Tropical Fungi and LULUCF: Synergies for Climate Mitigation Through Nature-Based Culture (NbC)
Abstract
1. Introduction
1.1. Background and Rationale of Study
1.2. Objectives of the Study
2. Methodology
2.1. Design and Scope
2.2. Information Sources and Search Strategy
| Scopus: TITLE-ABS-KEY (tropic* AND (fung* OR mycorrhiz*) AND (carbon OR sequestrat* OR “soil organic” OR methane OR CH4 OR N2O OR “greenhouse gas”) AND (forest* OR peat* OR mangrove*)) AND PUBYEAR > 2004 AND PUBYEAR < 2026 AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”)) AND (LIMIT-TO (LANGUAGE, “English”) OR LIMIT-TO (LANGUAGE, “Indonesian”)) Web of Science: TS = (tropic* AND (fung* OR mycorrhiz*) AND (carbon OR sequestrat* OR “soil organic” OR methane OR CH4 OR N2O OR “greenhouse gas”) AND (forest* OR peat* OR mangrove*)) Refined by: DOCUMENT TYPES: (ARTICLE OR REVIEW) Timespan: 1 January 2005 to 30 August 2025 (Publication Date); Languages: (English) |
2.3. Criteria for Listing and Exclusion
- Eligible studies must (1) focus on tropical forests, with climate change impacts as a core research theme; (2) prioritize LULUCF-related research, especially climate-smart regeneration technologies; and (3) include empirical data from Indonesian forests, where reforestation data gaps persist. Excluded are studies on forest ecology that lack climate linkages or do not have peer-reviewed, comprehensive data.
- This review applied three exclusion criteria to maintain focus and rigor: (1) non-tropical forest studies, ensuring geographical relevance; (2) research omitting fungal roles in ecosystem processes, particularly carbon and nutrient cycling; and (3) non-peer-reviewed or methodologically incomplete publications, safeguarding analytical reliability. The approach adhered to standardized evidence-synthesis protocols. However, due to the limited number of tropical studies on fungal-mediated GHG emissions, this review expanded its literature search to include non-tropical ecosystems. While acknowledging potential biogeographical differences in fungal communities and environmental drivers, such comparative analysis provides critical insights into underlying mechanisms. The inclusion of temperate and boreal studies serves to identify universal fungal traits in climate change mitigation.
2.4. Limitations of This Study
3. Results and Discussion
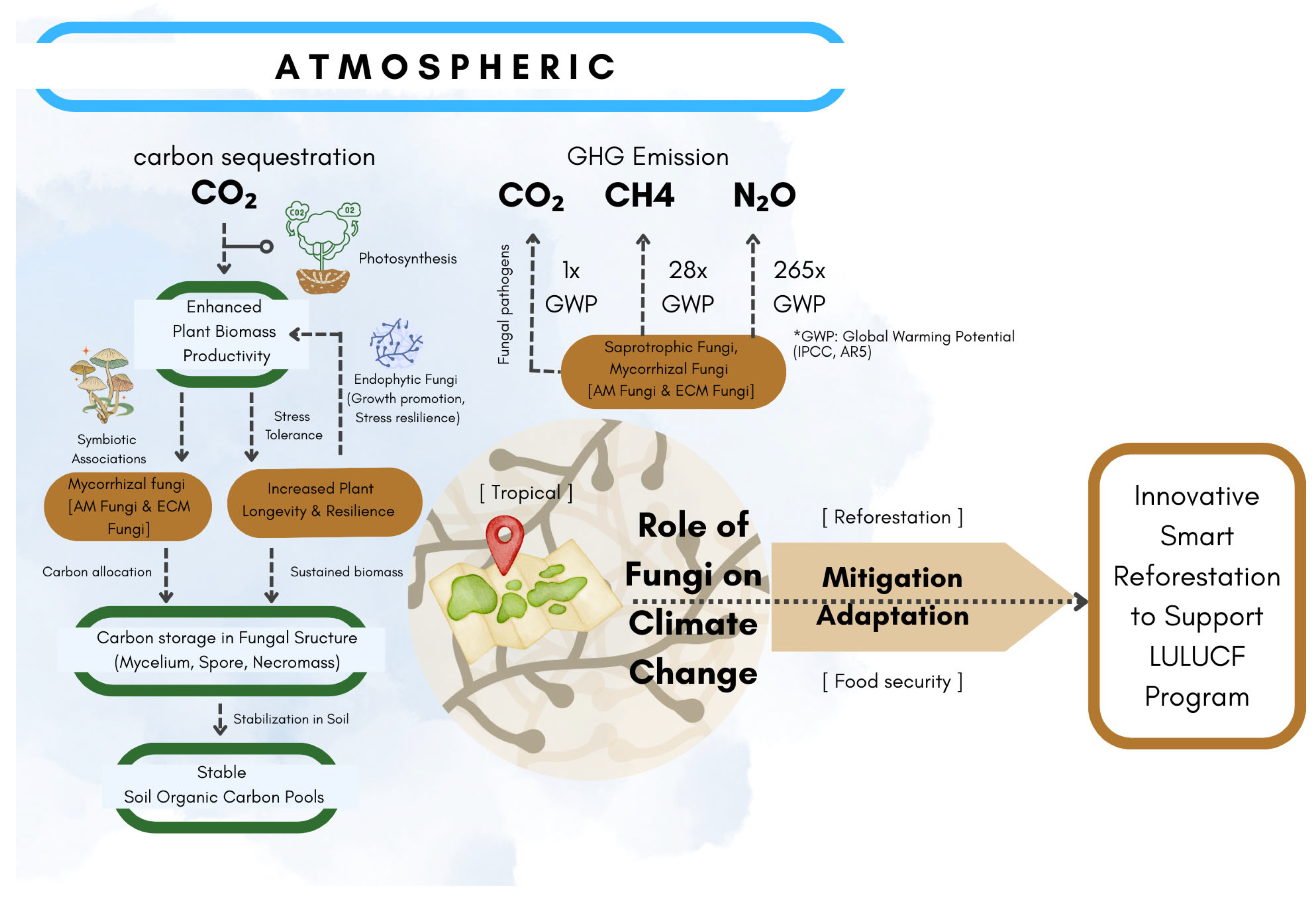
3.1. Fungi Enhance Carbon Sequestration and Storage
3.1.1. Fungal Biomass and Carbon Storage
3.1.2. Mycorrhizal Symbiosis, Enhanced Plant Growth, and Carbon Allocation
3.1.3. Contribution of Fungal Pathogens
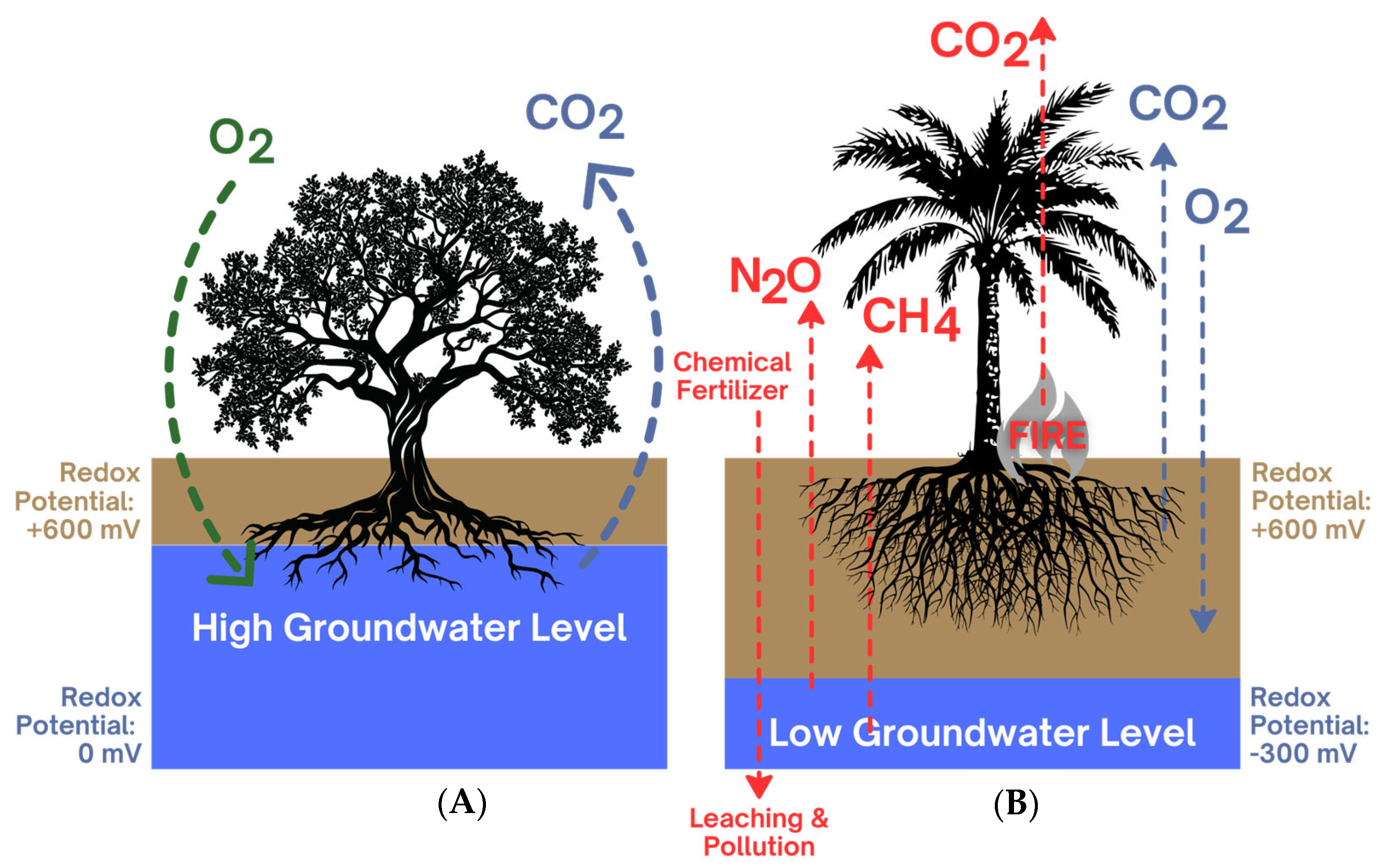
3.2. Fungal Regulation of Greenhouse Gases (GHGs)
3.3. Innovative Smart Reforestation to Support LULUCF Program
3.4. Socio-Environmental Dimension of Tropical Fungi in Climate Change
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassia, R.; Nocioni, M.; Correa-Aragunde, N.; Lamattina, L. Climate Change and the Impact of Greenhouse Gasses: CO2 and NO, Friends and Foes of Plant Oxidative Stress. Front. Plant Sci. 2018, 9, 273. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. Greenhouse Gas Emissions 2020. Available online: https://ourworldindata.org/greenhouse-gas-emissions (accessed on 30 August 2025).
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.I.D.; Brito, L.M.; Nunes, L.J.R. Soil Carbon Sequestration in the Context of Climate Change Mitigation: A Review. Soil Syst. 2023, 7, 64. [Google Scholar] [CrossRef]
- Wang, F.; Harindintwali, J.D.; Wei, K.; Shan, Y.; Mi, Z.; Costello, M.J.; Grunwald, S.; Feng, Z.; Wang, F.; Guo, Y.; et al. Climate Change: Strategies for Mitigation and Adaptation. Innov. Geosci. 2023, 1, 100015. [Google Scholar] [CrossRef]
- Griscom, B.W.; Adams, J.; Ellis, P.W.; Houghton, R.A.; Lomax, G.; Miteva, D.A.; Schlesinger, W.H.; Shoch, D.; Siikamäki, J.V.; Smith, P.; et al. Natural Climate Solutions. Proc. Natl. Acad. Sci. USA 2017, 114, 11645–11650. [Google Scholar] [CrossRef]
- Sasmito, S.D.; Taillardat, P.; Adinugroho, W.C.; Krisnawati, H.; Novita, N.; Fatoyinbo, L.; Friess, D.A.; Page, S.E.; Lovelock, C.E.; Murdiyarso, D.; et al. Half of Land Use Carbon Emissions in Southeast Asia Can Be Mitigated through Peat Swamp Forest and Mangrove Conservation and Restoration. Nat. Commun. 2025, 16, 740. [Google Scholar] [CrossRef]
- Anthony, M.A.; Tedersoo, L.; De Vos, B.; Croisé, L.; Meesenburg, H.; Wagner, M.; Andreae, H.; Jacob, F.; Lech, P.; Kowalska, A.; et al. Fungal Community Composition Predicts Forest Carbon Storage at a Continental Scale. Nat. Commun. 2024, 15, 2385. [Google Scholar] [CrossRef]
- Basuki, I.; Adinugroho, W.C.; Utomo, N.A.; Syaugi, A.; Tryanto, D.H.; Krisnawati, H.; Cook-Patton, S.C.; Novita, N. Reforestation Opportunities in Indonesia: Mitigating Climate Change and Achieving Sustainable Development Goals. Forests 2022, 13, 447. [Google Scholar] [CrossRef]
- Locatelli, B.; Catterall, C.P.; Imbach, P.; Kumar, C.; Lasco, R.; Marín-Spiotta, E.; Mercer, B.; Powers, J.S.; Schwartz, N.; Uriarte, M. Tropical Reforestation and Climate Change: Beyond Carbon. Restor. Ecol. 2015, 23, 337–343. [Google Scholar] [CrossRef]
- He, J.-D.; Chi, G.-G.; Zou, Y.-N.; Shu, B.; Wu, Q.-S.; Srivastava, A.K.; Kuča, K. Contribution of Glomalin-Related Soil Proteins to Soil Organic Carbon in Trifoliate Orange. Appl. Soil Ecol. 2020, 154, 103592. [Google Scholar] [CrossRef]
- Garg, S.; Kim, M.; Romero-Suarez, D. Current Advancements in Fungal Engineering Technologies for Sustainable Development Goals. Trends Microbiol. 2025, 33, 285–301. [Google Scholar] [CrossRef]
- Hawkins, H.-J.; Cargill, R.I.M.; Van Nuland, M.E.; Hagen, S.C.; Field, K.J.; Sheldrake, M.; Soudzilovskaia, N.A.; Kiers, E.T. Mycorrhizal Mycelium as A Global Carbon Pool. Curr. Biol. 2023, 33, R560–R573. [Google Scholar] [CrossRef]
- Waheed, A.; Haxim, Y.; Islam, W.; Ahmad, M.; Muhammad, M.; Alqahtani, F.M.; Hashem, M.; Salih, H.; Zhang, D. Climate Change Reshaping Plant-Fungal Interaction. Environ. Res. 2023, 238, 117282. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gunina, A.; Zhang, F.; Cui, Z.; Tian, J. Fungal Necromass Increases Soil Aggregation and Organic Matter Chemical Stability Under Improved Cropland Management and Natural Restoration. Sci. Total Environ. 2023, 858, 159953. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of Arbuscular Mycorrhizal Fungi on Photosynthesis, Water Status, and Gas Exchange of Plants Under Salt Stress–A Meta-Analysis. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Zaman, F.; Hassan, M.U.; Khattak, W.A.; Ali, A.; Awad, M.F.; Chen, F.-S. The Pivotal Role of Arbuscular Mycorrhizal Fungi in Enhancing Plant Biomass and Nutrient Availability Under Drought Stress Conditions: A Global Meta-Analysis. Sci. Total Environ. 2024, 955, 176960. [Google Scholar] [CrossRef]
- Heinonsalo, J.; Juurola, E.; Linden, A.; Pumpanen, J. Ectomycorrhizal Fungi Affect Scots Pine Photosynthesis through Nitrogen and Water Economy, Not Only through Increased Carbon Demand. Environ. Exp. Bot. 2015, 109, 103–112. [Google Scholar] [CrossRef]
- Kong, D.; Cui, L.; Wang, X.; Wo, J.; Xiong, F. Fungus-Derived Opine Enhances Plant Photosynthesis. J. Adv. Res. 2025, 75, 65–77. [Google Scholar] [CrossRef]
- Akter, S.; Kamruzzaman, M.; Sarder, M.P.; Amin, M.S.; Joardar, J.C.; Islam, M.S.; Nasrin, S.; Islam, M.U.; Islam, F.; Rabbi, S.; et al. Mycorrhizal Fungi Increase Plant Nutrient Uptake, Aggregate Stability and Microbial Biomass in the Clay Soil. Symbiosis 2024, 93, 163–176. [Google Scholar] [CrossRef]
- Brabcová, V.; Štursová, M.; Baldrian, P. Nutrient Content Affects the Turnover of Fungal Biomass in Forest Topsoil and the Composition of Associated Microbial Communities. Soil Biol. Biochem. 2018, 118, 187–198. [Google Scholar] [CrossRef]
- Hagenbo, A.; Hadden, D.; Clemmensen, K.E.; Grelle, A.; Manzoni, S.; Mölder, M.; Ekblad, A.; Fransson, P. Carbon Use Efficiency of Mycorrhizal Fungal Mycelium Increases during the Growing Season but Decreases with Forest Age across a Pinus Sylvestris Chronosequence. J. Ecol. 2019, 107, 2808–2822. [Google Scholar] [CrossRef]
- Sae-Tun, O.; Bodner, G.; Rosinger, C.; Zechmeister-Boltenstern, S.; Mentler, A.; Keiblinger, K. Fungal Biomass and Microbial Necromass Facilitate Soil Carbon Sequestration and Aggregate Stability Under Different Soil Tillage Intensities. Appl. Soil Ecol. 2022, 179, 104599. [Google Scholar] [CrossRef]
- Robinson, S.J.B.; Elias, D.; Johnson, D.; Both, S.; Riutta, T.; Goodall, T.; Majalap, N.; McNamara, N.P.; Griffiths, R.; Ostle, N. Soil Fungal Community Characteristics and Mycelial Production Across a Disturbance Gradient in Lowland Dipterocarp Rainforest in Borneo. Front. For. Glob. Change 2020, 3, 64. [Google Scholar] [CrossRef]
- Wang, X.; Yu, G.-H.; Kuzyakov, Y.; Yin, B.-H.; Kappler, A.; Liu, C.-Q. Contribution of Fungal Biomass to Persistent Soil Carbon Across Natural Ecosystems. Sci. China Earth Sci. 2025, 68, 444–456. [Google Scholar] [CrossRef]
- Fernandez, C.W.; Kennedy, P.G. Revisiting the ‘Gadgil Effect’: Do Interguild Fungal Interactions Control Carbon Cycling in Forest Soils? New Phytol. 2016, 209, 1382–1394. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, N.; Wang, J.; Yao, H.; Qiu, Q.; Chapman, S.J. High Turnover Rate of Free Phospholipids InSoil Confirms the Classic Hypothesis of PLFA Methodology. Soil Biol. Biochem. 2019, 135, 323–330. [Google Scholar] [CrossRef]
- Beidler, K.V.; Phillips, R.P.; Andrews, E.; Maillard, F.; Mushinski, R.M.; Kennedy, P.G. Substrate Quality Drives Fungal Necromass Decay and Decomposer Community Structure under Contrasting Vegetation Types. J. Ecol. 2020, 108, 1845–1859. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How Mycorrhizal Associations Drive Plant Population and Community Biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Frey, S.D. Mycorrhizal Fungi as Mediators of Soil Organic Matter Dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Lehmann, A.; Zheng, W.; Ryo, M.; Soutschek, K.; Roy, J.; Rongstock, R.; Maaß, S.; Rillig, M.C. Fungal Traits Important for Soil Aggregation. Front. Microbiol. 2020, 10, 2904. [Google Scholar] [CrossRef]
- Qiang, W.; Gunina, A.; Kuzyakov, Y.; He, L.; Zhang, Y.; Liu, B.; Pang, X. Contributions of Mycorrhizal Fungi to Soil Aggregate Formation During Subalpine Forest Succession. CATENA 2023, 221, 106800. [Google Scholar] [CrossRef]
- Köhl, L.; van der Heijden, M.G.A. Arbuscular Mycorrhizal Fungal Species Differ in Their Effect on Nutrient Leaching. Soil Biol. Biochem. 2016, 94, 191–199. [Google Scholar] [CrossRef]
- Wilkes, T.I.; Warner, D.J.; Edmonds-Brown, V.; Davies, K.G.; Denholm, I. Zero Tillage Systems Conserve Arbuscular Mycorrhizal Fungi, Enhancing Soil Glomalin and Water Stable Aggregates with Implications for Soil Stability. Soil Syst. 2021, 5, 4. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Understanding Mechanisms of Soil Biota Involvement in Soil Aggregation: A Way Forward with Saprobic Fungi? Soil Biol. Biochem. 2015, 88, 298–302. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Qu, C.; Huang, Q.; Cai, P. Microbial Extracellular Polymeric Substances (EPS) in Soil: From Interfacial Behaviour to Ecological Multifunctionality. Geo-Bio Interfaces 2024, 1, e4. [Google Scholar] [CrossRef]
- Jo, I.; Fei, S.; Oswalt, C.M.; Domke, G.M.; Phillips, R.P. Shifts in Dominant Tree Mycorrhizal Associations in Response to Anthropogenic Impacts. Sci. Adv. 2019, 5, eaav6358. [Google Scholar] [CrossRef]
- Crowther, T.W.; van den Hoogen, J.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The Global Soil Community and Its Influence on Biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Mrnka, L.; Koukol, O.; Hrabal, R.; Novák, F. Interactions of Saprotrophic and Root Symbiotic Fungi Control the Transformation of Humic Substances and Phosphorus in Norway Spruce Needle Litter. Soil Biol. Biochem. 2020, 149, 107919. [Google Scholar] [CrossRef]
- Pellitier, P.T.; Zak, D.R. Ectomycorrhizal Fungi and the Enzymatic Liberation of Nitrogen from Soil Organic Matter: Why Evolutionary History Matters. New Phytol. 2018, 217, 68–73. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial Necromass as the Source of Soil Organic Carbon in Global Ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Forest Soil Bacteria: Diversity, Involvement in Ecosystem Processes, and Response to Global Change. Microbiol. Mol. Biol. Rev. 2017, 81, e00063-16. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte Roles in Nutrient Acquisition, Root System Architecture Development and Oxidative Stress Tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Chen, L.; Xin, H.-L.; Zheng, C.-J.; Rahman, K.; Han, T.; Qin, L.-P. A Friendly Relationship between Endophytic Fungi and Medicinal Plants: A Systematic Review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in Fungal Communities and Associated Enzyme Activities Along an Age Gradient of Managed Pinus Sylvestris Stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Finlay, R.D.; Dahlberg, A.; Stenlid, J.; Wardle, D.A.; Lindahl, B.D. Carbon Sequestration Is Related to Mycorrhizal Fungal Community Shifts During Long-Term Succession in Boreal Forests. New Phytol. 2015, 205, 1525–1536. [Google Scholar] [CrossRef]
- Ekblad, A.; Mikusinska, A.; Ågren, G.I.; Menichetti, L.; Wallander, H.; Vilgalys, R.; Bahr, A.; Eriksson, U. Production and Turnover of Ectomycorrhizal Extramatrical Mycelial Biomass and Necromass Under Elevated CO2 and Nitrogen Fertilization. New Phytol. 2016, 211, 874–885. [Google Scholar] [CrossRef]
- Terrer, C.; Phillips, R.P.; Hungate, B.; Rosende, J.; Pett-Ridge, J.; Craig, M.; van Groenigen, K.; Keenan, T.; Sulman, B.; Stocker, B.; et al. A Trade-off between Plant and Soil Carbon Storage under Elevated CO2. Nature 2021, 591, 599–603. [Google Scholar] [CrossRef]
- Adinugroho, W.C.; Turjaman, M.; Irianto, R.S.B.; Rachmat, H.H.; Imanuddin, R.; Hidayat, A.; Apriyanto, D.; Prihartini, J.N.; Wahno, I.; Rochmania, L.; et al. Bridging Productivity and Conservation: Peatland Native Tree Species Plantation with Arbuscular Mycorrhizal Innovation. Oryx 2025, 59, 145–146. [Google Scholar] [CrossRef]
- Husna, H.; Tuheteru, F.D.; Arif, A. Arbuscular Mycorrhizal Fungi to Enhance the Growth of Tropical Endangered Species Pterocarpus Indicus and Pericopsis Mooniana in Post Gold Mine Field in Southeast Sulawesi, Indonesia. Biodiversitas J. Biol. Divers. 2021, 22, 3844–3853. [Google Scholar] [CrossRef]
- Tuheteru, F.D.; Arif, A.; Husna, H.; Mansur, I.; Tuheteru, E.J.; Jusniar, J.; Basrudin, B.; Albasri, A.; Hadijah, M.H.; Karepesina, S. Arbuscular mycorrhizal fungal inoculation improves Nauclea orientalis L. growth and phosphorus uptake in gold mine tailing soil media. J. Degrad. Min. Lands Manag. 2020, 7, 2193–2200. [Google Scholar] [CrossRef]
- Prematuri, R.; Turjaman, M.; Tawaraya, K. Effect of arbuscular mycorrhiza fungal inoculation on growth of tropical tree species under nursery and post-opencast bauxite mining field in Bintan Island, Indonesia. Int. J. Plant. Soil Sci. 2020, 32, 1–13. [Google Scholar] [CrossRef]
- Barua, A.; Gupta, S.D.; Mridha, M.A.U.; Bhuiyan, M.K. Effect of arbuscular mycorrhizal fungi on growth of Gmelina arborea in arsenic-contaminated soil. J. For. Res. 2010, 21, 423–432. [Google Scholar] [CrossRef]
- Ghaida, S.H.; Wasis, B.; Budi, S.W. Application of arbuscular mycorrhizal fungi and soil ameliorant on the growth of Leucaena leucocephala in limestone post-mining soil media. J. Man. Hut. Trop. 2020, 26, 282–290. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of Arbuscular Mycorrhizal Fungi in Regulating Growth, Enhancing Productivity, and Potentially Influencing Ecosystems under Abiotic and Biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef]
- Janoušková, M.; Remke, M.; Johnson, N.C.; Blažková, A.; Rydlová, J.; Kolaříková, Z.; Bowker, M.A. Transferred Communities of Arbuscular Mycorrhizal Fungal Persist in Novel Climates and Soils. Soil Biol. Biochem. 2023, 187, 109190. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-Morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Gomdola, D.; Bhunjun, C.; Hyde, K.; Jeewon, R.; Pem, D.; Jayawardena, R. Ten Important Forest Fungal Pathogens: A Review on Their Emergence and Biology. Mycosphere 2022, 13, 612–671. [Google Scholar] [CrossRef]
- Copeland, C.A.; Harper, R.W.; Brazee, N.J.; Bowlick, F.J. A Review of Dutch Elm Disease and New Prospects for Ulmus Americana in the Urban Environment. Arboric. J. 2023, 45, 3–29. [Google Scholar] [CrossRef]
- Harwood, T.D.; Tomlinson, I.; Potter, C.A.; Knight, J.D. Dutch Elm Disease Revisited: Past, Present and Future Management in Great Britain. Plant Pathol. 2011, 60, 545–555. [Google Scholar] [CrossRef]
- Gibbs, J.N. Intercontinental Epidemiology of Dutch Elm Disease. Annu. Rev. Phytopathol. 1978, 16, 287–307. [Google Scholar] [CrossRef]
- Westbrook, J.W.; Holliday, J.A.; Newhouse, A.E.; Powell, W.A. A Plan to Diversify a Transgenic Blight-tolerant American Chestnut Population Using Citizen Science. Plants People Planet 2020, 2, 84–95. [Google Scholar] [CrossRef]
- Rigling, D.; Prospero, S. Cryphonectria Parasitica, the Causal Agent of Chestnut Blight: Invasion History, Population Biology and Disease Control. Mol. Plant Pathol. 2018, 19, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dahlsjö, C.A.L.; Malhi, Y. Evaluating the Impact of an Invasive Pathogen on Tree Population Decline: An Evidence Based Modelling Approach. For. Ecol. Manag. 2024, 566, 122098. [Google Scholar] [CrossRef]
- Williams, G.M.; Ginzel, M.D.; Ma, Z.; Adams, D.C.; Campbell, F.; Lovett, G.M.; Pildain, M.B.; Raffa, K.F.; Gandhi, K.J.K.; Santini, A.; et al. The Global Forest Health Crisis: A Public-Good Social Dilemma in Need of International Collective Action. Annu. Rev. Phytopathol. 2023, 61, 377–401. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Sniezko, R.A. Quantitative Disease Resistance to White Pine Blister Rust at Southwestern White Pine’s (Pinus strobiformis) Northern Range. Front. For. Glob. Change 2021, 4, 765871. [Google Scholar] [CrossRef]
- Coker, T.L.R.; Rozsypálek, J.; Edwards, A.; Harwood, T.P.; Butfoy, L.; Buggs, R.J.A. Estimating Mortality Rates of European Ash (Fraxinus excelsior) under the Ash Dieback (Hymenoscyphus fraxineus) Epidemic. Plants People Planet 2019, 1, 48–58. [Google Scholar] [CrossRef]
- Grünwald, N.J.; LeBoldus, J.M.; Hamelin, R.C. Ecology and Evolution of the Sudden Oak Death Pathogen Phytophthora Ramorum. Annu. Rev. Phytopathol. 2019, 57, 301–321. [Google Scholar] [CrossRef]
- Syazwan, S.A.; Mohd-Farid, A.; Wan-Muhd-Azrul, W.-A.; Syahmi, H.M.; Zaki, A.M.; Ong, S.P.; Mohamed, R. Survey, Identification, and Pathogenicity of Ceratocystis Fimbriata Complex Associated with Wilt Disease on Acacia mangium in Malaysia. Forests 2021, 12, 1782. [Google Scholar] [CrossRef]
- Tarigan, M.; Roux, J.; Van Wyk, M.; Tjahjono, B.; Wingfield, M.J. A New Wilt and Die-Back Disease of Acacia mangium Associated with Ceratocystis manginecans and C. Acaciivora Sp. Nov. in Indonesia. South Afr. J. Bot. 2011, 77, 292–304. [Google Scholar] [CrossRef]
- Roux, J.; Wingfield, M. Ceratocystis Species: Emerging Pathogens of Non-Native Plantation Eucalyptus and Acacia Species. South. For. A J. For. Sci. 2009, 71, 115–120. [Google Scholar] [CrossRef]
- Hardiyanto, E.B.; Inail, M.A.; Nambiar, S.; Mendham, D.S. Sustaining Plantation Forest Productivity in Sumatra over Three Decades: From Acacias to Eucalypts. For. Ecol. Manag. 2024, 553, 121613. [Google Scholar] [CrossRef]
- Nambiar, E.K.S.; Harwood, C.E.; Mendham, D.S. Paths to Sustainable Wood Supply to the Pulp and Paper Industry in Indonesia after Diseases Have Forced a Change of Species from Acacia to Eucalypts. Aust. For. 2018, 81, 148–161. [Google Scholar] [CrossRef]
- Barry, K.; Irianto, R.S.; Santoso, E.; Turjaman, M.; Widyati, E.; Sitepu, I.; Mohammed, C. Incidence of Heartrot in Harvest-Age Acacia mangium in Indonesia, Using a Rapid Survey Method. For. Ecol. Manag. 2004, 190, 273–280. [Google Scholar] [CrossRef]
- Loquez, M.O.; Amper, C.D.; Tulod, A.M.; Gilbero, D.M. Teliospore Morphology Characterization of Uromycladium falcatariae in Falcata Plantations at Different Elevations in Mindanao Island, Philippines. Biodiversitas J. Biol. Divers. 2025, 26, 296–305. [Google Scholar] [CrossRef]
- Tulod, A.M.; Casas, J.V.; Rojo, M.J.A.; Marin, R.A.; Talisay, B.A.M.; Bruno, E.N.; Branzuela, N.E.; Solis, E.B.; Bayang, S.S.; Gilbero, J.S.; et al. Strategies for Falcata (Falcataria falcata (L.) Greuter and R. Rankin) Farmers to Mitigate Gall Rust Severity across Elevations. Davao Res. J. 2024, 15, 45–50. [Google Scholar] [CrossRef]
- Lelana, N.E.; Wiyono, S.; Giyanto; Siregar, I.Z.; Anggraeni, I. Phylogenetic and Morphological Characteristics of Uromycladium falcatariae, the Fungal Pathogen That Causes Gall Rust Epidemics of Falcataria moluccana in Indonesia. J. Phytopathol. 2022, 170, 598–604. [Google Scholar] [CrossRef]
- Lelana, N.E.; Darmawan, U.W.; Nuroniah, H.S.; Puspitaningtyas, D.M.; Irianto, R.S.B. Rapid Spread and High Prevalence of the Gall Rust Pathogen Uromycladium falcatariae in Stands of Falcataria falcata. Forest Sci. Technol. 2024, 20, 309–315. [Google Scholar] [CrossRef]
- Doungsa-ard, C.; McTaggart, A.R.; Geering, A.D.W.; Shivas, R.G. Diversity of Gall-Forming Rusts (Uromycladium, Pucciniales) on Acacia in Australia. Persoonia -Mol. Phylogeny Evol. Fungi 2018, 40, 221–238. [Google Scholar] [CrossRef]
- Rahayu, S.; Lee, S.S.; Shukor, N.A.A. Uromycladium tepperianum, the Gall Rust Fungus from Falcataria moluccana in Malaysia and Indonesia. Mycoscience 2010, 51, 149–153. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhao, Y.; Zhang, X.; Wang, Y.; Cao, Q.; Liu, J. Microbial Necromass Carbon Contributed to Soil Organic Carbon Accumulation and Stabilization in the Newly Formed Inland Wetlands. Environ. Res. 2025, 264, 120397. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shu, Q.; Guo, W.; Shang, Z.; Qi, L. Secondary Succession Altered the Diversity and Co-Occurrence Networks of the Soil Bacterial Communities in Tropical Lowland Rainforests. Plants 2022, 11, 1344. [Google Scholar] [CrossRef]
- Janzen, D.H. Herbivores and the Number of Tree Species in Tropical Forests. Am. Nat. 1970, 104, 501–528. [Google Scholar] [CrossRef]
- Connell, J. On the Role of Natural Enemies in Preventing Competitive Exclusion in Some Marine Animals and in Rain Forest Trees. In Dynamics of Population; Den Boer, P.J., Gradwell, G., Eds.; Pudoc: Wageningen, The Netherlands, 1970. [Google Scholar]
- Țopa, D.-C.; Căpșună, S.; Calistru, A.-E.; Ailincăi, C. Sustainable Practices for Enhancing Soil Health and Crop Quality in Modern Agriculture: A Review. Agriculture 2025, 15, 998. [Google Scholar] [CrossRef]
- Poorter, L.; van der Sande, M.T.; Thompson, J.; Arets, E.J.M.M.; Alarcón, A.; Álvarez-Sánchez, J.; Ascarrunz, N.; Balvanera, P.; Barajas-Guzmán, G.; Boit, A.; et al. Diversity Enhances Carbon Storage in Tropical Forests. Glob. Ecol. Biogeogr. 2015, 24, 1314–1328. [Google Scholar] [CrossRef]
- Albert-Saiz, M.; Lamentowicz, M.; Rastogi, A.; Juszczak, R. Unveiling Water Table Tipping Points in Peatland Ecosystems: Implications for Ecological Restoration. CATENA 2025, 257, 109149. [Google Scholar] [CrossRef]
- Kohler, A.; Kuo, A.; Nagy, L.G.; Morin, E.; Barry, K.W.; Buscot, F.; Canbäck, B.; Choi, C.; Cichocki, N.; Clum, A.; et al. Convergent Losses of Decay Mechanisms and Rapid Turnover of Symbiosis Genes in Mycorrhizal Mutualists. Nat. Genet. 2015, 47, 410–415. [Google Scholar] [CrossRef]
- Algora Gallardo, C.; Baldrian, P.; López-Mondéjar, R. Litter-Inhabiting Fungi Show High Level of Specialization Towards Biopolymers Composing Plant and Fungal Biomass. Biol. Fertil. Soils 2021, 57, 77–88. [Google Scholar] [CrossRef]
- Schroll, M.; Lenhart, K.; Bender, T.; Hötten, P.; Rudolph, A.; Sörensen, S.; Keppler, F. Fungal Methane Production Controlled by Oxygen Levels and Temperature. Methane 2024, 3, 257–275. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, M.; Du, X.; Feng, K.; Gu, S.; Zhou, Y.; Yang, X.; Zhang, Z.; Wang, Y.; Zhang, Z.; et al. Fungi and Cercozoa Regulate Methane-Associated Prokaryotes in Wetland Methane Emissions. Front. Microbiol. 2023, 13, 1076610. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.P.; Schilling, J.S. Harnessing Fungi to Mitigate CH4 in Natural and Engineered Systems. Appl. Microbiol. Biotechnol. 2018, 102, 7365–7375. [Google Scholar] [CrossRef] [PubMed]
- Yasuri, A.K. Innovative Solutions to Climate Change: Evaluating the Most Effective Strategies. Environ. Claims J. 2025, 37, 333–365. [Google Scholar] [CrossRef]
- Leamdum, C.; Phruksaphithak, N.; Chanthong, S.; Niyasom, C. Methane Oxidation Rates and Efficiencies Across Four Distinct Soil Environments: Implications for Greenhouse Gas Mitigation. ASEAN J. Sci. Technol. Rep. 2024, 28, e255939. [Google Scholar] [CrossRef]
- Moslehpour, M.; Aldeehani, T.M.; Sibghatullah, A.; Tai, T.D.; Phan, T.T.H.; Ngo, T.Q. Dynamic Association between Technological Advancement, Green Finance, Energy Efficiency and Sustainable Development: Evidence from Vietnam. Econ. Res. Istraživanja 2023, 36, 2190796. [Google Scholar] [CrossRef]
- Kržišnik, D.; Gonçalves, J. Environmentally Conscious Technologies Using Fungi in a Climate-Changing World. Earth 2023, 4, 69–77. [Google Scholar] [CrossRef]
- Leifheit, E.F.; Veresoglou, S.D.; Lehmann, A.; Morris, E.K.; Rillig, M.C. Multiple Factors Influence the Role of Arbuscular Mycorrhizal Fungi in Soil Aggregation—A Meta-Analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- Bender, S.F.; van der Heijden, M.G.A. Soil Biota Enhance Agricultural Sustainability by Improving Crop Yield, Nutrient Uptake and Reducing Nitrogen Leaching Losses. J. Appl. Ecol. 2015, 52, 228–239. [Google Scholar] [CrossRef]
- Kip, N.; van Winden, J.F.; Pan, Y.; Bodrossy, L.; Reichart, G.-J.; Smolders, A.J.P.; Jetten, M.S.M.; Damsté, J.S.S.; Op den Camp, H.J.M. Global Prevalence of Methane Oxidation by Symbiotic Bacteria in Peat-Moss Ecosystems. Nat. Geosci. 2010, 3, 617–621. [Google Scholar] [CrossRef]
- Rachmat, H.H.; Ginoga, K.L.; Lisnawati, Y.; Hidayat, A.; Imanuddin, R.; Fambayun, R.A.; Yulita, K.S.; Susilowati, A. Generating Multifunctional Landscape through Reforestation with Native Trees in the Tropical Region: A Case Study of Gunung Dahu Research Forest, Bogor, Indonesia. Sustainability 2021, 13, 11950. [Google Scholar] [CrossRef]
- Liu, P.; Wen, S.; Zhu, S.; Hu, X.; Wang, Y. Microbial Degradation of Soil Organic Pollutants: Mechanisms, Challenges, and Advances in Forest Ecosystem Management. Processes 2025, 13, 916. [Google Scholar] [CrossRef]
- Nazaries, L.; Murrell, J.C.; Millard, P.; Baggs, L.; Singh, B.K. Methane, Microbes and Models: Fundamental Understanding of the Soil Methane Cycle for Future Predictions. Environ. Microbiol. 2013, 15, 2395–2417. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, J.; Lian, X.; Wei, B.; Ma, X.; Wu, P. Effect of Glomus Intraradices on Root Morphology, Biomass Production and Phosphorous Use Efficiency of Chinese Fir Seedlings under Low Phosphorus Stress. Front. Plant Sci. 2023, 13, 1095772. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Yu, F.; Zhang, Y.; Liu, K.; Zhuo, X.; Qiu, Y.; Zhang, H.; Gu, J.; Wang, W.; et al. Reducing Methane Emission by Promoting Its Oxidation in Rhizosphere through Nitrogen-Induced Root Growth in Paddy Fields. Plant Soil 2022, 474, 541–560. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Z.; Liu, R.; Cai, B. Funneliformis Mosseae Enhances the Function of C, N and P Cycling Bacteria in Continuous Soybean Rhizosphere Soil. J. Soil Sci. Plant Nutr. 2024, 24, 8263–8279. [Google Scholar] [CrossRef]
- Hao, B.; Zhang, Z.; Bao, Z.; Hao, L.; Diao, F.; Li, F.Y.; Guo, W. Claroideoglomus Etunicatum Affects the Structural and Functional Genes of the Rhizosphere Microbial Community to Help Maize Resist Cd and La Stresses. Environ. Pollut. 2022, 307, 119559. [Google Scholar] [CrossRef]
- Venice, F.; de Pinto, M.C.; Novero, M.; Ghignone, S.; Salvioli, A.; Bonfante, P. Gigaspora Margarita with and without Its Endobacterium Shows Adaptive Responses to Oxidative Stress. Mycorrhiza 2017, 27, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Cui, X.; Zuo, X.; Li, K.; Wang, S.; Jia, Y.; Misselbrook, T.; Liu, X. The Contribution of Arbuscular Mycorrhizal Fungi to Ecosystem Respiration and Methane Flux in an Ephemeral Plants-dominated Desert. Land Degrad. Dev. 2021, 32, 1844–1853. [Google Scholar] [CrossRef]
- Malyan, S.K.; Bhatia, A.; Kumar, A.; Gupta, D.K.; Singh, R.; Kumar, S.S.; Tomer, R.; Kumar, O.; Jain, N. Methane Production, Oxidation and Mitigation: A Mechanistic Understanding and Comprehensive Evaluation of Influencing Factors. Sci. Total Environ. 2016, 572, 874–896. [Google Scholar] [CrossRef] [PubMed]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of Soil Enzymes in Sustainable Crop Production. In Enzymes in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 569–589. [Google Scholar]
- Pu, S.; Liu, S. Global Climate Change and Enzyme Activities. In Extracellular Enzymes in Environments; Elsevier: Amsterdam, The Netherlands, 2023; pp. 65–93. [Google Scholar]
- Neethirajan, S. Innovative Strategies for Sustainable Dairy Farming in Canada amidst Climate Change. Sustainability 2023, 16, 265. [Google Scholar] [CrossRef]
- Vieira, R.I.M.; Peixoto, A.d.S.; Monclaro, A.V.; Ricart, C.A.O.; Filho, E.X.F.; Miller, R.N.G.; Gomes, T.G. Fungal Coculture: Unlocking the Potential for Efficient Bioconversion of Lignocellulosic Biomass. J. Fungi 2025, 11, 458. [Google Scholar] [CrossRef]
- Jo, C.; Zhang, J.; Tam, J.M.; Church, G.M.; Khalil, A.S.; Segrè, D.; Tang, T.-C. Unlocking the Magic in Mycelium: Using Synthetic Biology to Optimize Filamentous Fungi for Biomanufacturing and Sustainability. Mater. Today Bio 2023, 19, 100560. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. The Significance of Microbial Transformation of Nitrogen Compounds in the Light of Integrated Crop Management. Agronomy 2021, 11, 1415. [Google Scholar] [CrossRef]
- Hodge, A.; Fitter, A.H. Microbial Mediation of Plant Competition and Community Structure. Funct. Ecol. 2013, 27, 865–875. [Google Scholar] [CrossRef]
- Gui, H.; Gao, Y.; Wang, Z.; Shi, L.; Yan, K.; Xu, J. Arbuscular Mycorrhizal Fungi Potentially Regulate N2O Emissions from Agricultural Soils via Altered Expression of Denitrification Genes. Sci. Total Environ. 2021, 774, 145133. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Sharma, P.L.; Paul, P.; Baruah, N.R.; Choudhury, J.; Begum, T.; Karmakar, R.; Khan, T.; Kalita, J. Harnessing Endophytes: Innovative Strategies for Sustainable Agricultural Practices. Discov. Bact. 2025, 2, 1. [Google Scholar] [CrossRef]
- Basiru, S.; Mhand, K.A.S.; Hijri, M. Deciphering the Mechanisms through Which Arbuscular Mycorrhizal Symbiosis Reduces Nitrogen Losses in Agroecosystems. Appl. Soil Ecol. 2025, 206, 105799. [Google Scholar] [CrossRef]
- Shoun, H.; Fushinobu, S.; Jiang, L.; Kim, S.-W.; Wakagi, T. Fungal Denitrification and Nitric Oxide Reductase Cytochrome P450nor. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1186–1194. [Google Scholar] [CrossRef]
- Schweigert, M.; Herrmann, S.; Miltner, A.; Fester, T.; Kästner, M. Fate of Ectomycorrhizal Fungal Biomass in a Soil Bioreactor System and Its Contribution to Soil Organic Matter Formation. Soil Biol. Biochem. 2015, 88, 120–127. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; Heijden, M.G.A. van der The Role of Arbuscular Mycorrhizas in Reducing Soil Nutrient Loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef]
- Wei, W.; Isobe, K.; Shiratori, Y.; Nishizawa, T.; Ohte, N.; Otsuka, S.; Senoo, K. N2O Emission from Cropland Field Soil through Fungal Denitrification after Surface Applications of Organic Fertilizer. Soil Biol. Biochem. 2014, 69, 157–167. [Google Scholar] [CrossRef]
- Zhou, Z.; Takaya, N.; Sakairi, M.A.C.; Shoun, H. Oxygen Requirement for Denitrification by the Fungus Fusarium Oxysporum. Arch. Microbiol. 2001, 175, 19–25. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, S.; Peng, B.; Tan, D.; Wu, M.; Wei, J.; Wang, Y.; Luo, H. Ganoderma Lucidum: A Comprehensive Review of Phytochemistry, Efficacy, Safety and Clinical Study. Food Sci. Hum. Wellness 2024, 13, 568–596. [Google Scholar] [CrossRef]
- Seo, D.C.; DeLaune, R.D. Effect of Redox Conditions on Bacterial and Fungal Biomass and Carbon Dioxide Production in Louisiana Coastal Swamp Forest Sediment. Sci. Total Environ. 2010, 408, 3623–3631. [Google Scholar] [CrossRef]
- Britto Martins de Oliveira, J.; Corrêa Junior, D.; Parente, C.E.T.; Frases, S. Fungi in Mangrove: Ecological Importance, Climate Change Impacts, and the Role in Environmental Remediation. Microorganisms 2025, 13, 878. [Google Scholar] [CrossRef] [PubMed]
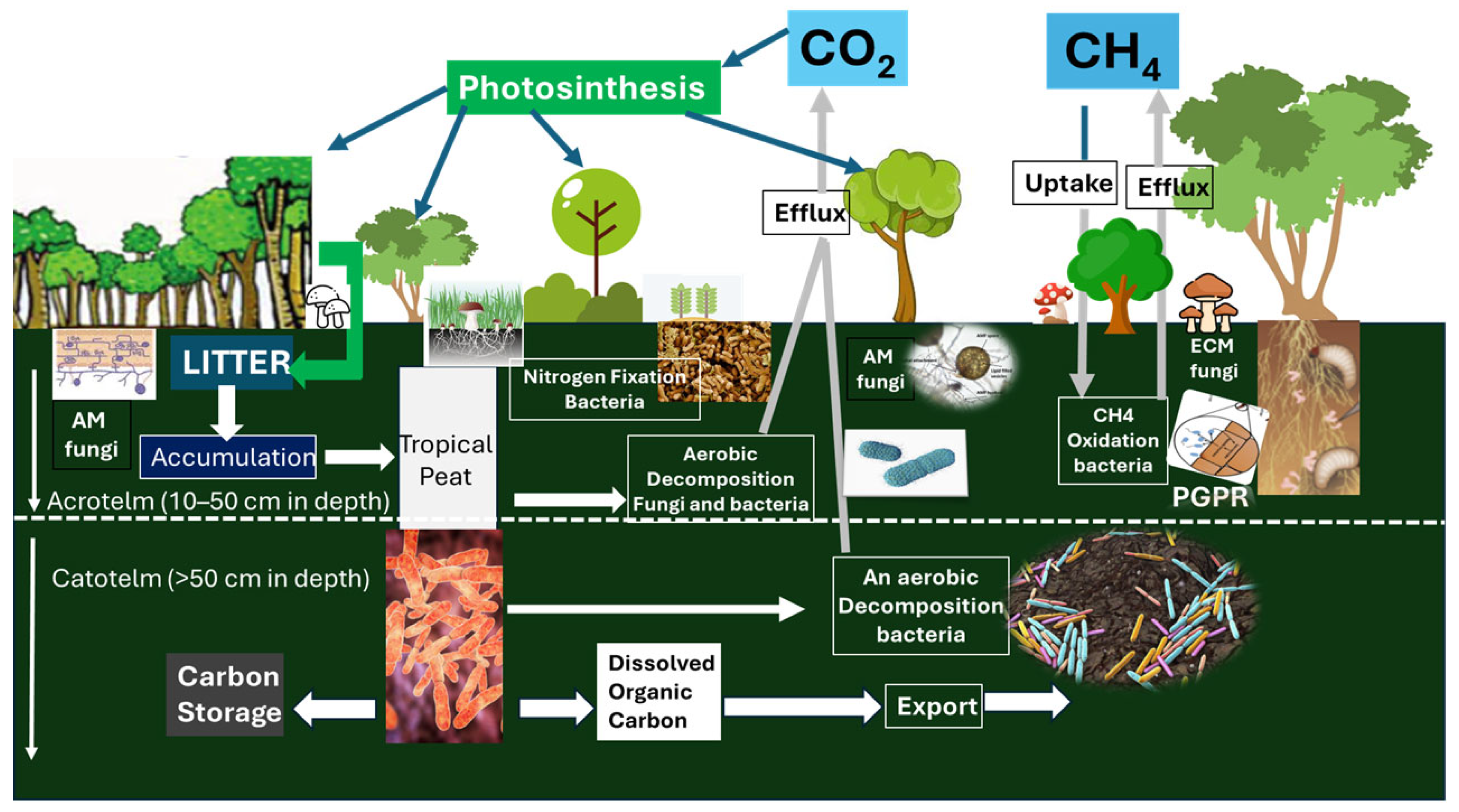
- Osaki, M.; Tsuji, N.; Kato, T. Innovative Eco-Evaluation System for Tropical Peatlands. In Tropical Peatland Eco-Evaluation; Springer Nature: Singapore, 2023; pp. 3–67. [Google Scholar]
- Osaki, M.; Kato, T.; Kohyama, T.; Takahashi, H.; Haraguchi, A.; Yabe, K.; Tsuji, N.; Shiodera, S.; Rahajoe, J.S.; Atikah, T.D.; et al. Basic Information About Tropical Peatland Ecosystems. In Tropical Peatland Eco-Management; Springer: Singapore, 2021; pp. 3–62. [Google Scholar]
- Osaki, M.; Kato, T.; Takahashi, H.; Sulaiman, A.; Tsuji, N. Triharmony/Trilemma of Carbon Assets in Tropical Peatland. Res. Outreach 2023. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal Laccases—Occurrence and Properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Pollegioni, L.; Tonin, F.; Rosini, E. Lignin-degrading Enzymes. FEBS J. 2015, 282, 1190–1213. [Google Scholar] [CrossRef]
- Martinez, D.; Larrondo, L.F.; Putnam, N.; Gelpke, M.D.S.; Huang, K.; Chapman, J.; Helfenbein, K.G.; Ramaiya, P.; Detter, J.C.; Larimer, F.; et al. Genome Sequence of the Lignocellulose Degrading Fungus Phanerochaete Chrysosporium Strain RP78. Nat. Biotechnol. 2004, 22, 695–700. [Google Scholar] [CrossRef]
- Ruiz-Dueñas, F.J.; Martínez, Á.T. Microbial Degradation of Lignin: How a Bulky Recalcitrant Polymer Is Efficiently Recycled in Nature and How We Can Take Advantage of This. Microb. Biotechnol. 2009, 2, 164–177. [Google Scholar] [CrossRef]
- Aguilar-Trigueros, C.A.; Hempel, S.; Powell, J.R.; Anderson, I.C.; Antonovics, J.; Bergmann, J.; Cavagnaro, T.R.; Chen, B.; Hart, M.M.; Klironomos, J.; et al. Branching out: Towards a Trait-Based Understanding of Fungal Ecology. Fungal Biol. Rev. 2015, 29, 34–41. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomycology: Biogeochemical Transformations of Rocks, Minerals, Metals and Radionuclides by Fungi, Bioweathering and Bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef]
- de Boer, W.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a Fungal World: Impact of Fungi on Soil Bacterial Niche Development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- Van der Wal, A.; Geydan, T.D.; Kuyper, T.W.; de Boer, W. A Thready Affair: Linking Fungal Diversity and Community Dynamics to Terrestrial Decomposition Processes. FEMS Microbiol. Rev. 2013, 37, 477–494. [Google Scholar] [CrossRef]
- Morel, M.; Meux, E.; Mathieu, Y.; Thuillier, A.; Chibani, K.; Harvengt, L.; Jacquot, J.; Gelhaye, E. Xenomic Networks Variability and Adaptation Traits in Wood Decaying Fungi. Microb. Biotechnol. 2013, 6, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Valášková, V. Degradation of Cellulose by Basidiomycetous Fungi. FEMS Microbiol. Rev. 2008, 32, 501–521. [Google Scholar] [CrossRef] [PubMed]
- Lundell, T.K.; Mäkelä, M.R.; Hildén, K. Lignin-modifying Enzymes in Filamentous Basidiomycetes—Ecological, Functional and Phylogenetic Review. J. Basic Microbiol. 2010, 50, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms Underlying Beneficial Plant–Fungus Interactions in Mycorrhizal Symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Eastwood, D.C.; Floudas, D.; Binder, M.; Majcherczyk, A.; Schneider, P.; Aerts, A.; Asiegbu, F.O.; Baker, S.E.; Barry, K.; Bendiksby, M.; et al. The Plant Cell Wall–Decomposing Machinery Underlies the Functional Diversity of Forest Fungi. Science 2011, 333, 762–765. [Google Scholar] [CrossRef]
- Osono, T. Ecology of Ligninolytic Fungi Associated with Leaf Litter Decomposition. Ecol. Res. 2007, 22, 955–974. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal Associations and Other Means of Nutrition of Vascular Plants: Understanding the Global Diversity of Host Plants by Resolving Conflicting Information and Developing Reliable Means of Diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a PH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C; Cambridge University Press: Cambridge, UK, 2022; pp. 1–24. [Google Scholar]
- Kabir, M.; Habiba, U.E.; Khan, W.; Shah, A.; Rahim, S.; Rios-Escalante, P.R.D.l.; Farooqi, Z.-U.-R.; Ali, L.; Shafiq, M. Climate Change Due to Increasing Concentration of Carbon Dioxide and Its Impacts on Environment in 21st Century; a Mini Review. J. King Saud Univ. -Sci. 2023, 35, 102693. [Google Scholar] [CrossRef]
- Tawaraya, K.; Turjaman, M. Use of Arbuscular Mycorrhizal Fungi for Reforestation of Degraded Tropical Forests. In Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration; Springer: Berlin/Heidelberg, Germany, 2014; pp. 357–373. [Google Scholar]
- Chen, J.; Tang, X.; Xu, H.; Li, Y.; Corrales, A.; Li, Y.; Kuzyakov, Y.; Liu, Z.; Liu, S. Mycorrhizal and Nutrient Controls of Carbon Sequestration in Tropical Rainforest Soil. Geoderma 2025, 454, 117188. [Google Scholar] [CrossRef]
- Imanuddin, R.; Hidayat, A.; Rachmat, H.H.; Turjaman, M.; Pratiwi; Nurfatriani, F.; Indrajaya, Y.; Susilowati, A. Reforestation and Sustainable Management of Pinus Merkusii Forest Plantation in Indonesia: A Review. Forests 2020, 11, 1235. [Google Scholar] [CrossRef]
- Rachmat, H.H.; Adinugroho, W.C.; Imanuddin, R.; Lestari, N.S.; Subiakto, A.; Purwanto; Brata, B.; Gaman, S.; Rossita, A. Promoting the 4N Concept for Tropical Peatland Restoration and Greenhouse Gas Emission Reduction. IPB Press: Bogor, Indonesia, 2023; ISBN 9786231110831. [Google Scholar]
- Lampela, M.; Jauhiainen, J.; Sarkkola, S.; Vasander, H. Promising Native Tree Species for Reforestation of Degraded Tropical Peatlands. For. Ecol. Manag. 2017, 394, 52–63. [Google Scholar] [CrossRef]
- Arifanti, V.B.; Sidik, F.; Mulyanto, B.; Susilowati, A.; Wahyuni, T.; Subarno, S.; Yulianti, Y.; Yuniarti, N.; Aminah, A.; Suita, E.; et al. Challenges and Strategies for Sustainable Mangrove Management in Indonesia: A Review. Forests 2022, 13, 695. [Google Scholar] [CrossRef]
- Wetadewi, R.I.; Osaki, M.; Turjaman, M.; Antonius, S.; Goenadi, D.H.; Nursyamsi, D.; Maswar; Surayah, L.; Kato, T. Principles of AeroHydro Culture. In Tropical Peatland Eco-Management; Springer: Singapore, 2021; pp. 249–283. [Google Scholar]
- Wulandari, D.; Nufus, M.; Faridah, E.; Maulana, A.F.; Tawaraya, K. Native Arbuscular Mycorrhizal Fungi and Nauclea Orientalis for Potential Reclamation of Tropical Coal Mining Areas. Environ. Adv. 2024, 15, 100462. [Google Scholar] [CrossRef]
- Adinugroho, W.C.; Rachmat, H.H.; Lestari, N.S.; Brata, B.; Santoso, P.B.; Imanuddin, R.; Aryanto; Rahayu, L.M.; Hidayat, A.; Turjaman, M.; et al. Revolutionizing Tropical Peatland Restoration in Indonesia: The 4N Approach. Oryx 2024, 58, 10–11. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of Soil Organic Matter as an Ecosystem Property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef]
- Kasel, S.; Singh, S.; Sanders, G.J.; Bennett, L.T. Species-Specific Effects of Native Trees on Soil Organic Carbon in Biodiverse Plantings across North-Central Victoria, Australia. Geoderma 2011, 161, 95–106. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Hamelin, M.; Navarrete, M.; Debaeke, P.; Henri, A. Emerging Agroscience. In Sustainable Agriculture Volume 2; Springer: Dordrecht, The Netherlands, 2011; pp. 3–14. [Google Scholar]
- Berthrong, S.T.; Piñeiro, G.; Jobbágy, E.G.; Jackson, R.B. Soil C and N Changes with Afforestation of Grasslands across Gradients of Precipitation and Plantation Age. Ecol. Appl. 2012, 22, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Clymo, R.S. Productivity and Decomposition of Peatland Ecosystems. In Peatland Ecosystems and Man: An Impact Assessment; Bragg, O., Hulme, P.D., Ingram, H.A.P., Robertson, R.A., Eds.; Department of Biological Sciences Dundee University: Dundee, UK, 1992; pp. 3–16. [Google Scholar]
- Varenius, K. Interactions Between Fungi, Forest Management, and Ecosystem Services; Swedish University: Sundsvall, Sweden, 2017. [Google Scholar]
- Monteverde, C.; De Sales, F.; Jones, C. Evaluation of the CMIP6 Performance in Simulating Precipitation in the Amazon River Basin. Climate 2022, 10, 122. [Google Scholar] [CrossRef]
- Thormann, M.N. The Role of Fungi in Boreal Peatlands. In Boreal Peatland Ecosystems; Springer: Berlin/Heidelberg, Germany, 2006; pp. 101–123. [Google Scholar]
- Tiko, J.M.; Ndjadi, S.S.; Obandza-Ayessa, J.L.; Mweru, J.P.M.; Michel, B.; Beeckman, H.; Rakotondrasoa, O.L.; Hulu, J.P.M.T. Carbon Sequestration Potential in Rubber Plantations: A Complementary Approach to Tropical Forest Conservation Strategies, A Review. Earth 2025, 6, 21. [Google Scholar] [CrossRef]
- Dwiyanti, F.G.; Rosdayanti, H.; Yulita, K.S.; Rachmat, H.H.; Ayyasy, Y.; Muharam, K.F.; Rahman, M.M.; Adzkia, U.; Siregar, I.Z. Growth and Wood Traits Evaluation of 15-Year-Old Tengkawang (Shorea Spp.) Tree Stands in Gunung Walat University Forest, West Java, Indonesia. Indones. J. For. Res. 2024, 11, 243–258. [Google Scholar] [CrossRef]
- Milenge Kamalebo, H.; De Kesel, A. Wild Edible Ectomycorrhizal Fungi: An Underutilized Food Resource from the Rainforests of Tshopo Province (Democratic Republic of the Congo). J. Ethnobiol. Ethnomed. 2020, 16, 8. [Google Scholar] [CrossRef]
- Sharma, E.; Bairwa, R.; Lal, P.; Pattanayak, S.; Chakrapani, K.; Poorvasandhya, R.; Kumar, A.; Altaf, M.A.; Tiwari, R.K.; Lal, M.K.; et al. Edible Mushrooms Trending in Food: Nutrigenomics, Bibliometric, from Bench to Valuable Applications. Heliyon 2024, 10, e36963. [Google Scholar] [CrossRef]
- Oyanedel, R.; Hinsley, A.; Dentinger, B.T.M.; Milner-Gulland, E.J.; Furci, G. A Way Forward for Wild Fungi in International Sustainability Policy. Conserv. Lett. 2022, 15, e12882. [Google Scholar] [CrossRef]
- Chamberlain, J.; Small, C.; Baumflek, M. Sustainable Forest Management for Nontimber Products. Sustainability 2019, 11, 2670. [Google Scholar] [CrossRef]
- Li, X.; Qu, Z.; Zhang, Y.; Ge, Y.; Sun, H. Soil Fungal Community and Potential Function in Different Forest Ecosystems. Diversity 2022, 14, 520. [Google Scholar] [CrossRef]
- Shackleton, C.M. Multiple Roles of Non-Timber Forest Products in Ecologies, Economies and Livelihoods. In Routledge Handbook of Forest Ecology; Peh, K.S.-H., Corlett, R.T., Bergeron, Y., Eds.; Routledge: Oxford, UK, 2015; p. 12. ISBN 9781317816447. [Google Scholar]
- Olarewaju, O.O.; Fawole, O.A.; Baiyegunhi, L.J.S.; Mabhaudhi, T. Integrating Sustainable Agricultural Practices to Enhance Climate Resilience and Food Security in Sub-Saharan Africa: A Multidisciplinary Perspective. Sustainability 2025, 17, 6259. [Google Scholar] [CrossRef]
- Boa, E. Wild Edible Fungi: A Global Overview of Their Use and Importance to People; Food and Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- Amirta, R.; Herawati, E.; Suwinarti, W.; Watanabe, T. Two-Steps Utilization of Shorea Wood Waste Biomass for the Production of Oyster Mushroom and Biogas—A Zero Waste Approach. Agric. Agric. Sci. Procedia 2016, 9, 202–208. [Google Scholar] [CrossRef]
- Neeraj, A.; Jarial, R.S.; Jarial, K.; Bhatia, J.N. Comprehensive Review on Oyster Mushroom Species (Agaricomycetes): Morphology, Nutrition, Cultivation and Future Aspects. Heliyon 2024, 10, e26539. [Google Scholar] [CrossRef] [PubMed]
- Yuwa-amornpitak, T.; Yeunyaw, P.-N. Diversity Wild Mushrooms in the Community Forest of Na Si Nuan Sub-District, Thailand. J. Biochem. Technol. 2020, 11, 28–36. [Google Scholar] [CrossRef]
- Bahram, M.; Netherway, T. Fungi as Mediators Linking Organisms and Ecosystems. FEMS Microbiol. Rev. 2022, 46, fuab058. [Google Scholar] [CrossRef] [PubMed]
- Turjaman, M.; Rachmat, H.; Hidayat, A.; Christita, M.; Faizal, A.; Istiandari, P.; Esyanti, R.; Siregar, I.; Dwiyanti, F.; Hermaty, D. Indonesian Agarwood: Sustainability and Global Markets; ITB Press: Bandung, Indonesia, 2025; ISBN 978-623-297-678-8. [Google Scholar]
- Thompson, I.D.; Lim, T.; Turjaman, M. Expensive, Exploited and Endangered: A Review of the Agarwood-Producing Genera Aquilaria and Gyrinops: CITES Considerations, Trade Patterns, Conservation, and Management; ITTO Technical Series; International Tropical Timber Organization (ITTO): Yokohama, Japan, 2022; Volume 12. [Google Scholar]
- Helbert; Turjaman, M.; Nara, K. Ectomycorrhizal Fungal Communities of Secondary Tropical Forests Dominated by Tristaniopsis in Bangka Island, Indonesia. PLoS ONE 2019, 14, e0221998. [Google Scholar] [CrossRef]
- Putra, I.P.; Nurhayat, O.D.; Taridala, S.A.A.; Herliyana, E.N.; Hartati, A.T.; Andi, B. Patadjai Systematics of Hemioporus Retisporus (PAT. & C.f. Baker) E. Horak (Agaricomycetes: Boletaceae), an Edible Mushroom from Southeast Sulawesi, Indonesia. Taprobanica 2025, 14, 58–64. [Google Scholar]
- Retnowati, A.; Faulina, S.A.; Helbert, H.; Riffiani, R.; Saskiawan, I.; Hidayat, A.; Turjaman, M.; Surya Hakim, S.; Rahayu, L.M.; Aryanto, A.; et al. Morchella rinjaniensis: A Novel Species of Tropical Morchella (Ascomycota, Pezizales, and Morchellaceae) Discovered in UNESCO Rinjani-Lombok Biosphere Reserve, Indonesia. Mycobiology 2025, 53, 367–378. [Google Scholar] [CrossRef]
- Bitzer, V.; Moździerz, M.; Kuijpers, R.; Schouten, G.; Juju, D.B. Gender and Forest Resources in Low- and Middle-Income Countries: A Systematic Literature Review. For. Policy Econ. 2024, 163, 103226. [Google Scholar] [CrossRef]
- Zaman, M.; Jabeen, A.; Waheed, M.; Haq, S.M.; Hashem, A.; Almutairi, K.F.; Abd_Allah, E.F.; Bussmann, R.W. Gendered Ethnobotanical Practices and Their Influence on Livelihoods: Non-Timber Forest Product Collection around Ayubia National Park. Trees For. People 2025, 19, 100752. [Google Scholar] [CrossRef]
- Turjaman, M.; Hidayat, A.; Santoso, E. Development of Agarwood Induction Technology Using Endophytic Fungi. In Agarwood: Science Behind the Fragrance; Springer: Singapore, 2016; pp. 57–71. [Google Scholar]







| Fungal Type | Mechanism | Effect on Plant Biomass/Carbon Pool | Impact on Carbon Storage/Sequestration | References |
|---|---|---|---|---|
| Arbuscular Mycorrhizal Fungi (AM fungi) | Enhanced nutrient/water uptake; increased photosynthesis efficiency; carbon allocation via hyphal networks | Increased shoot and root biomass, increased belowground carbon allocation | Moderate–high; substantial transfer of carbon belowground via hyphal networks | [16,17,30] |
| Ectomycorrhizal Fungi (ECM fungi) | Nutrient uptake and cycling (N, P); extensive mycelial networks transfer carbon deeper into soils; direct carbon storage in fungal tissues | Increased woody biomass and tree productivity; enhanced belowground biomass | Very high; significantly enhances carbon sequestration, especially in forest ecosystems | [18,21,29,37,38] |
| Saprotrophic Fungi | Decomposition of lignin, cellulose, and hemicellulose; formation of stable humic substances | Conversion of plant litter to stabilized organic matter, indirectly increasing stable carbon pools | High; enhances stable, long-term carbon storage through humic substance formation. | [26,27,28,39,40,41,42] |
| Endophytic Fungi | Plant growth promotion via phytohormone production; enhanced stress resistance | Increased plant biomass and resilience to environmental stress | Moderate–high; indirectly enhances carbon storage through sustained plant productivity | [43,44] |
| Fungal Species | Role in Methane Oxidation | Associated Methanotrophs | Ecosystem | Reference |
|---|---|---|---|---|
| Russula spp. | ECM fungi hosting methanotrophic bacteria | Methylocystis, Methylobacter | Boreal, tropical forests | [100,101] |
| Laccaria bicolor | Forms ECM symbiosis with methanotrophs, enhancing methane oxidation in root zones | Methylocystis | Forest soil (mycorrhizal) | [100] |
| Paxillus involutus | Promotes methane oxidation via ECM interaction | Methylobacter | Northern coniferous forest | [100] |
| Phanerochaete chrysosporium | White-rot fungus that promotes soil aeration, possibly enhancing methanotroph activity | Not directly associated | Decaying wood, forest soil | [102] |
| Trichoderma harzianum | Produces extracellular enzymes, supports carbon cycling; indirect methane influence | Not directly associated | Soil and rhizosphere | [103] |
| Penicillium spp. | Ascomycetes involved in methanol turnover support the microbial community | Not directly associated | Soil | [103] |
| Aspergillus spp. | Similar to Penicillium, it may facilitate secondary carbon transformation | Not directly associated | Soil | [103] |
| Glomus intraradices | Boosts methanotroph activity in the rhizosphere and encourages the production of methane oxidation enzymes through the effects of root exudates | Methylocystis sp., Methylosinus sp. | Agricultural soils (e.g., maize, rice rhizosphere) | [104] |
| Rhizophagus intraradices | Increases oxygen availability in the rhizosphere and stimulates methanotroph activity by modifying root exudates | Methylocystis sp., Methylobacter sp. | Paddy soils and terrestrial rhizosphere environments | [105] |
| Funneliformis mosseae | Contributes to increased methane monooxygenase activity through mutualistic interactions with host plants | Methylocystis sp. | Soybean rhizosphere; agricultural systems | [106] |
| Claroideoglomus etunicatum | Boosts the population and activity of methanotrophic bacteria through soil chemical changes and increased plant exudates | Not yet specifically identified: Methylomonas sp., Methylobacter sp. | Maize and cadmium-contaminated soils | [107] |
| Gigaspora margarita | Potentially creates microaerobic zones around roots that facilitate methane oxidation | Not yet specifically identified, possibly linked to Methylocapsa sp. | Legume-associated soils and stress-adapted environments | [108] |
| Diversispora versiformis | Alters soil microbial communities; may indirectly support methane oxidation | No specific genera have been confirmed; the effects on methanotrophs remain speculative | Remediated contaminated soils (e.g., cadmium) | [109] |
| Fungal Species | Role in Reducing N2O Emission | Reference |
|---|---|---|
| Mucor spp. | Some Mucor species contribute to N2O reduction through their involvement in the decomposition of organic matter, which reduces nitrogen availability for denitrifying bacteria. | [121] |
| Rhizophagus irregularis | R. irregularis improves nitrogen uptake by plants, reducing available nitrogen for nitrifiers and denitrifiers, thus mitigating N2O emissions. | [122] |
| Funneliformis mosseae | In symbiosis with plants, F. mosseae reduces the nitrogen pool in the rhizosphere, limiting N2O production by denitrifying bacteria. | [122] |
| Glomus spp. | Glomus species increases nitrogen assimilation by plants, which limits the nitrogen available for microbial processes that produce N2O. | [123] |
| Mortierella spp. | Particular species of Mortierella may influence N2O reduction by decomposing organic matter, reducing nitrogen compounds in the soil, which can lead to N2O production. | [124] |
| Fusarium oxysporum | Conducts dissimilatory nitrate reduction, converting N2O to N2 under low oxygen. | [125] |
| Aspergillus terreus | A. terreus reduces N2O via fungal denitrification pathways in soils. | [121] |
| Trichoderma asperellum | T. asperellum decreases soil N2O emission. | [126] |
| Chaetomium globosum | This species immobilizes nitrogen, thereby reducing the substrate available for bacterial denitrification. | [122] |
| Fungal Species | Metabolic Activities Contributing to Redox Potential | Redox Potential (mV) | Role | Reference |
|---|---|---|---|---|
| Trametes versciolor | Laccase production, lignin degradation, | +400 to +600 | Forest ecosystems, decomposing wood | [132,133] |
| Phanerochaete chrysosporium | Lignin peroxidase activity, manganese peroxidase production | +600 to +800 | Deciduous forest litter, woody debris | [134,135] |
| Aspergilus niger | Organic acid production, glucose oxidase activity | +100 to +300 | Soil ecosystems, agricultural environments | [136,137] |
| Trichoderma harzianum | Cellulase production, biocontrol metabolites | +200 to +400 | Agricultural soils, rhizosphere | [138,139] |
| Penicilum chrysogenum | Secondary metabolite production, organic acid synthesis | +150 to +350 | Soil environments, organic substrates | [137,140] |
| Pleurotus astreatus | Laccase activity, cellulose degradation | +300 to +500 | Forest ecosystems, agricultural waste | [141,142] |
| Fusarium oxysporum | Root colonization, iron reduction | +50 to +250 | Plant rhizosphere, agricultural soils | [136,143] |
| Agaricus bisporus | Phenol oxidase activity, organic matter decomposition | +250 to +450 | Compost ecosystems, agricultural soils | [144,145] |
| Reforestation Method | Type of Forest (Location) | Reforested Area (Ha) | Tree Species | References |
|---|---|---|---|---|
| ECM fungi (tablet) | Mountain Forest Plantation (Takengon, Aceh Tengah) | 97,300 * | Pinus merkusii | [152] |
| ECM fungi (tablet, alginate, spores) | Forest plantation (Jasinga-West Java, Majenang-Central Java, Muria Mountain-Central Java, Ponorogo Selatan-East-Java) | 17 | P. merkusii, Shorea leprosula | [152] |
| 4N Concept | Peatland (South Sumatra, Central Kalimantan (Indonesia) | 115.6 | Native species | [153,154] |
| ECM fungi (soil from host trees) | Mountain forest (Gunung Dahu, West Java, Indonesia) | 250 | Shorea spp. (6 species) | [101] |
| AM fungi, soil from the host tree coated on the seedball | Mangroves (Indramayu, West Java, Indonesia, 2 Ha; Teluk Prima, Bali Barat National Park, Bali Province, 2 Ha) | 4 | Avicennia marina | [155] |
| AeroHydro Culture (AM fungi + PGPR) | Peatland (Riau and Central Kalimantan) | 2 | Oil palm, S. balangeran, sago | [156] |
| AM fungi | Forest Plantation Industry, Perawang, Riau | 3 | Melaleuca cajuputi, Cratoxylon arborescens, Lophostemon suaveolens | [50] |
| AM fungi | Post gold mining (Kendari, Southeast Sulawesi) | <1 | Pterocarpus indicus, Pericopsis moniana | [51] |
| AM fungi | Post-opencast coal mining (East Kalimantan) | <1 | Albizia saman Paraserianthes falcataria | [157] |
| Fungal Species | Use | Regional Sources | Economic Value | Trading and Market Demand | Sources |
|---|---|---|---|---|---|
| Cordyceps sinenisis | Edible mushroom | Asia | High | Regional food markets | [170] |
| Termitomyces spp. | Edible mushroom (NTFPs) | Africa, Asia | High local market value | Local/regional food markets | [176] |
| Ganoderma lucidum | Medicinal (anti-inflammatory) | China, South Asia, Southeast Asia | High in herbal medicine | Global nutraceutical industry | [126] |
| Pleurotus ostreatus | Edible, bioremediation | Worldwide | Medium | Commercial mushroom farming | [177,178] |
| Astraeus hygrometricus | Edible mushroom | Thailand | Very high | High-end restaurants/export | [179] |
| Aspergillus spp. | Industrial enzymes (biofuels) | Global | Medium–high | Biotechnology sector | [180] |
| Volvariella volvacea | Edible mushroom | Malaysia, Indonesia, South-east Asia | High | Mushroom farming | [38] |
| Fusarium solani | Agarwood inoculant | India, Bangladesh, Sri Lanka, China, Southeast Asia | High | Agarwood chips and oil, incense, and herbal medicine market | [181,182] |
| Hemiosporus retisporus | Edible mushroom | Indonesia | High | National food market | [183,184] |
| Morchella rinjaniensis | Edible mushroom | Indonesia | High | National food market | [185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prayudyaningsih, R.; Turjaman, M.; Christita, M.; Lelana, N.E.; Irianto, R.S.B.; Antonius, S.; Hakim, S.S.; Putri, A.I.; Rachmat, H.H.; Arifanti, V.B.; et al. Tropical Fungi and LULUCF: Synergies for Climate Mitigation Through Nature-Based Culture (NbC). Climate 2025, 13, 208. https://doi.org/10.3390/cli13100208
Prayudyaningsih R, Turjaman M, Christita M, Lelana NE, Irianto RSB, Antonius S, Hakim SS, Putri AI, Rachmat HH, Arifanti VB, et al. Tropical Fungi and LULUCF: Synergies for Climate Mitigation Through Nature-Based Culture (NbC). Climate. 2025; 13(10):208. https://doi.org/10.3390/cli13100208
Chicago/Turabian StylePrayudyaningsih, Retno, Maman Turjaman, Margaretta Christita, Neo Endra Lelana, Ragil Setio Budi Irianto, Sarjiya Antonius, Safinah Surya Hakim, Asri Insiana Putri, Henti Hendalastuti Rachmat, Virni Budi Arifanti, and et al. 2025. "Tropical Fungi and LULUCF: Synergies for Climate Mitigation Through Nature-Based Culture (NbC)" Climate 13, no. 10: 208. https://doi.org/10.3390/cli13100208
APA StylePrayudyaningsih, R., Turjaman, M., Christita, M., Lelana, N. E., Irianto, R. S. B., Antonius, S., Hakim, S. S., Putri, A. I., Rachmat, H. H., Arifanti, V. B., Adinugroho, W. C., Fahmi, S., Imanuddin, R., Suharti, S., Sari, U. K., Hidayat, A., Suhartana, S., Wahyuni, T., Silsigia, S., ... Osaki, M. (2025). Tropical Fungi and LULUCF: Synergies for Climate Mitigation Through Nature-Based Culture (NbC). Climate, 13(10), 208. https://doi.org/10.3390/cli13100208










