climate Change; Sejian, V., Bhatta, R., Gaughan, J., Malik, P.K., Naqvi, S.M.K., Lal, R., Eds.; Springer: Singapore, 2017; pp. 235–265. ISBN 978-981-10-4713-8. [Google Scholar]
- Ayanlade, A.; Oluwaranti, A.; Ayanlade, O.S.; Borderon, M.; Sterly, H.; Sakdapolrak, P.; Jegede, M.O.; Weldemariam, L.F.; Ayinde, A.F.O. Extreme Climate Events in Sub-Saharan Africa: A Call for Improving Agricultural Technology Transfer to Enhance Adaptive Capacity. Clim. Serv. 2022, 27, 100311. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.-Z.; Liu, B.; Shen, C.; Tu, J.; Wang, S.; Lei, R.; Peng, S.; Xiao, X.; Zhao, Y.; et al. The Effects and Mechanisms of Heat Stress on Mammalian Oocyte and Embryo Development. J. Therm. Biol. 2024, 124, 103927. [Google Scholar] [CrossRef]
- Song, J.; Tong, G.; Chao, J.; Chung, J.; Zhang, M.; Lin, W.; Zhang, T.; Bentler, P.M.; Zhu, W. Data Driven Pathway Analysis and Forecast of Global Warming and Sea Level Rise. Sci. Rep. 2023, 13, 5536. [Google Scholar] [CrossRef]
- Mavule, B.S.; Sarti, F.M.; Lasagna, E.; Kunene, N.W. Morphological Differentiation amongst Zulu Sheep Populations in KwaZulu-Natal, South Africa, as Revealed by Multivariate Analysis. Small Rumin. Res. 2016, 140, 50–56. [Google Scholar] [CrossRef]
- Alhidary, I.A.; Shini, S.; Al Jassim, R.A.M.; Gaughan, J.B. Physiological Responses of Australian Merino Wethers Exposed to High Heat Load. J. Anim. Sci. 2012, 90, 212–220. [Google Scholar] [CrossRef]
- Romo-Barron, C.B.; Diaz, D.; Portillo-Loera, J.J.; Romo-Rubio, J.A.; Jimenez-Trejo, F.; Montero-Pardo, A. Impact of Heat Stress on the Reproductive Performance and Physiology of Ewes: A Systematic Review and Meta-Analyses. Int. J. Biometeorol. 2019, 63, 949–962. [Google Scholar] [CrossRef]
- Narayan, E.; Sawyer, G.; Parisella, S. Faecal Glucocorticoid Metabolites and Body Temperature in Australian Merino Ewes (Ovis aries) during Summer Artificial Insemination (AI) Program. PLoS ONE 2018, 13, e0191961. [Google Scholar] [CrossRef] [PubMed]
- McManus, C.M.; Lucci, C.M.; Maranhão, A.Q.; Pimentel, D.; Pimentel, F.; Rezende Paiva, S. Response to Heat Stress for Small Ruminants: Physiological and Genetic Aspects. Livest. Sci. 2022, 263, 105028. [Google Scholar] [CrossRef]
- Erasmus, L.M.; Van Marle-Köster, E. Heat Stress in Dairy Cows: A Review of Abiotic and Biotic Factors, with Reference to the Subtropics. S. Afr. J. Anim. Sci. 2025, 55, 10–23. [Google Scholar] [CrossRef]
- Brivio, F.; Zurmühl, M.; Grignolio, S.; Von Hardenberg, J.; Apollonio, M.; Ciuti, S. Forecasting the Response to Global Warming in a Heat-Sensitive Species. Sci. Rep. 2019, 9, 3048. [Google Scholar] [CrossRef]
- García-Casillas, A.C.; Prado-Rebolledo, O.F.; Carrillo-Díaz, M.I.; Zepeda-Batista, J.L.; Barajas-Saucedo, C.E.; Hernández-Rivera, J.A. Reproductive Activity of Socorro Island Merino Ewes and Their Crosses with Pelibuey under Heat Stress Conditions. Animals 2024, 14, 1405. [Google Scholar] [CrossRef] [PubMed]
- Gastelum-Delgado, M.A.; Avendaño-Reyes, L.; Álvarez-Valenzuela, F.D.; Correa-Calderón, A.; Meza-Herrera, C.A.; Mellado, M.; Macías-Cruz, U. Conducta estral circanual en ovejas Pelibuey bajo condiciones áridas del noroeste de México. Rev. Mex. Cienc. Pecu. 2015, 6, 109–118. [Google Scholar] [CrossRef][Green Version]
- Ben Moula, A.; Moussafir, Z.; Hamidallah, N.; El Amiri, B. Heat Stress and Ram Semen Production and Preservation: Exploring Impacts and Effective Strategies. J. Therm. Biol. 2024, 119, 103794. [Google Scholar] [CrossRef]
- Mazlishah, M.S.H.; Fauzi, N.M.; Nor, M.F.F.M.; Hashim, N.H. Influence of Management Systems on Severity of Heat Stress and Reproductive Performance of Rams in the Tropics—A Review. Ann. Anim. Sci. 2024, 24, 1081–1092. [Google Scholar] [CrossRef]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Increases in Extreme Heat Stress in Domesticated Livestock Species during the Twenty-first Century. Glob. Change Biol. 2021, 27, 5762–5772. [Google Scholar] [CrossRef]
- Wang, Q.-J.; Yi, H.-M.; Ou, J.-Y.; Wang, R.; Wang, M.-M.; Wang, P.-H.; He, X.-L.; Tang, W.-H.; Chen, J.-H.; Yu, Y.; et al. Environmental Heat Stress Decreases Sperm Motility by Disrupting the Diurnal Rhythms of Rumen Microbes and Metabolites in Hu Rams. Int. J. Mol. Sci. 2024, 25, 11161. [Google Scholar] [CrossRef]
- Eccles, R.; Zhang, H.; Hamilton, D. A Review of the Effects of Climate Change on Riverine Flooding in Subtropical and Tropical Regions. J. Water Clim. Change 2019, 10, 687–707. [Google Scholar] [CrossRef]
- Cherchi, A.; Ambrizzi, T.; Behera, S.; Freitas, A.C.V.; Morioka, Y.; Zhou, T. The Response of Subtropical Highs to Climate Change. Curr. Clim. Change Rep. 2018, 4, 371–382. [Google Scholar] [CrossRef]
- Ngcobo, J.N.; Nephawe, K.A.; Maqhashu, A.; Nedambale, T.L. Seasonal Variations in Semen Parameters of Zulu Rams Preserved at 10 °C for 72 H During Breeding and Non-Breeding Season. Am. J. Anim. Vet. Sci. 2020, 15, 226–239. [Google Scholar] [CrossRef]
- Kumar, S.; Magotra, A.; Kumar, N.; Bangar, Y.C.; Dahiya, S.P. Physiological Responses of Munjal Sheep to Variations in Temperature Humidity Index in Subtropical Climate. Trop. Anim. Health Prod. 2025, 57, 163. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture: Trends and Challenges; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; ISBN 978-92-5-109551-5. [Google Scholar]
- Poverty and Equity Briefs. Available online: https://www.worldbank.org/en/topic/poverty/publication/poverty-and-equity-briefs (accessed on 2 April 2025).
- Moyer, J.D.; Hedden, S. Are We on the Right Path to Achieve the Sustainable Development Goals? World Dev. 2020, 127, 104749. [Google Scholar] [CrossRef]
- Atangana, E. With the Continuing Increase in Sub-Saharan African Countries, Will Sustainable Development of Goal 1 Ever Be Achieved by 2030? Sustainability 2022, 14, 10304. [Google Scholar] [CrossRef]
- Nhemachena, C.; Nhamo, L.; Matchaya, G.; Nhemachena, C.R.; Muchara, B.; Karuaihe, S.T.; Mpandeli, S. Climate Change Impacts on Water and Agriculture Sectors in Southern Africa: Threats and Opportunities for Sustainable Development. Water 2020, 12, 2673. [Google Scholar] [CrossRef]
- Kunene, N.W.; Bezuidenhout, C.C.; Nsahlai, I.V. Genetic and Phenotypic Diversity in Zulu Sheep Populations: Implications for Exploitation and Conservation. Small Rumin. Res. 2009, 84, 100–107. [Google Scholar] [CrossRef]
- Dannevig, H.; Korsbrekke, M.H.; Hovelsrud, G.K. Advancements of Sustainable Development Goals in Co-Production for Climate Change Adaptation Research. Clim. Risk Manag. 2022, 36, 100438. [Google Scholar] [CrossRef]
- Natamba, L.; Zhang, W.; Zhang, J.; Zhao, X. Climate Change Causing Food Insecurity in East Africa: Traditional and Non-Traditional Strategies to Solve the Problem. Appl. Ecol. Env. Res. 2018, 16, 2233–2254. [Google Scholar] [CrossRef]
- Millington, N.; Scheba, S. Day Zero and The Infrastructures of Climate Change: Water Governance, Inequality, and Infrastructural Politics in Cape Town’s Water Crisis. Int. J. Urban. Reg. Res. 2021, 45, 116–132. [Google Scholar] [CrossRef]
- Naqvi, S.M.K.; Sejian, V. Global Climate Change: Role of Livestock. Asian J. Agric. Sci. 2011, 3, 19–25. [Google Scholar]
- Igbal, M.R.; Iqbaal, U.B.; Kishore, R.; Magiri, R.B. The Effects of Climate Change on Animal Production in Fiji. J. Agric. Sci. 2022, 14, 191. [Google Scholar] [CrossRef]
- Goma, A.A.; Phillips, C.J.C. ‘Can They Take the Heat?’—The Egyptian Climate and Its Effects on Livestock. Animals 2022, 12, 1937. [Google Scholar] [CrossRef]
- Van Wettere, W.H.E.J.; Kind, K.L.; Gatford, K.L.; Swinbourne, A.M.; Leu, S.T.; Hayman, P.T.; Kelly, J.M.; Weaver, A.C.; Kleemann, D.O.; Walker, S.K. Review of the Impact of Heat Stress on Reproductive Performance of Sheep. J. Anim. Sci. Biotechnol. 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Binuni Rebez, E.; Sejian, V.; Silpa, M.V.; Kalaignazhal, G.; Devaraj, C.; Nikhil, K.T.; Ninan, J.; Tüfekci, H.; Fonsêca, V.F.C.; Chauhan, S.S.; et al. Feed Additives Supplementation: A Potential Strategy to Ameliorate Heat Stress in Sheep. Ann. Anim. Sci. 2024, 24. [Google Scholar] [CrossRef]
- World of Change: Global Temperatures. Available online: https://earthobservatory.nasa.gov/world-of-change/global-temperatures (accessed on 15 April 2025).
- Gupta, M.; Vaidya, M.; Kumar, S.; Singh, G.; Osei-Amponsah, R.; Chauhan, S.S. Heat Stress: A Major Threat to Ruminant Reproduction and Mitigating Strategies. Int. J. Biometeorol. 2025, 69, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Saeed, O.A.; Abdulghafoor, R.T.; Al-Salmany, S.S.; Ali, F.M.; Samsudin, A.A.; Mahmood, E.K. Effect of Temperature on the Physiological Characteristics of Awassi and Crossbred Sheep. J. Anim. Behav. Biometeorol. 2023, 11, 2023031. [Google Scholar] [CrossRef]
- Macías-Cruz, U.; Gastélum, M.A.; Álvarez, F.D.; Correa, A.; Díaz, R.; Meza-Herrera, C.A.; Mellado, M.; Avendaño-Reyes, L. Effects of Summer Heat Stress on Physiological Variables, Ovulation and Progesterone Secretion in Pelibuey Ewes under Natural Outdoor Conditions in an Arid Region. Anim. Sci. J. 2016, 87, 354–360. [Google Scholar] [CrossRef]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological Traits as Affected by Heat Stress in Sheep—A Review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Mascarenhas, N.M.H.; Furtado, D.A.; Fonsêca, V.D.F.C.; De Souza, B.B.; De Oliveira, A.G.; Leal Morais, F.T.; Silva, R.D.S.; Silva, M.R.D.; Batista, L.F.; Dornelas, K.C.; et al. Thermal Stress Index for Native Sheep. J. Therm. Biol. 2023, 115, 103607. [Google Scholar] [CrossRef] [PubMed]
- Čukić, A.; Cincović, M.; Đoković, R.; Rakonjac, S.; Petrović, M.; Petrović, M. Heat Stress Impact on Sheep Production. In Proceedings of the Zbornik Radova 26. Medunarodni Kongres Mediteranske Federacije za Zdravlje i Produkciju Preživara—FeMeSPRum—Zbornik Radova, Novi Sad, Serbia, 20–23 June 2024; Poljoprivredni Fakultet Novi Sad: Novi Sad, Serbia, 2024; p. 7. [Google Scholar]
- Ngcobo, J.N.; Nedambale, T.L.; Nephawe, K.A.; Mpofu, T.J.; Chokoe, T.C.; Ramukhithi, F.V. An Update on South African Indigenous Sheep Breeds’ Extinction Status and Difficulties during Conservation Attempts: A Review. Diversity 2022, 14, 516. [Google Scholar] [CrossRef]
- Aurambout, J.-P.; Benke, K.K.; O’Leary, G.J. Accumulative Heat Stress in Ruminants at the Regional Scale under Changing Environmental Conditions. Environments 2024, 11, 55. [Google Scholar] [CrossRef]
- Casey, N.H. Livestock Adaptation to Climate. Anim. Front. 2023, 13, 3–5. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Zhang, X.X.; Li, F.D.; Li, C.; Li, G.Z.; Zhang, D.Y.; Song, Q.Z.; Li, X.L.; Zhao, Y.; Wang, W.M. Characterization of the Rumen Microbiota and Its Relationship with Residual Feed Intake in Sheep. Animal 2021, 15, 100161. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Singh, G.; Verma, A.K.; Dutta, N.; Sejian, V. Impact of Heat Stress on Rumen Functions. Vet. World 2013, 6, 992–996. [Google Scholar] [CrossRef]
- Chen, S.; Yong, Y.; Ju, X. Effect of Heat Stress on Growth and Production Performance of Livestock and Poultry: Mechanism to Prevention. J. Therm. Biol. 2021, 99, 103019. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, D.K.; Liu, S.-M.; Chua, S.C.; Schwartz, G.J.; Jo, Y.-H. Activation of Temperature-Sensitive TRPV1-like Receptors in ARC POMC Neurons Reduces Food Intake. PLoS Biol. 2018, 16, e2004399. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Effects of Chronic Heat Exposure on Growth Performance, Intestinal Epithelial Histology, Appetite-Related Hormones and Genes Expression in Broilers. J. Sci. Food Agric. 2018, 98, 4471–4478. [Google Scholar] [CrossRef]
- Wang, M.; Ren, C.; Wang, P.; Cheng, X.; Chen, Y.; Huang, Y.; Chen, J.; Sun, Z.; Wang, Q.; Zhang, Z. Microbiome–Metabolome Reveals the Contribution of the Gut–Testis Axis to Sperm Motility in Sheep (Ovis aries). Animals 2023, 13, 996. [Google Scholar] [CrossRef]
- Chen, W.; Zou, H.; Xu, H.; Cao, R.; Zhang, H.; Zhang, Y.; Zhao, J. The Potential Influence and Intervention Measures of Gut Microbiota on Sperm: It Is Time to Focus on Testis-Gut Microbiota Axis. Front. Microbiol. 2024, 15, 1478082. [Google Scholar] [CrossRef]
- Arfuso, F.; Acri, G.; Piccione, G.; Sansotta, C.; Fazio, F.; Giudice, E.; Giannetto, C. Eye Surface Infrared Thermography Usefulness as a Noninvasive Method of Measuring Stress Response in Sheep during Shearing: Correlations with Serum Cortisol and Rectal Temperature Values. Physiol. Behav. 2022, 250, 113781. [Google Scholar] [CrossRef]
- Wojtas, K.; Cwynar, P.; Kolacz, R.; Kupczynski, R. Effect of Heat Stress on Acid-Base Balance in Polish Merino Sheep. Arch. Anim. Breed. 2013, 56, 917–923. [Google Scholar] [CrossRef]
- Thumfart, K.M.; Mansuy, I.M. What Are Sertoli Cells? Historical, Methodological, and Functional Aspects. Andrology 2023, 11, 849–859. [Google Scholar] [CrossRef]
- Hassaneen, A.S.A.; Anis, A.; Nour, S.Y.; Mohamed, R.S.; Wassif, I.M.; El-kattan, A.M.; Abdelgawad, H.A.; Mohamed, R.H. Poor Semen Quality Is Associated with Impaired Antioxidant Response and Acute Phase Proteins and Is Likely Mediated by High Cortisol Levels in Brucella-Seropositive Dromedary Camel Bulls. Sci. Rep. 2024, 14, 27816. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Evans, G.; Maxwell, W.M.C.; Marti, J.I. Seminal Plasma Proteins Do Not Consistently Improve Fertility after Cervical Insemination of Ewes with Non-Sorted or Sex-Sorted Frozen—Thawed Ram Spermatozoa. Reprod. Fertil. Dev. 2010, 22, 606. [Google Scholar] [CrossRef] [PubMed]
- Rahim, A.; El Amiri, B. Effects of Heat Stress and Chemical Pollutants on Sheep Reproduction and Strategies to Mitigate Them. In Advanced Technology for Smart Environment and Energy; Mabrouki, J., Mourade, A., Irshad, A., Chaudhry, S.A., Eds.; Environmental Science and Engineering; Springer International Publishing: Cham, Switzerland, 2023; pp. 173–185. ISBN 978-3-031-25661-5. [Google Scholar]
- Pasha, M.M.H.; Rahman, M.Z.; Sultana, N.; Moniruzzaman, M. Impact of Heat Stress on Female Reproduction in Farm Animals: Challenges and Possible Remedies. Bang. J. Anim. Sci. 2024, 53, 77–100. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Zhang, H.; Zhang, P.; Shahzad, M.; Du, W.; Zhao, X. Heat-Stress Impacts on Developing Bovine Oocytes: Unraveling Epigenetic Changes, Oxidative Stress, and Developmental Resilience. Int. J. Mol. Sci. 2024, 25, 4808. [Google Scholar] [CrossRef]
- Teixeira, M.B.; Ferreira, J.C.P.; Codognoto, V.M.; Rossi, E.S.; Pupulim, A.G.R.; De Carvalho, J.C.; Rattes, P.Z.; Oba, E.; Navolar, F.M.N.; Di Santis, G.W.; et al. Heat Stress Induced by Testicular Insulation for 24 or 48 h Rapidly Impairs Epididymal Sperm Quality and Reduces Spermatogenesis in Rams. Small Rumin. Res. 2025, 243, 107443. [Google Scholar] [CrossRef]
- Rizzoto, G.; Kastelic, J.P. A New Paradigm Regarding Testicular Thermoregulation in Ruminants? Theriogenology 2020, 147, 166–175. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, M.; Wang, Y.; Pan, Q.; Annandale, H.; Irons, P.C.; Dong, H. Semen Quality, Testicular Cell Apoptosis, and Transcriptome Analysis Following Mild Scrotal Heat Stress in Wugu–Hu Crossbred and Hu Rams. Animals 2025, 15, 724. [Google Scholar] [CrossRef]
- López Armengol, M.F.; Rubio, N.; Sabino, G.A.; Bérgamo, N.S.; Pelufo, V. Microscopic Sperm Head Damage and Abnormalities as Heat Stress Indicators in Australian Merino Rams (Ovis aries) in Northern Patagonia, Argentina. Braz. J. Vet. Res. Anim. Sci. 2018, 55, 1–11. [Google Scholar] [CrossRef]
- Berihulay, H.; Abied, A.; He, X.; Jiang, L.; Ma, Y. Adaptation Mechanisms of Small Ruminants to Environmental Heat Stress. Animals 2019, 9, 75. [Google Scholar] [CrossRef]
- Lees, A.M.; Sullivan, M.L.; Olm, J.C.W.; Cawdell-Smith, A.J.; Gaughan, J.B. A Panting Score Index for Sheep. Int. J. Biometeorol. 2019, 63, 973–978. [Google Scholar] [CrossRef]
- Silanikove, N. Effects of Heat Stress on the Welfare of Extensively Managed Domestic Ruminants. Livest. Prod. Sci. 2000, 67, 1–18. [Google Scholar] [CrossRef]
- Department of Agriculture, Land Reform and Rural Development—Statistic & Economic Analysis. Available online: https://www.dlrrd.gov.za/index.php/publication/324-publication-statistical-abstract (accessed on 2 April 2025).
- Molieleng, L.; Fourie, P.; Nwafor, I. Adoption of Climate Smart Agriculture by Communal Livestock Farmers in South Africa. Sustainability 2021, 13, 10468. [Google Scholar] [CrossRef]
- Plessis, A. Du South Africa’s Water Predicament: Freshwater’s Unceasing Decline; Springer Nature: Berlin/Heidelberg, Germany, 2023; ISBN 978-3-031-24019-5. [Google Scholar]
- Halimani, T.; Marandure, T.; Chikwanha, O.C.; Molotsi, A.H.; Abiodun, B.J.; Dzama, K.; Mapiye, C. Smallholder Sheep Farmers’ Perceived Impact of Water Scarcity in the Dry Ecozones of South Africa: Determinants and Response Strategies. Clim. Risk Manag. 2021, 34, 100369. [Google Scholar] [CrossRef]
- Marcone, G.; Kaart, T.; Piirsalu, P.; Arney, D.R. Panting Scores as a Measure of Heat Stress Evaluation in Sheep with Access and with No Access to Shade. Appl. Anim. Behav. Sci. 2021, 240, 105350. [Google Scholar] [CrossRef]
- Shorten, P.R.; Schütz, K.E. Development of a Heat Load Index and Risk Map for Grazing Sheep. N. Z. J. Agric. Res. 2024, 1–25. [Google Scholar] [CrossRef]
- Schütz, K.E.; Saunders, L.-R.; Huddart, F.J.; Watson, T.; Latimer, B.; Cox, N.R. Effects of Shade on the Behaviour and Physiology of Sheep in a Temperate Climate. Appl. Anim. Behav. Sci. 2024, 272, 106185. [Google Scholar] [CrossRef]
- Knight, M.I.; Linden, N.P.; Butler, K.L.; Rice, M.; Ponnampalam, E.N.; Behrendt, R.; Jongman, E.C. The Effect of Shade on Sheep Grazing Pasture during Summer Conditions. J. Vet. Behav. 2023, 64–65, 16–24. [Google Scholar] [CrossRef]
- Taofik, A.; Yusuf, M. Oxidative Stress Status in Heat Shock Sheep Controlled Shearing and Ascorbyl Palmitate Administration. J. Peternak. 2024, 21, 90. [Google Scholar] [CrossRef]
- Shi, L.; Xu, Y.; Mao, C.; Wang, Z.; Guo, S.; Jin, X.; Yan, S.; Shi, B. Effects of Heat Stress on Antioxidant Status and Immune Function and Expression of Related Genes in Lambs. Int. J. Biometeorol. 2020, 64, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, H.R.; El-Shalofy, A.S.; Samir, H. Exogenous L-Carnitine Administration Ameliorates the Adverse Effects of Heat Stress on Testicular Hemodynamics, Echotexture, and Total Antioxidant Capacity in Rams. Front. Vet. Sci. 2022, 9, 860771. [Google Scholar] [CrossRef]
- Fadl, A.M.; Abdelnaby, E.A.; El-Sherbiny, H.R. Supplemental Dietary Zinc Sulphate and Folic Acid Combination Improves Testicular Volume and Haemodynamics, Testosterone Levels and Semen Quality in Rams under Heat Stress Conditions. Reprod. Domest. Anim. 2022, 57, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; Celi, P.; Fahri, F.T.; Leury, B.J.; Dunshea, F.R. Dietary Antioxidants at Supranutritional Doses Modulate Skeletal Muscle Heat Shock Protein and Inflammatory Gene Expression in Sheep Exposed to Heat Stress. J. Anim. Sci. 2014, 92, 4897–4908. [Google Scholar] [CrossRef]
- Kobeisy, M.; Kamal, S.; Hayder, M.; Abo El-Wafa, G. Impact of Antioxidant (Astaxanthin) Supplementation on Farafra Ewes Reproductive Performance and Growth Performance of Their Lambs Exposed to Heat Stress. Asian J. Appl. Sci. 2024, 55, 27–37. [Google Scholar] [CrossRef]
- Khnissi, S.; Ben Salem, I.; Bejaoui, B.; Fattouch, S.; Mustapha, S.B.; Haj-Kacem, R.; M’Hamdi, N.; Martin, P.; Dattena, M.; Lassoued, N. Antioxidant Capacity of Thyme (Thymus vulgaris) Essential Oil and Its Effect on In Vivo Fertility of Rams Subjected to Testicle Heat Stress. Anim. Physiol. Nutr. 2025, 109, 437–448. [Google Scholar] [CrossRef]
- Samara, E.M.; Bahadi, M.A.; Khan, M.A.; Al-Badwi, M.A.; Abdoun, K.A.; Afzal, M.; Alghamdi, S.S.; Al-Haidary, A.A. Thermo-Physiological and Molecular Profiling of Two Indigenous Purebred Saudi Sheep under Acute Heat Stress Conditions. Trop. Anim. Sci. J. 2024, 47, 300–311. [Google Scholar] [CrossRef]
- Saadatabadi, L.M.; Mohammadabadi, M.; Nanaei, H.A.; Ghanatsaman, Z.A.; Stavetska, R.V.; Kalashnyk, O.; Kochuk-Yashchenko, O.A.; Kucher, D.M. Unraveling Candidate Genes Related to Heat Tolerance and Immune Response Traits in Some Native Sheep Using Whole Genome Sequencing Data. Small Rumin. Res. 2023, 225, 107018. [Google Scholar] [CrossRef]
- Ibrahim, S.; Al-Sharif, M.; Younis, F.; Ateya, A.; Abdo, M.; Fericean, L. Analysis of Potential Genes and Economic Parameters Associated with Growth and Heat Tolerance in Sheep (Ovis aries). Animals 2023, 13, 353. [Google Scholar] [CrossRef]
- Gaspa, G.; Cesarani, A.; Pauciullo, A.; Peana, I.; Macciotta, N.P.P. Genomic Analysis of Sarda Sheep Raised at Diverse Temperatures Highlights Several Genes Involved in Adaptations to the Environment and Heat Stress Response. Animals 2024, 14, 3585. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, X.; Aihemaiti, A.; Haire, A.; Gao, Y.; Niu, C.; Yang, P.; Liu, G.; Jia, G.; Wusiman, A. The Mechanism of Heat Stress Resistance During Spermatogenesis in Turpan Black Sheep. Front. Vet. Sci. 2022, 9, 846981. [Google Scholar] [CrossRef]
- FAO (Ed.) Food Aid for Food Security? The State of Food and Agriculture; FAO: Rome, Italy, 2006; ISBN 978-92-5-105600-4. [Google Scholar]
- FAO (Ed.) Biofuels: Prospects, Risks and Opportunities; The State of Food and Agriculture; FAO: Rome, Italy, 2008; ISBN 978-92-5-105980-7. [Google Scholar]
- Cowley, F.C.; Barber, D.G.; Houlihan, A.V.; Poppi, D.P. Immediate and Residual Effects of Heat Stress and Restricted Intake on Milk Protein and Casein Composition and Energy Metabolism. J. Dairy Sci. 2015, 98, 2356–2368. [Google Scholar] [CrossRef]
- Cloete, S.W.P.; Greeff, J.C.; Nel, C.L.; Scholtz, A.J. Breeds and Lines of Sheep Suitable for Production in Challenging Environments. Anim. Front. 2023, 13, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Molotsi, A.H.; Dube, B.; Cloete, S.W.P. The Current Status of Indigenous Ovine Genetic Resources in Southern Africa and Future Sustainable Utilisation to Improve Livelihoods. Diversity 2019, 12, 14. [Google Scholar] [CrossRef]
- Domestic Animal Diversity Information System (DAD-IS)|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/dad-is/en/ (accessed on 2 April 2025).
- Ates, S.; Keles, G.; Inal, F.; Gunes, A.; Dhehibi, B. Performance of Indigenous and Exotic×indigenous Sheep Breeds Fed Different Diets in Spring and the Efficiency of Feeding System in Crop–Livestock Farming. J. Agric. Sci. 2015, 153, 554–569. [Google Scholar] [CrossRef]
- Wojtas, K.; Cwynar, P.; Kołacz, R. Effect of Thermal Stress on Physiological and Blood Parameters in Merino Sheep. Bull. Vet. Inst. Pulawy 2014, 58, 283–288. [Google Scholar] [CrossRef]
- Cloete, S.W.P.; Brand, T.S. Responses to Heat in Ewes from Indigenous and Commercial South African Sheep Breeds: Preliminary Results. In Proceedings of the 24th Association for the Advancement of Animal Breeding and Genetics Conference (AAABG), Adelaide, SA, Australia, 2–4 November 2021. [Google Scholar]
- Rana, M.; Hashem, M.; Sakib, M.; Kumar, A. Effect of Heat Stress on Blood Parameters in Indigenous Sheep. J. Bangladesh Agric. Univ. 2014, 12, 91–94. [Google Scholar] [CrossRef]
- Phaladi, A.M.; Tyasi, T.L.; Tada, O.; Mogashoa, S. Breeding Practices and Trait Preferences of Sheep Farmers from Two Villages in Lepelle-Nkumpi Municipality, Limpopo Province, South Africa. S. Afr. J. Anim. Sci. 2025, 55, 74–84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngcobo, J.N.; Egerszegi, I.; Nephawe, K.A. Recent Advances in Understanding the Impact of Environmental Heat Stress on Sheep Production and Reproductive Performance: A Subtropical Climate Perspective. Climate 2025, 13, 130. https://doi.org/10.3390/cli13060130
Ngcobo JN, Egerszegi I, Nephawe KA. Recent Advances in Understanding the Impact of Environmental Heat Stress on Sheep Production and Reproductive Performance: A Subtropical Climate Perspective. Climate. 2025; 13(6):130. https://doi.org/10.3390/cli13060130
Chicago/Turabian StyleNgcobo, Jabulani Nkululeko, István Egerszegi, and Khathutshelo Agree Nephawe. 2025. "Recent Advances in Understanding the Impact of Environmental Heat Stress on Sheep Production and Reproductive Performance: A Subtropical Climate Perspective" Climate 13, no. 6: 130. https://doi.org/10.3390/cli13060130
APA StyleNgcobo, J. N., Egerszegi, I., & Nephawe, K. A. (2025). Recent Advances in Understanding the Impact of Environmental Heat Stress on Sheep Production and Reproductive Performance: A Subtropical Climate Perspective. Climate, 13(6), 130. https://doi.org/10.3390/cli13060130








