Abstract
Oak forests are an important part of temperate European ecosystems, where they are actively improving biodiversity, carbon storage, and ecological stability. However, current concerns such as climatic changes, and especially rising temperatures and changing precipitation patterns, are impacting their resilience. In this context, our study intends to evaluate the impact of climatic variability on temperate oak forests, focusing on the influence of temperature and precipitation. This covers different sites that have different environmental conditions. By using both a bibliometric approach and a systematic analysis of publications that have studied the influence of climate change on oak forests, our study has identified specific species and site responses to climate stressors. Furthermore, we have also evaluated trends in drought sensitivity. All these aspects have allowed us to understand and suggest improvements for the impact of climate change on the resilience and productivity of oak ecosystems. We have analyzed a total number of 346 publications that target the impact of climate change on oak forests. The articles were published between 1976 and 2024, with the majority originating from the USA, Spain, Germany, and France. These studies were published in leading journals from Forestry, Environmental Sciences, and Plant Sciences, among which the most cited journals were Forest Ecology and Management, the Journal of Biogeography, and Global Change Biology. As for the keywords, the most frequent ones were climate change, drought, growth, forest, and oak. However, we have observed a trend towards drought sensitivity, which indicates the intensification of climate changes on oak ecosystems. Moreover, this trend was more present in central and southern regions, which further highlights the impact of regional conditions. As such, certain local factors (soil properties, microclimate) were also taken into account in our study. Our literature review focused on the following aspects: Oak species affected by climate change; Impact of drought on oak forests; Influence of climate change on mixed forests containing oaks; Effects of climate change on other components of oak ecosystems; Radial growth of oaks in response to climate change; Decline of oak forests due to climate change. Our results indicate that oak forests decline in a process caused by multiple factors, with climate change being both a stressor and a catalyst. Across the globe, increasing temperatures and declining precipitation affect these ecosystems in their growth, functions, and resistance to pathogens. This can only lead to an increased forest decline. As such, our results indicate the need to implement forest management plans that take into account local conditions, species, and climate sensitivity. This approach is crucial in improving the adaptivity of oak forests and mitigating the impact of future climate extremes.
1. Introduction
Climate change is a widely acknowledged phenomenon, though it continues to generate debate in some circles. It remains a central focus of scientific discourse, with many questions still unresolved. Among the most frequently explored areas is the relationship between climate change and forest ecosystems. As of 20 February 2025, a search revealed 68,736 scholarly articles addressing this broad topic.
Oaks, belonging to the genus Quercus in the beech family (Fagaceae), comprise approximately 500 to 600 species globally. They are particularly abundant in parts of Europe (e.g., Bulgaria, Portugal, Italy, Spain, and Romania), North America (Canada, the United States, and Mexico), and Asia (including Afghanistan, China, Mongolia, Nepal, Russia, Japan, India, and Pakistan) [1,2,3,4]. In the United States, 93 oak species are distributed across nearly the entire country [5]. In Mexico, oaks (Quercus spp.) are prominent in both lowland and montane forests, where they often represent the tallest tree species [6].
Recent studies and observations regarding climate changes have emphasized clear regional variability patterns. These are crucial in analyzing the response of oak forests located in temperate areas [7].
In Europe, climate variability has evidently increased, with a focus on precipitation patterns. Greater annual precipitations are recorder in high altitude areas from Northern Europe, while drying phenomena are prevalent in Southern and Eastern Europe. SPI studies have showcased fewer and less severe droughts in Northeastern Europe, with an opposite pattern in Southern Europe, namely severe droughts during spring and summer. The north-south difference is also proved by SPEI (which includes both temperature and precipitation). The past three decades have showcased wetter conditions in Northern Europe and increased droughts in Central and Southern Europe [8,9,10].
In North America, divergent regional trends were indicated by many climate studies and projections. For example, warming and droughts are projected to decrease soil moisture and affect ecosystem processes in areas such as the southern Great Plains, the Southwest, and northern Mexico. On the other hand, warmer and wetter conditions are projected to positively impact northern areas such as the northern Plains and southern Canada. Increased temperatures will also appear in the Pacific Northwest, but with no significant changes in annual precipitation. However, it is expected that summer precipitations will decline sharply. Rising temperatures are expected to cause more frequent and extreme hydrological events and droughts. This proves the suggestion of some others that temperatures will become more important than precipitation in affecting future drought risks [11,12].
In Asia, an increased drought was recorded in Northern East Asia (NEA). This was mainly caused by rising temperatures. Compared with precipitation trends (that recorded no significant changes), warming indicators are directly linked with drought intensification. Some studies (1902–2018) have shown that extreme droughts are predominantly increasing during the growth seasons. China and Mongolia have shown the highest number and duration of droughts, becoming the driest parts of East Asia. On the other hand, Japan, South Korea, and North Korea have wetter summers, which lead to fewer droughts [13,14].
In this context of regions affected by regional climate conditions (increased precipitations, increased droughts caused by higher temperatures, consistent dryings in southern areas), it is essential to understand how oak forests are adapting and responding to these climate influences.
In the Central Himalayas, oak forests constitute the climax evergreen vegetation between 1000 and 3600 m elevation [15]. Holm oak (Quercus ilex L.) is a dominant species in the Mediterranean, occupying transition zones between temperate forests and shrublands [16]. English oak (Quercus robur L.) is a foundational species in mixed and broadleaved forests across Eastern Europe [17,18]. In Asia, the Mongolian oak (Quercus mongolica) is widespread [19], while in Pakistan, seven oak species have been recorded [1].
Despite their adaptability, oaks often face strong competition from more competitive species, such as European beech (Fagus sylvatica L.), particularly in optimal growing sites [20]. Conversely, oaks are more successful under challenging ecological conditions—such as arid environments, clay soils, or floodplains—where they can establish natural stands and occupy specific ecological niches [21,22]. Nonetheless, monospecific oak stands growing under ideal conditions remain rare.
Bibliometrics—a method that uses statistical analysis to evaluate the impact and structure of the academic literature—has become an essential tool in scientific research. It allows researchers to systematically examine large bodies of literature, revealing historical trends, knowledge gaps, and emerging areas of interest. In recent years, the development of advanced software tools has greatly enhanced the efficiency and accuracy of bibliometric analysis [23,24]. Unlike traditional literature reviews, bibliometric methods enable rapid mapping of a research field and the identification of key publications and research directions.
By utilizing citation and content analysis, bibliometric techniques have gained increasing prominence, supported by the growth of digital databases and computational tools. Their effectiveness depends on the availability of large datasets, making them especially suitable for analyzing well-established research areas [25].
Although numerous reviews have addressed the broader implications of climate change [26,27,28,29,30] and forestry-related topics [31,32,33,34,35], no comprehensive review has specifically examined the intersection of climate change and oak forests.
To fill this gap, we conducted an in-depth review of the ISI-indexed literature using both traditional and bibliometric approaches. The results offer an integrated overview of current knowledge, highlight research trends, and provide a valuable foundation for future studies exploring how climate change affects oak-dominated ecosystems.
The primary goal of our research was to assess the sensitivity of temperate oak forests to climatic variability, with a particular focus on the impacts of temperature and precipitation on these ecosystems. By analyzing published articles, we aimed to identify species-specific and site-specific growth responses to climate stressors, evaluate temporal trends in drought sensitivity, and improve our understanding of how ongoing climate change may affect the resilience and productivity of oak-dominated ecosystems.
2. Materials and Methods
The first part of this study involved a bibliometric analysis to assess global scientific research on the impact of climate change on oak forests from 1976 to 2024. We conducted our analysis using the Science Citation Index Expanded (SCI-Expanded) within the Web of Science database, as well as Scopus, to identify relevant publications.
After carefully evaluating different search strategies and using various keywords, the phrase “influence of climate change on oak forests” was selected as the primary search term. This search yielded 695 articles from Web of Science and 442 articles from Scopus. However, only research articles and review papers were included in our analysis. Following a thorough examination of each article, the final dataset was reduced to 346 articles after eliminating duplicates (articles appearing in both databases), publications unrelated to the topic, and those lacking abstracts.
The articles were filtered based on these criteria: (1) peer-reviewed article; (2) includes an abstract; (3) focuses on the influence of climate change on oak forests. On the other hand, the articles that were excluded were (1) publications that used keywords in unrelated contexts (for example, using the term “climate change” in forests in general); (2) the terms were used in non-scientific content (editorial notes, errata); and (3) non-English sources or the gray literature (technical reports, NGO publications).
The bibliometric analysis focused on ten key aspects: (1) publication types, (2) research areas, (3) publication years, (4) countries, (5) authors, (6) institutions, (7) language, (8) journals, (9) publishers, and (10) keywords.
Data processing was performed using the Web of Science Core Collection tools, Scopus, Excel, and Geochart [36,37,38,39]. To generate visual maps and conduct cluster analyses, we utilized VOSviewer (version 1.6.20) [40].
The second part of this study employed a traditional literature review approach, providing an in-depth analysis of the 346 selected articles. The findings were categorized into six main research areas: (1) Oak species affected by climate change; (2) Impact of drought on oak forests; (3) Influence of climate change on mixed forests containing oaks; (4) Effects of climate change on other components of oak ecosystems; (5) Radial growth of oaks in response to climate change; (6) Decline of oak forests due to climate change.
The first part of this study involved a bibliometric analysis from Web of Science as well as Scopus to identify relevant publications (Figure 1).
Figure 1.
Methodology used.
3. Results
3.1. Classical Review—Synthesis of the Literature
From the analysis of the types of publications (898) in which the articles on the impact of climate change on oak forests are classified, it is observed that the largest number are research articles (638 articles, 94% of the total publications), followed by 32 reviews (4%), while proceedings papers and book chapters represent 1% (Figure 2).
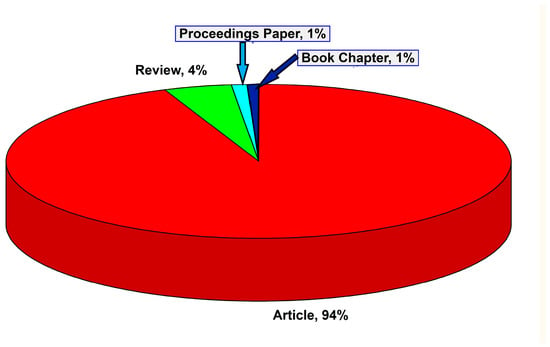
Figure 2.
Distribution of the main publication types related to the impact of climate change on oak forests.
We have identified 36 research areas in which we can frame the published articles. The most representative areas are Forestry (41%), Environmental Sciences (29%), and Plant Sciences (12%) (Figure 3).
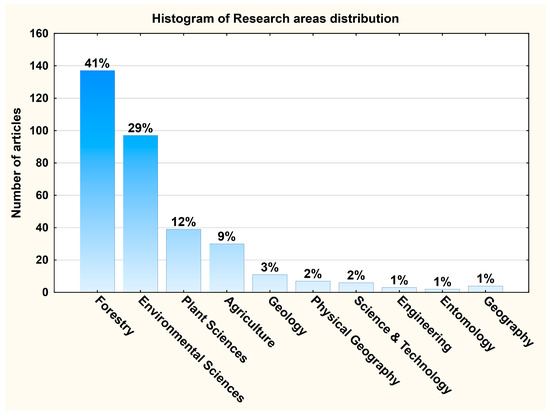
Figure 3.
Distribution of the primary research areas in publications analyzed bibliometrically.
Starting from 1986 (when the first article on this topic was published), the number of publications has steadily increased, reaching 21 in 2020 and 41 in 2024 (Figure 4).
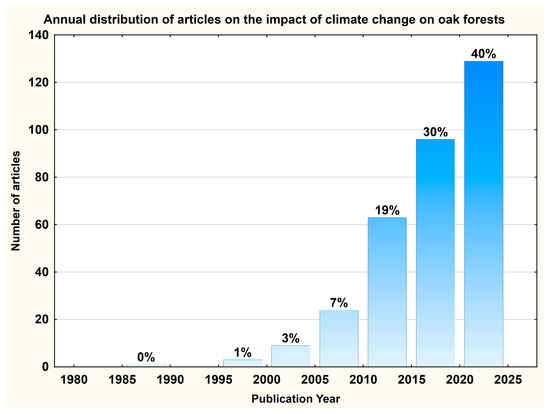
Figure 4.
Annual distribution of articles on the impact of climate change on oak forests.
Authors from 77 countries have published articles on this topic. The most well-represented countries are the USA, Spain, Germany, France, and China (Figure 5).
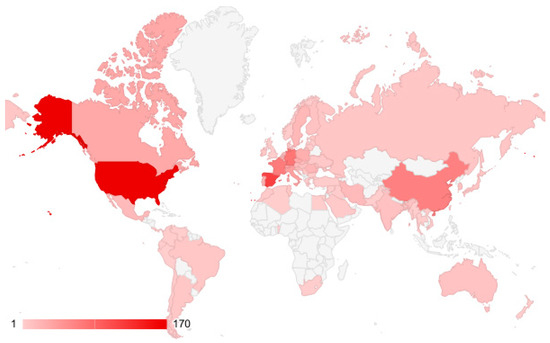
Figure 5.
Countries with contributing authors of articles on the impact of climate change on oak forests.
Countries can be grouped into five clusters, two of which contain the most countries; the first cluster includes Croatia, Denmark, Estonia, Finland, Germany, Israel, Italy, the Netherlands, Poland, and Sweden; the second cluster includes Argentina, Brazil, Hungary, India, Mexico, Saudi Arabia, Spain, Tunisia, and the USA (Figure 6).
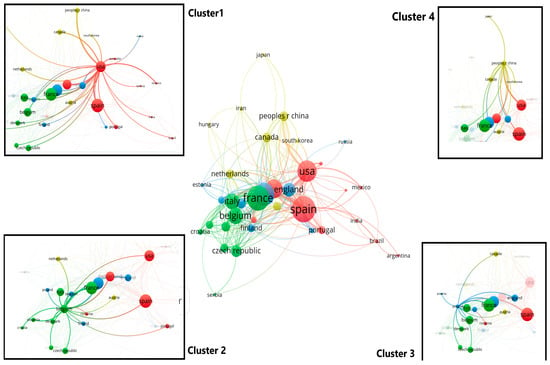
Figure 6.
Country clusters of authors publishing on the impact of climate change on oak forests.
The vast majority of articles (95%) are written in English. However, we have also identified articles written in other languages, such as French and Spanish (three articles each), Croatian, German, and Russian (two articles each), and Slovak (one article).
The journals with the greatest number and most significant articles published on the impact of climate change on oak forests are Forest Ecology and Management, Forests, Science of the Total Environment, and Agricultural and Forest Meteorology (Table 1 and Figure 7).

Table 1.
Leading journals publishing articles on the impact of climate change on oak forests.
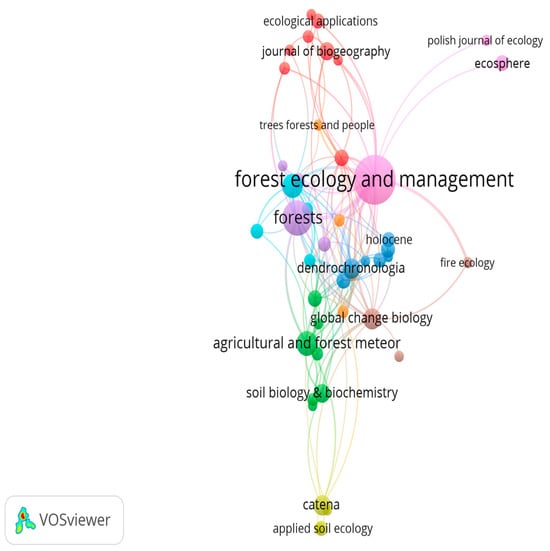
Figure 7.
Key journals featuring articles on the impact of climate change on oak forests.
The institutions to which the authors of these articles belong, ranked by importance, are as follows: Chinese Academy of Sciences (nine articles); United States Department of Agriculture (USDA) (nine articles); Centro de Investigacion Ecologica y Aplicaciones Forestales (CREAF-CERCA) (seven articles); Consejo Superior de Investigaciones Cientificas (CSIC) (seven articles); Swiss Federal Institutes of Technology (seven articles); Czech University of Life Sciences Prague (five articles).
Among the 93 publishers who have published articles on this subject, the most representative are Elsevier (219 articles), Springer Nature (104 articles), Wiley (101 articles), and MDPI (52 articles).
In these articles, the most frequently used keywords are climate change, drought, growth, forest, and oak (Table 2).

Table 2.
Most frequently used keywords in articles on the impact of climate change on oak forests.
Based on their connections, keywords can be grouped into several clusters, the most important being the first cluster includes dendrochronology, drought, forest management, forests, oak, radial growth, stands, and tree mortality; the second cluster includes climate change, impacts, model, mortality, phenology, precipitation, responses, and trees (Figure 8).
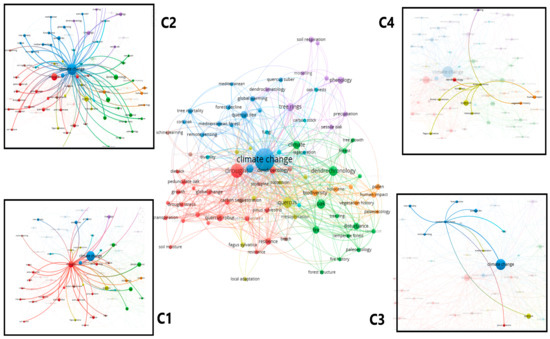
Figure 8.
Authors’ keywords related to the impact of climate change on oak forests C1 = first cluster: C2 = second cluster; C3 = third cluster; C4 = fourth cluster.
Regarding the evolution over time of keyword usage, it can be observed that in the early period (2015–2016), the most commonly used words were vegetation, disturbance, carbon, and fire. In the middle period (2017–2018), the predominant keywords were climate change, oak, biodiversity, patterns, and tree. In the most recent period (2019–2024), the keywords used were temperature, precipitation, drought, radial growth, and soil (Figure 9).
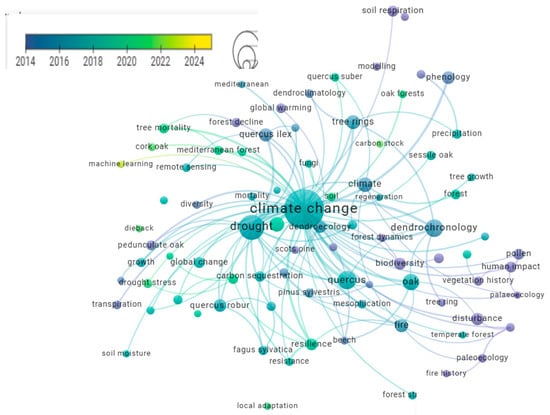
Figure 9.
Annual distribution of keywords related to the impact of climate change on oak forests.
3.2. Oak Species’ Climate Sensitivity Across Regions
A significant number of oak species are cited in studies concerning the impact of climate change on forests. The main of these species are presented in Table 3.

Table 3.
Oak species affected by climate change.
3.3. Physiological and Growth Responses of Oak Forests to Drought and Temperature
Our analysis has shown that drought can reduce carbon sequestration rates (CSR) in oak forests. This happened even in areas that recorded modest temperature raises. An example is the northeastern range limits of Quercus robur [84], which is relatively sensitive to climatic changes, making it susceptible to climate proxy. Growth rates were mostly correlated with precipitation and water balances in both Q. robur and Q. petraea. This happened especially during summer months [87]. We did not observe any significant latitudinal, longitudinal, or elevation patterns, with the exception of a decreased water balance during summer months in the case of Q. petraea with increasing latitude. In addition, both species did not maintain their pre-drought growth during drought years, although they did show a fast recovery after summer droughts. This contrasted specifically with sluggish or incomplete recoveries recorded after spring droughts [97].
Q. cerris showcased a higher drought tolerance and a stronger climate-related growth when compared with Q. robur from the lowland mixed forests of Serbia and Bulgaria [51,52,98]. In Hungary, Q. petraea and Q. cerris were limited in their growth because of precipitation [50]. Q. robur growths were correlated with climatic changes in floodplains, including scPDSI, groundwater levels, and river discharge [99].
In North America, chestnut and white oaks were the species that recorded the best resilience towards drought in comparison with black and northern red oaks. For example, the stem size and density of black oaks has caused a negative influence on their resistance to drought [96]. A similar situation was recorded in Tunisia, where Q. suber recorded higher mortality rates (up to 63,622 trees) during the prolonged droughts recorded between 1988 and 1995 [100].
In Central Europe, radial growth was the factor most positively associated with spring and summer temperatures, although it was moderated by groundwater [101]. In East Asia, Quercus spp. was more resilient to warming temperatures compared with Pinus densiflora. Some projections suggest possible compositional shifts in the future [102,103].
Q. liaotungensis and Q. robur were very influenced by the hydroclimate conditions recorded during late summers and autumns [67,82]. Negative correlations between radial growth and potential evapotranspiration were also recorded for Q. robur and Q. petraea in June [75]. Growths were mainly caused by prolonged droughts that lasted more than two years in the Moldavian Plateau [104].
3.4. Mixed and Transitional Forest Dynamics Under Climate Stress
In the transition zone between oak and beech forests, where drought is the most limiting factor, oaks with less favorable stem diameter position (Weisse’s middle stems) show slower radial growth, have less pronounced climatic precipitation signals, and have milder responses to extreme climatic events [105].
In oak–beech forests in the Swiss lowlands, at most sites, the relative importance of oak decreased gradually compared to beech. Mortality increased over time for both oak and beech, but the increase was stronger for oak. Oak and beech mortality decreased with increasing DBH. Additionally, oak mortality increased with stand basal area, whereas no such trend was found for beech [106]. Also for oak–beech, in Italy: After the 1990s, beech trees showed a climate-driven decrease in growth compared to oak, especially after 2003 (−20% of basal area increment), with a significant growth trend reversal between the species. Q. cerris trees showed a significantly higher ability to recover their growth levels after extreme droughts [49].
A currently warmer and drier climate seemed to favor the height growth superiority of European beech, whereas it decreased the superiority of Scots pine. The results indicated that even if the climate changes as predicted, the growth of oak will depend upon silvicultural promotion [107].
Dendrochronological studies on mixed stands of Oregon white oak and Douglas-fir on Southern Vancouver Island revealed species-specific differences in radial growth sensitivity to drought. Oregon white oak trees, particularly those growing at high densities or competing with Douglas-fir for moisture, were found to be more sensitive to drought and more sensitive to growing conditions during the prior year. The response of Douglas-fir to drought was less variable, possibly due to the relatively low conifer densities at the study site, as well as the species’ ability to root graft, its higher shade tolerance than Oregon white oak, and its rapid growth rates that allow it to achieve a more dominant canopy position. The non-stationary response to climate exhibited by Oregon white oak provides insights into the mechanisms by which Oregon white oak savannas are being converted to coniferous woodland but also suggests that tree-ring reconstructions of climate need to explicitly address changes in stand dynamics that could influence the growth–climate relationship [59].
Examined species interactions in mixtures of Scots pine (Pinus sylvestris) and pedunculate oak (Quercus robur) for varying soil conditions in the Netherlands over a period of 30 years, showing lower productivity of oak and higher productivity of Scots pine under climate change [85].
Both species, broadleaf Quercus mongolica (QUMO) and coniferous Abies koreana (ABKO), grow at the southern end of their distributional range, and they have adjacent altitudinal ranges in South Korea. This suggests an upward migration potential for QUMO due to warming-enhanced growth at higher elevations. In the elevational zone where ABKO and QUMO coexist, warming plays a primary role in ABKO growth reduction, while QUMO growth increases and thus induces a potential upward migration of QUMO. This combined effect can lead to population decline of ABKO [72].
3.5. Ecosystem-Level Effects and Structural Shifts of Oak Forests
In oak–beech forests of the Swiss lowlands, oak has gradually lost its relative dominance compared to beech at most sites. While mortality rates have increased for both species over time, the increase has been more pronounced in oak. The mortality of both species decreased with increasing diameter at breast height (DBH) but tended to rise with higher precipitation levels. Additionally, oak mortality increased with stand basal area, whereas no such correlation was observed for beech [106]. A similar trend was observed in oak–beech forests in Italy, where beech trees experienced a climate-driven reduction in growth compared to oak after the 1990s, especially after 2003 (−20% basal area increment). A significant growth trend reversal was noted between the species, with Quercus cerris displaying a significantly higher ability to recover growth levels after extreme droughts [49].
A warmer and drier climate appears to favor the height growth superiority of European beech while reducing the growth dominance of Scots pine. These findings suggest that even if climate projections materialize, oak growth will be strongly influenced by silvicultural management [107].
Dendrochronological studies on mixed stands of Oregon white oak and Douglas-fir on Southern Vancouver Island revealed species-specific differences in radial growth sensitivity to drought. Oregon white oak, particularly in high-density stands or when competing with Douglas-fir for moisture, showed heightened sensitivity to drought and growing conditions from the prior year. In contrast, Douglas-fir exhibited a more stable response to drought, likely due to its lower density, root grafting capabilities, higher shade tolerance, and rapid growth rate, allowing it to attain canopy dominance. The non-stationary climate response of Oregon white oak provides insights into its transition from savanna to coniferous woodland and suggests that tree-ring-based climate reconstructions should consider stand dynamics that influence growth–climate relationships [59].
A study on Scots pine (Pinus sylvestris) and pedunculate oak (Quercus robur) mixtures in the Netherlands over a 30-year period showed that climate change and varying soil conditions resulted in reduced oak productivity while increasing Scots pine productivity compared to current climatic conditions [85].
In South Korea, both broadleaf Quercus mongolica and coniferous Abies koreana (ABKO) occupy the southernmost part of their range with adjacent altitudinal distributions. Warming has enhanced Quercus mongolica growth at higher elevations, suggesting an upward migration potential for the species. In areas where Abies koreana and Quercus mongolica coexist, warming has primarily led to a reduction in Abies koreana growth while promoting Quercus mongolica growth, potentially leading to a population decline of Abies koreana in the future.
3.6. Drivers and Patterns of Decline in Oak Forests
The decline of oak forests is a multifaceted process influenced by a combination of biotic and abiotic stressors, with climate change playing a central and increasingly prominent role. Recent studies have emphasized the synergistic effects of environmental stressors and biological agents in accelerating oak decline across diverse biogeographical regions.
In Mediterranean-type climate regions, a review by Marques et al. [108] identified pathogens (48%) and climate factors (17%) as the most frequently studied drivers of oak decline. Notably, interactions between multiple stressors—particularly those involving climatic variables—were often reported, suggesting that combined effects may exacerbate decline symptoms more severely than isolated stressors.
Phytophthora cinnamomi, a soil-borne oomycete, has been strongly associated with the widespread decline and mortality of Quercus suber and Q. ilex across the Mediterranean. Its impact is particularly pronounced when coupled with prolonged drought conditions, which are believed to act as predisposing factors in the Iberian Peninsula’s oak decline [61]. Similarly, Ruiz-Gómez et al. [62] identified extreme climatic events and the incursion of alien invasive species as primary drivers of Q. ilex loss in Mediterranean open woodlands.
In North Africa, particularly Tunisia, cork oak (Q. suber) forests have experienced marked reductions over the past century. Historical analyses report a decline of 40,000 hectares between 1952 and 1992 [109] and a 45% decrease in forest area between 1920 and 2005—from 148,000 to 70,000 hectares [110]. Modeling studies predict that up to 40% of climatically suitable habitat for cork oak could be lost by 2070, with the most pronounced impacts expected in northern Africa and the southern Iberian Peninsula [93]. The decline is closely linked to reduced precipitation, which is the key positive driver of cork productivity, while rising temperatures enhance evaporative demand, exacerbating water stress. Moreover, the encroachment of drought-tolerant species such as Aleppo pine into cork oak habitats indicates a broader ecological shift likely driven by climate change.
Despite cork oak’s adaptation to the Mediterranean climate—characterized by wet winters and hot, dry summers—this species remains vulnerable to long-term climate variability. Extended periods of high temperature and/or low precipitation can reduce soil water availability, triggering physiological stress and increasing susceptibility to pathogen attacks [111].
In Central and Eastern Europe, similar patterns of climate-induced decline have been documented. In Poland, oak decline (Q. robur) is attributed to the synergistic effects of biotic and abiotic stressors, with a notable increase in drought frequency—especially in May and June—contributing significantly to reduced vigor and increased mortality [112]. Long-term comparisons using Walter’s climate diagrams and dendrochronological data confirm the major role of climatic variability in driving these trends.
In Romania, the decline of oak species has been substantiated through a combination of historical reports and interdisciplinary research from intensive monitoring plots (Level II), where climatic stress was identified as the primary causal factor [113].
Northern latitudes are not exempt from these patterns. In Finland, long-term monitoring of oak populations revealed that trees affected by dieback had ceased growth during a sequence of dry summers between 2005 and 2007. These declining or dead trees exhibited reduced radial growth beginning as early as the 1990s, indicating a lagged response to prolonged drought conditions. In contrast, healthy individuals showed greater adaptive capacity and resilience to climatic fluctuations [114].
Overall, the accumulated evidence highlights that climate change—especially increasing temperatures and decreasing precipitation—interacts with biotic stressors to accelerate the decline of oak forests. This phenomenon is regionally variable but globally consistent in its attribution to climate-induced physiological stress, reduced growth, and heightened vulnerability to secondary agents such as pathogens and invasive species.
4. Discussion
4.1. Classical Review—Synthesis of the Literature
Most of the studied articles are scientific articles (94%), with 4% review articles. This indicates a maturity of this topic, reaching a point where meta- and synthesis studies are in place. This is also a reflection of climate change and its impact on oak forests. The fact that this has reached a research levels only indicates the need to evaluate and integrate studies and methodologies.
The articles covered 36 research areas, highlighting the interdisciplinary aspect of this study. Most of the articles covered Forestry (41%), Environmental Sciences (29%), and Plant Sciences (12%), reflecting the primary approach of oak forests from an ecological and environmental point of view. However, the numerous disciplines that have tackled oak forests also imply the large impact of this topic. This ranges from biodiversity conservation, land-use change, and ecosystem services to social, climatic, and biological domains.
The number of publications has grown significantly, with a notable rise of 41 in 2024. This again highlights the international concern caused by climate change and its impact on forest ecosystems. This fact consolidates the growing number of policies and public concerns raised by climate resilience, carbon sequestration, and biodiversity preservation. Furthermore, this indicates a future expansion and even diversification of sub-topics (assisted migration, genomic adaptation, and socio-ecological resilience).
Our research shows a larger input from countries such as the USA, Spain, Germany, France, and China. We can attribute this fact to a series of factors. The first one is that these countries possess significant oak forests, followed by established research and funding mechanisms in the climate and environmental areas. However, this can also reflect regional vulnerabilities, such as the particularly sensitive nature of Mediterranean countries (France, Spain), especially under drought conditions, while temperate areas (the USA and Germany) are affected by growth and mortality in the context of warming and seasonal variations. In addition, these are the countries that are usually very active in international forest monitoring networks and research programs, which fuels their need for studies and consistent data gathering.
International collaborations and themes are further enriched by identifying research clusters among these countries. For example, Cluster 1 (e.g., Germany, Sweden, and the Netherlands) focuses on temperate countries and boreal forests with strong environmental research agendas. On the other hand, Cluster 2 (e.g., USA, Spain, and India) includes countries that must deal with complex ecological situations and regional concerns caused by climate change, which makes them interested in adopting and implementing resilience strategies. This clustering can also reflect different research priorities—from the decline of temperate European forests to resilience toward drought from subtropical areas.
Our language analysis also showcased a larger number of English articles. This is expected in a global scientific community, but articles in other languages (Spanish, French, and German) can suggest a second layer of articles that can reveal studies that are more focused or niched. This aspect can be further studied in future reviews and comparative articles.
Our article’s subject is of high academic importance, proven by the high number of renowned journals that tackle this topic at a global research level (e.g., Forest Ecology and Management and Science of the Total Environment). These publications are known for providing studies that are relevant and rigorous while tackling high ecological concerns. This only highlights even more our concern towards the decline of oak forests.
The same importance can also be found in the institutions that promote monitoring programs (e.g., Chinese Academy of Sciences, USDA, and CREAF). This can come from their access to large forest plots, experimental infrastructure, or modeling capabilities. More importantly, institutions such as CREAF and CSIC are focused on Mediterranean forest ecology, while USDA and the Chinese Academy of Sciences cover more ecologically diverse regions.
Next, our keyword clustering offers important data about the evolution and focus of methodologies. Terms such as “drought”, “radial growth”, “mortality”, and “phenology” suggest that the majority of studies are focused on the functional aspects of oak trees under climate change. On the other hand, the presence of modeling-related keywords (e.g., “model”, “responses”, and “impacts”) suggests a growth towards predictive tools needed to simulate future scenarios. If we consider the time spectrum, we can see that descriptive ecological studies have been replaced by process-based and predictive approaches, integrating physiological, meteorological, and management data.
The exponential growth of published articles over the years has been observed in other topics as well [24,115].
In conclusion, our bibliometric analysis highlights a research field that is continuously expanding. This area is shifting towards a more integrative and predictive approach, mainly caused by climate change and the need to conserve these forests. Future research will definitely benefit from a collaboration that exceeds borders and by integrating social, economic, and genomic concerns in order to address the resilience of oak forests under these climatic pressures.
4.2. Oak Species’ Climate Sensitivity Across Regions
From the total number of Quercus species (estimated at 500–600 globally), our review identified 48 species explicitly cited in the scientific literature as being affected by climate change (Table 1). These species span five continents and are reported in 29 countries, reflecting both the wide distribution of oaks and the global concern regarding their vulnerability to climatic shifts.
A notable pattern emerging from the reviewed literature is the geographic and taxonomic concentration of research efforts. For instance, a significant number of studies focus on East Asia—particularly China and Japan—where numerous endemic oak species, such as Quercus guyavaefolia, Quercus spinosa, and Quercus schottkyana, are located in climatically sensitive mountainous regions. These regions are especially vulnerable to climate-induced habitat shifts, as shown by Sun et al. [69], who found that oak species richness in southwestern China is predicted to contract under intensified climate scenarios. High-elevation ecosystems may serve as temporary refugia; however, upward or poleward migration is limited by topography and habitat fragmentation.
In Europe, Mediterranean and temperate oak species such as Quercus ilex, Q. suber, and Q. robur have been extensively studied, with mixed responses to climate stressors. Q. ilex, for example, has demonstrated resilience to drought conditions [62], yet there are also concerns about declining productivity and increased vulnerability to pests in certain Mediterranean regions. Meanwhile, Q. cerris (Turkey oak) appears to exhibit better drought tolerance than Q. robur (pedunculate oak), as shown by Kostić et al. [51], suggesting a potential shift in species composition in mixed oak forests under future drought regimes.
In North America, multiple oak species, such as Q. alba, Q. garryana, Q. rubra, and Q. stellata, have been analyzed in relation to temperature increase, altered precipitation patterns, and fire regimes. For example, Q. garryana in the Pacific Northwest shows signs of expansion potential in response to warming, although dependent on fire dynamics and land use [58]. Conversely, Q. rubra, though widely distributed, has shown growth reductions under extreme drought [89].
The Neotropical and Mesoamerican oaks, such as Q. brandegeei and Q. meavei, are also highlighted in recent studies [44,55], yet data remain limited. These regions are recognized as biodiversity hotspots with high endemism but low resilience due to restricted ranges and limited altitudinal migration options.
In the Middle East and Central Asia, species such as Q. brantii, Q. castaneifolia, and Q. libani face increasing aridification and extreme heat events, with studies from Iran and Iraq indicating significant physiological stress responses [45,65]. These regions are expected to become increasingly inhospitable for current oak populations, raising concerns about their long-term persistence without conservation intervention.
Taken together, the evidence underscores that while some oak species may adapt, persist, or even expand under changing climatic conditions, many others—especially those with narrow ecological niches or restricted geographic distributions—are likely to face habitat loss, demographic decline, or local extinction.
4.3. Physiological and Growth Responses to Drought and Temperature
Drought is the most important climatic stress factor that impacts both the health and productivity of oak forests [116,117]. This can lead to accelerated decline and increased mortality [118,119] and biotic stress caused by pest and pathogen interactions [120,121]. Many Quercus species have drought-adaptive traits such as deep rooting and efficient water use [47,122]. However, their response to stress is dependent on the species and the local context.
Q. robur and Q. petraea did not manage to maintain their growth after drought periods, which emphasized the importance of phenological timing. Mixed stress effects are recorded during early season droughts, which are connected with leaf expansion and cambial activities [97]. However, post-summer-drought recoveries show a more flexible response, as these periods are less phenologically constrained.
Q. cerris showed an increased tolerance to droughts, especially in southeastern Europe. This supports our view that precipitation has a more important role than temperature in oak growths [51,52]. This suggests the resilience to future precipitation changes, which is important in our climate-changing context.
The sensitivity of species to drought did not record any consistent geographic trends, meaning that local conditions (soil moisture, microclimates, groundwater availability) are more important than climatic conditions. This only reinforces the need to implement monitoring and management strategies, especially in the context of extreme climatic events [101,104].
Hydroclimatic conditions from previous years had a carryover effect on the growth of oak species, emphasizing a physiological memory mechanism. Late-summer and autumn climates have a direct influence on the storage of carbohydrates and the development of buds. This emphasizes the long-term influence of seasonal variability [67,82,123,124].
Growth trends from Germany and France indicate an increased productivity [125,126]. This was likely caused by CO2 fertilization, nitrogen deposition, and longer growing seasons. However, these might also indicate latent vulnerabilities to heatwaves and droughts. Despite their adaptive growth patterns, Mediterranean species (Q. ilex) face reproductive challenges under chronic aridity [63,127].
In the context of warming scenarios from East Asia, Quercus spp. showed an advantage over P. densiflora. This suggests a future need for reconsidering forest composition and favoring oak species [102,103]. This not only impacts the local context but also biodiversity, ecosystem services, and the need for climate change-adapted forest management.
Overall, our findings prove that oak resilience is not uniform and not guaranteed in climatic contexts. Future silviculture strategies should take into account both the traits of species as well as climatic conditions and the ecohydrological conditions of the sites. This is needed to maintain both the stability and productivity of oak forests and mixed-oak forests under modern climatic conditions.
4.4. Mixed and Transitional Forest Dynamics Under Climate Stress
Oak species frequently coexist with other tree species, particularly European beech, Scots pine, Douglas-fir, and various conifers, forming complex mixed forest stands. The response of oaks within these ecologically and structurally diverse communities to climate change is often species-specific and highly context-dependent, reflecting both biotic competition and abiotic stressors such as drought and temperature extremes.
In transition zones between oak- and beech-dominated forests, such as those described by Sedmakova et al. [105], drought emerges as the primary limiting factor for oak performance. Middle-stemmed oaks (per Weisse’s classification), with less favorable stem diameter positions, exhibit reduced radial growth and a diminished response to both precipitation signals and extreme climatic events. This suggests that in mixed stands under drought stress, competitive position within the canopy structure significantly affects oak resilience.
Studies from the Swiss lowlands [106] further indicate a gradual decline in oak dominance relative to beech, with oak experiencing higher mortality rates over time—especially in denser stands and wetter conditions. The increased mortality with precipitation may seem counterintuitive, but it likely reflects associated pathogen or competitive stress under humid conditions, highlighting the complexity of oak response when biotic pressures are intensified by climatic variability.
Contrastingly, in southern Europe, a different pattern emerges. In Italy, beech experienced a more pronounced decline in growth than oak after the 1990s, with Q. cerris showing strong recovery capabilities post-drought, especially after 2003 [49]. This reversal of growth trends suggests that in warmer, drier climates, oaks—particularly drought-tolerant species like Q. cerris—may gain a competitive advantage over mesic species such as beech. These findings align with broader trends pointing to potential species composition shifts in mixed forests under progressive warming.
Silvicultural management appears to be a critical factor influencing oak performance in mixed stands. As Stimm et al. [107] noted, even under climate scenarios that predict warmer and drier conditions, oak growth is highly dependent on human intervention and promotion. This reinforces the idea that natural resilience alone may not be sufficient to ensure oak persistence or dominance in changing climates—management practices will likely determine future forest composition.
Outside Europe, dynamics within North American mixed forests reveal additional complexities. In British Columbia, Oregon white oak exhibits heightened drought sensitivity in competitive conditions, particularly when in proximity to Douglas-fir [59]. The dominance of Douglas-fir, with its higher shade tolerance and rooting strategies, contributes to the gradual replacement of oak savannas by coniferous woodland. These changes underscore how interspecific interactions and shifting forest structure—not just climate—can drive species declines or range shifts.
This notion is echoed in long-term simulation studies from the Netherlands, where mixed stands of Scots pine and Q. robur under climate change conditions resulted in reduced oak productivity and increased Scots pine growth [85]. These results suggest that in the absence of targeted silviculture or natural disturbance that favors broadleaf regeneration, oaks may lose ground to faster-growing or more drought-adapted conifers.
At the biogeographic edges of their distributions, climate-driven shifts are also becoming more apparent. In Korea, Quercus mongolica shows upward migration potential due to enhanced growth at higher elevations, while its coniferous neighbor, Abies koreana, experiences reduced growth in the same zones (ABKO). This suggests that climate warming may drive oak expansion into higher altitudes while displacing less tolerant species, potentially altering entire forest zones over time.
Collectively, these studies highlight the increasingly dynamic nature of mixed oak forests in a changing climate. The interaction between climate stressors, interspecific competition, and forest structure determines not only the growth and survival of oaks but also their ability to retain or gain dominance within mixed stands. These interactions are further modulated by site-specific conditions such as soil moisture availability, elevation, and stand density.
Importantly, oak resilience in mixed stands cannot be considered in isolation. Competitive interactions with co-occurring species, historical land-use patterns, and the intensity of management practices all play crucial roles in mediating the effects of climate change. Going forward, forest managers and conservationists must recognize these complex interdependencies when developing adaptive strategies for maintaining biodiversity, ecosystem function, and the long-term sustainability of mixed oak forests.
4.5. Ecosystem-Level Effects and Structural Shifts
It is important to note that climate change affects all components of an ecosystem, not just trees. A wide range of examples highlighting these effects can be found throughout the published literature. The results compiled in this review indicate that oak ecosystems, particularly those located in ecotonal zones or mixed-species stands, are experiencing complex and sometimes contradictory responses to changing climate variables such as temperature, precipitation, and drought frequency.
In transition zones between oak and beech forests, such as the one examined by Sedmakova et al. [105], the interaction between topography, species composition, and climate-induced stress reveals the vulnerability of oaks with less favorable stem positions. These trees show not only slower growth but also reduced sensitivity to climatic signals, possibly due to chronic stress or reduced vigor. This highlights how microhabitat conditions and intra-stand competition can modulate species’ climate responses.
The comparative mortality data from Swiss oak–beech forests [106] further emphasize the species-specific vulnerabilities under climate stress. The finding that oak mortality is more sensitive to stand basal area and precipitation patterns than beech suggests that oak may be at a competitive disadvantage in increasingly dense or wetter sites. Beech’s ability to maintain stable mortality rates regardless of basal area might be indicative of its greater competitive or physiological resilience in those contexts.
Conversely, in some regions like Italy [49], oak appears to outperform beech under warmer and drier conditions, particularly following extreme drought events. The significant recovery of Quercus cerris after 2003 points to inherent drought-adaptive traits, such as deep rooting or efficient water use, which may become more advantageous under future climates. These contrasting patterns between northern and southern Europe underline the importance of regional context and species composition when interpreting climate impacts.
The study by Stimm et al. [107] reinforces this context dependency by showing how forest management continues to play a pivotal role. Despite climatic pressures, silvicultural practices can influence growth outcomes—either mitigating or exacerbating the effects of climate change. This supports the idea that forest resilience is not solely determined by climate factors but also by anthropogenic interventions.
Evidence from North America also illustrates species-specific responses, particularly in mixed stands. Gedalof and Franks [59] reported that Oregon white oak shows heightened drought sensitivity compared to Douglas-fir. This finding highlights the importance of interspecific competition and site history in shaping growth dynamics. Douglas-fir’s physiological traits give it a competitive edge, while Oregon white oak’s response varies with density and previous-year conditions, emphasizing the need to consider legacy effects and stand dynamics in ecological forecasting.
Similar dynamics are observed in the Netherlands, where Bouwman et al. [85] found that Scots pine benefits more from changing climate conditions than oak, especially on certain soil types. This again illustrates the shifting competitive balance among co-occurring species and the strong modulating influence of edaphic factors.
In East Asia, the case of Quercus mongolica and Abies koreana reveals a clear elevational response to warming (South Korea). As Quercus mongolica expands upward and Abies koreana declines, this suggests a long-term risk of local extinction for the latter species, especially in areas with limited elevational range. Such changes not only alter forest structure and composition but may also affect biodiversity, microclimate regulation, and other ecosystem services.
Taken together, these studies provide a compelling picture of how climate change is reconfiguring oak forest ecosystems across biogeographic regions. Key themes include the importance of species-specific traits, competition dynamics, microenvironmental factors, and human management. The cumulative effect of these interacting drivers may lead to significant shifts in forest composition, structure, and function, with potentially cascading impacts on associated flora and fauna.
4.6. Drivers and Patterns of Decline in Oak Forests
The large-scale decline of Quercus species across temperate and Mediterranean regions has emerged as one of the most striking ecological symptoms of ongoing climate change. Numerous studies have documented this pattern in both Europe [112,128,129] and North America [130,131], underscoring a global phenomenon that transcends ecological and geographical boundaries.
As shown in this review, drought—both as a chronic stressor and an acute disturbance event—plays a pivotal role in oak decline. Experimental studies confirm that drought reduces stem and shoot growth and overall leaf area [132], which in turn limits photosynthetic capacity and carbon assimilation. Although moderate climate warming may not directly impact leaf nitrogen or carbohydrate storage [133], prolonged or intense drought significantly disrupts water uptake and physiological function, contributing to tree decline and mortality [112].
The Mediterranean region provides a particularly vivid example of how climate-induced stress and biotic agents interact to drive oak decline. According to Marques et al. [108], although pathogens like Phytophthora cinnamomi are frequently identified as direct causes of mortality, their effects are often potentiated by climatic stressors, especially prolonged droughts. In the Iberian Peninsula and North Africa, where cork oak (Quercus suber) and holm oak (Quercus ilex) dominate, the decline has become both spatially widespread and temporally persistent. The sharp reduction in cork oak area—up to 45% in Tunisia over the past century [110]—signals not just the loss of individual trees but a broader ecological shift, including species replacement by drought-tolerant competitors such as Aleppo pine.
This replacement dynamic suggests that oak species, despite their traditional adaptation to Mediterranean climates, are reaching the limits of their tolerance under current climate trajectories. As demonstrated by Mechergui et al. [93], modeling projections predict a substantial reduction—up to 40%—in climatically suitable habitat for cork oak by 2070. These changes are tightly linked to precipitation decline and increasing evaporative demand, both of which elevate physiological stress and reduce resilience.
Central and Eastern Europe exhibit similar trends, although the specific stressors and timings differ. In Poland, the increase in late-spring droughts correlates with diminished tree vigor and elevated mortality [112]. In Romania, long-term monitoring has confirmed that climatic stress, rather than pollution or pathogens, is now the principal driver of oak decline [113]. These findings highlight a critical temporal dimension: even relatively moderate shifts in climate variables can produce cumulative stress over decades, eventually tipping stands into decline.
In northern Europe, oak populations are not immune. For instance, in Finland, the delayed growth response observed in declining trees—years before visible symptoms—points to the importance of long-term drought sequences and legacy effects [114]. This suggests that oak decline is not merely a reaction to present-day extremes but often the outcome of prolonged sublethal stress that weakens trees and predisposes them to eventual dieback.
Cork oak decline, in particular, warrants urgent attention due to its ecological, economic, and cultural significance. The widespread reduction in cork production—by 40–60% in severely defoliated stands in Italy [134] and up to 30% forest loss in northwest Morocco [135]—represents a major challenge for Mediterranean land use systems. This is compounded by a decline in stand density and regeneration capacity, which could undermine the long-term sustainability of cork-based industries and the habitats they support.
In summary, the decline of oak forests is a multifactorial process, but climate change serves as both a direct stressor and a catalyst for other biotic threats. Across biogeographical zones, increasing temperatures and declining precipitation reduce growth, impair physiological function, and weaken resistance to pathogens. This leads to a compounded vulnerability that accelerates forest decline. Importantly, while the specific expression of oak decline varies by region, the underlying mechanism—climate-induced stress combined with secondary biotic factors—is globally consistent. Future forest management strategies must therefore incorporate proactive adaptation measures, including species selection, stand thinning, assisted migration, and long-term monitoring, to preserve oak ecosystems in an era of accelerating climate change. A high level of genetic diversity and maximum caution in the transfer of forest reproductive materials are also needed [136,137,138].
Management of drought stress in forests consists of the following:
Modeling the drought risk and mapping the sensitive lands for the next rotation (20 ± 100 yr, according to local oak species, climate, and policy/silviculture) may offer basic information for forest planning and future management [139].
Promoting drought-resistant oak species is the most accepted silvicultural option in short-, medium-, or long-term forest policy [140].
Silvicultural measures, such as regeneration prior to regeneration cuts, planting oak seedlings from local germplasm, shortening the rotation period of coppice stands, and gradual conversion of coppice towards high forests, were recommended in the Mediterranean zone [141]. In high stand density, the oak root system and crown biomass are low, and the competition for water and nutrients is high; therefore, thinning and other silvicultural measures that strengthen the trees improve the oak’s resistance to drought [142].
Selection of oak ecotypes and resistant genotypes was a constant goal of worldwide research, proving significant differences in oak genotypes regarding drought [143,144].
Controlling (treating) the harmful organisms (defoliators, sucking insects, bark beetles, leaf, root, and sapwood pathogens, etc.) was used on a large scale (i.e., survey and control of defoliators) or in particular oak stages (young plantations, special cultures, urban forests) [145,146]. Many studies present the dangerous impact of the invasive pest and pathogens on oak forests [147,148].
5. Conclusions
Our study provides strong evidence that temperate oak forests have different degrees of sensitivity towards climatic actions, especially temperature and precipitation. Our analysis showcases specific species and sites that have shown growth responses, proving the impact of local climate conditions on forest dynamics.
Our study emphasizes the response of pedunculate oak (Quercus robur) to climatic conditions, especially in its northeastern range limits. This sustains its potential as a proxy climate indicator. Our findings show that climate variability and warmer temperatures (caused by changing winter and summer temperatures) can affect oak populations from Eastern Europe.
Oak species react differently towards drought and recovery. For example, some species can adapt, persist, and even grow under changing climatic conditions. On the other hand, other species, especially those with restricted geographic distributions and from narrow ecological niches, deal with habitat losses, declines, and even extinction.
The resilience of oaks from mixed stands should also consider larger aspects, such as competitive interactions between species, the historical usage of the land, and the usage of management practices. In order to move forward, forest managers and conservationists should take into account these interactions when initiating maintenance strategies.
Our research sustains that climate change (especially increased temperatures and decreased precipitation) acts together with biotic stressors in accelerating the decline of oak forests. Even though this aspect varies locally, it is globally consistent if we take into account the climate stress, reduced growth, and heightened vulnerability to pathogens and invasive species. As such, the decline of oaks depends on each region, but the underlying mechanism (climatic stress) is globally consistent.
As we have observed, the last decades have indicated an increase in drought sensitivity, which poses a great threat to oak forests. The consequences can be damaging, as they cover forest productivity, biodiversity, and carbon sequestration. Our findings sustain the need to implement adaptive forest management strategies that take into account both the composition of species and local conditions.
Our study can be complemented in the future by long-term monitoring and integrative models that can predict the response of forests towards different climate change scenarios. Oak ecosystems can be conserved only if we understand the connection between biotic and abiotic factors in a fast-changing climate.
In conclusion, our study provides a base for understanding the impact of different climatic events on temperate oak forests. Our results can be used especially in areas where oak species are under-represented both locally and in studies. Our findings have emphasized key vulnerabilities and adaptive traits that can be used by other researchers in management strategies and in conserving oak biodiversity and the overall forest resilience under our current climatic changes.
While this study provides valuable insights into the climate sensitivity of oak forests, several limitations should be acknowledged: while we examined recent climatic trends and their influence on growth patterns, the study did not account for other potentially interacting factors such as pest outbreaks, competition dynamics, or forest management history, all of which may confound the observed climate-growth relationships. Also, our temporal analysis may be limited by the relatively short span of high-resolution climate data available for some sites, constraining our ability to detect longer-term trends or legacy effects. Future research addressing these limitations through expanded spatial sampling, multi-proxy approaches, and integration of biotic stressors will enhance our understanding of the resilience and adaptability of oak forests in the face of ongoing climate change.
Future research should focus on long-term monitoring and integrative modeling approaches to better predict forest responses under various climate change scenarios. Understanding the interplay between biotic and abiotic stressors will be crucial for conserving oak-dominated ecosystems in a rapidly changing climate.
Future forest management strategies must therefore incorporate proactive adaptation measures, including species selection, stand thinning, assisted migration, and long-term monitoring, to preserve oak ecosystems in an era of accelerating climate change.
Author Contributions
Conceptualization, L.D. and G.M.; methodology, C.C.; software, G.M.; validation, L.D. and G.M.; formal analysis, L.D.; investigation, C.C.; resources, I.B.; data curation, G.M.; writing—original draft preparation, L.D.; writing—review and editing, C.C.; visualization, I.B.; supervision, G.M.; project administration, L.D.; funding acquisition, I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC was funded by Lucian Blaga University of Sibiu through the research grant LBUS-IRG-2022-08. L.D. and C.C. were supported by the project PN 23090102 (contract no. 12N/2023) within the FORCLIMSOC program (Sustainable Forest Management Adapted to Climate Change and Societal Challenges) and financed by the Romanian National Authority for Research (ANC). The work of Gabriel Murariu was supported by a grant of the Ministry of Research, Innovation and Digitization, CNCS/CCCDI-UEFISCDI, project number PN-IV-P8-8.1-PRE-HE-ORG-2024-0212, within PNCDI IV.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Muhammad, N.; Castillejo, M.Á.; Rey, M.D.; Jorrín-Novo, J.V. An overview of oak species in Pakistan: Past, present, and future research perspectives. Forests 2023, 14, 777. [Google Scholar] [CrossRef]
- Mölder, A.; Meyer, P.; Nagel, R.V. Integrative management to sustain biodiversity and ecological continuity in Central European temperate oak (Quercus robur, Q. petraea) forests: An overview. For. Ecol. Manag. 2019, 437, 324–339. [Google Scholar] [CrossRef]
- Budeanu, M.; Popescu, F.; Şofletea, N. In situ conservation of forest genetic resources in Romania. In Forests of Southeast Europe Under a Changing Climate: Conservation of Genetic Resources; Springer: Cham, Switzerland, 2019; pp. 195–205. [Google Scholar]
- Apostol, E.N.; Stuparu, E.; Scarlatescu, V.; Budeanu, M. Testing Hungarian oak (Quercus frainetto Ten.) provenances in Romania. iForest 2020, 13, 9. [Google Scholar] [CrossRef]
- McShea, W.J.; Healy, W.M. (Eds.) Oak Forest Ecosystems: Ecology and Management for Wildlife; Johns Hopkins University Press: Baltimore, MD, USA, 2002. [Google Scholar]
- Nixon, K.C. Global and neotropical distribution and diversity of oak (genus Quercus) and oak forests. In Ecology and Conservation of Neotropical Montane Oak Forests; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–13. [Google Scholar]
- Dore, M.H. Climate change and changes in global precipitation patterns: What do we know? Environ. Int. 2005, 31, 1167–1181. [Google Scholar] [CrossRef]
- Spinoni, J.; Naumann, G.; Vogt, J.V. Pan-European seasonal trends and recent changes of drought frequency and severity. Glob. Planet. Change 2017, 148, 113–130. [Google Scholar] [CrossRef]
- Tsanis, I.; Tapoglou, E. Winter North Atlantic Oscillation impact on European precipitation and drought under climate change. Theor. Appl. Climatol. 2019, 135, 323–330. [Google Scholar] [CrossRef]
- Gudmundsson, L.; Seneviratne, S.I. European drought trends. Proc. Int. Assoc. Hydrol. Sci. 2015, 369, 75–79. [Google Scholar] [CrossRef]
- Zhao, C.; Brissette, F.; Chen, J.; Martel, J.L. Frequency change of future extreme summer meteorological and hydrological droughts over North America. J. Hydrol. 2020, 584, 124316. [Google Scholar] [CrossRef]
- Polley, H.W.; Briske, D.D.; Morgan, J.A.; Wolter, K.; Bailey, D.W.; Brown, J.R. Climate change and North American rangelands: Trends, projections, and implications. Rangel. Ecol. Manag. 2013, 66, 493–511. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, L.; Liu, Y.; Hao, Z.; Zhang, J. Changes in the drought condition over northern East Asia and the connections with extreme temperature and precipitation indices. Glob. Planet. Change 2021, 207, 103645. [Google Scholar] [CrossRef]
- Ali, S.; Basit, A.; Umair, M.; Makanda, T.A.; Shaik, M.R.; Ibrahim, M.; Ni, J. The role of climate change and its sensitivity on long-term standardized precipitation evapotranspiration index, vegetation and drought changing trends over East Asia. Plants 2024, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Singh, J.S. Structure and function of the Central Himalayan oak forests. Proc. Plant Sci. 1986, 96, 159–189. [Google Scholar] [CrossRef]
- Rodà, F.; Retana, J.; Gracia, C.A.; Bellot, J. (Eds.) Ecology of Mediterranean Evergreen Oak Forests; Springer: Dordrecht, The Netherlands, 2012; Volume 137. [Google Scholar]
- Kozharinov, A.V.; Borisov, P.V. Distribution of oak forests in Eastern Europe over the last 13,000 years. Contemp. Probl. Ecol. 2013, 6, 755–760. [Google Scholar] [CrossRef]
- Crisan, V.; Dinca, L.; Scarlatescu, V.; Braga, C.; Ienasoiu, G.; Crisan, C. Predictions about sessile oak forest ecosystems from Banat Mountains in the next 80 years. Sci. Pap. Ser. E Land Reclam. Earth Obs. Surv. Environ. Eng. 2023, 12, 44–51. [Google Scholar]
- Tang, Y.; Du, E.; Guo, H.; Wang, Y.; Peñuelas, J.; Reich, P.B. Rapid migration of Mongolian oak into the southern Asian boreal forest. Glob. Change Biol. 2024, 30, e17002. [Google Scholar] [CrossRef]
- Ellenberg, H.H.; Strutt, G.K. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Bauhus, J.; Forrester, D.I.; Pretzsch, H. From observations to evidence about effects of mixed-species stands. In Mixed-Species Forests: Ecology and Management; Springer: Cham, Switzerland, 2017; pp. 27–71. [Google Scholar]
- Dincă, L.; Achim, F. The management of forests situated on fields susceptible to landslides and erosion from the Southern Carpathians. Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural Dev. 2019, 19, 183–188. [Google Scholar]
- Kashani, M.M.; Homavandi, H.; Batooli, Z. Bibliometric studies of most-cited medical papers: A bibliometric analysis. Int. Arch. Health Sci. 2022, 9, 123–128. [Google Scholar]
- Zhang, X.; Liang, S.; Lu, J.; Cui, X. Evolution of research on global soil water content in the past 30 years based on ITGinsight bibliometric analysis. Int. J. Environ. Res. Public Health 2022, 19, 15476. [Google Scholar] [CrossRef]
- Ellegaard, O.; Wallin, J.A. The bibliometric analysis of scholarly production: How great is the impact? Scientometrics 2015, 105, 1809–1831. [Google Scholar] [CrossRef]
- VijayaVenkataRaman, S.; Iniyan, S.; Goic, R. A review of climate change, mitigation and adaptation. Renew. Sustain. Energy Rev. 2012, 16, 878–897. [Google Scholar] [CrossRef]
- Thornton, P.K.; Ericksen, P.J.; Herrero, M.; Challinor, A.J. Climate variability and vulnerability to climate change: A review. Global Change Biol. 2014, 20, 3313–3328. [Google Scholar] [CrossRef] [PubMed]
- Olaniyi, O.A.; Ojekunle, Z.O.; Amujo, B.T. Review of climate change and its effect on Nigeria ecosystem. Int. J. Afr. Asian Stud. 2013, 1, 57–65. [Google Scholar]
- Mawdsley, J.R.; O’Malley, R.; Ojima, D.S. A review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conserv. Biol. 2009, 23, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Dietz, T.; Shwom, R.L.; Whitley, C.T. Climate change and society. Annu. Rev. Sociol. 2020, 46, 135–158. [Google Scholar] [CrossRef]
- Sullivan, A. Bridging the divide between rural and urban community-based forestry: A bibliometric review. For. Policy Econ. 2022, 144, 102826. [Google Scholar] [CrossRef]
- Bullock, S.H.; Mooney, H.A.; Medina, E. (Eds.) Seasonally Dry Tropical Forests; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Estiarte, M.; Peñuelas, J. Alteration of the phenology of leaf senescence and fall in winter deciduous species by climate change: Effects on nutrient proficiency. Glob. Change Biol. 2014, 21, 1005–1017. [Google Scholar] [CrossRef]
- Dincă, L.; Crisan, V.; Ienasoiu, G.; Murariu, G.; Drasovean, R. Environmental Indicator Plants in Mountain Forests: A Review. Plants 2024, 13, 3358. [Google Scholar] [CrossRef]
- Timis-Gansac, V.; Dinca, L.; Constandache, C.; Murariu, G.; Cheregi, G.; Timofte, C.S.C. Conservation biodiversity in arid areas: A review. Sustainability 2025, 17, 2422. [Google Scholar] [CrossRef]
- Web of Science Core Collection. Available online: https://clarivate.com/products/scientific-and-academic-research/research-discovery-and-workflow-solutions/webofscience-platform/web-of-science-core-collection/ (accessed on 20 January 2025).
- Scopus. Available online: https://www.elsevier.com/products/scopus (accessed on 10 December 2024).
- Microsoft Corporation. Microsoft Excel. Available online: https://www.microsoft.com/en-us/microsoft-365/excel?legRedir=true&CorrelationId=3bb60ab0-fe13-41a4-812b-2627667cf346 (accessed on 1 February 2025).
- Geochart. Available online: https://developers.google.com/chart/interactive/docs/gallery/geochart (accessed on 13 March 2025).
- VOS Viewer. Available online: https://www.vosviewer.com/ (accessed on 7 December 2024).
- Chen, Y.; Li, Y.; Mao, L. Combining the effects of global warming, land use change and dispersal limitations to predict the future distributions of East Asian Cerris Oaks (Quercus Section Cerris, Fagaceae) in China. Forests 2022, 13, 367. [Google Scholar] [CrossRef]
- Liao, Z.; Nobis, M.P.; Xiong, Q.; Tian, X.; Wu, X.; Pan, K.; Zhang, L. Potential distributions of seven sympatric sclerophyllous oak species in Southwest China depend on climatic, non-climatic, and independent spatial drivers. Ann. For. Sci. 2021, 78, 5. [Google Scholar] [CrossRef]
- Marquis, R.J.; Lill, J.T.; Forkner, R.E.; Le Corff, J.; Landosky, J.M.; Whitfield, J.B. Declines and resilience of communities of leaf-chewing insects on Missouri oaks following spring frost and summer drought. Front. Ecol. Evol. 2019, 7, 396. [Google Scholar] [CrossRef]
- Brinckwirth, C.; Klimas, C.A.; Cortez, C.; Nuñez, J.; Perez-Morales, D.W.; Breceda, A.; Álvarez-Clare, S. Environmental factors can influence spatial aggregation and acorn production in the endemic and endangered oak Quercus brandegeei in Mexico. Bot. Sci. 2023, 101, 761–774. [Google Scholar] [CrossRef]
- Jafarian, N.; Mirzaei, J.; Omidipour, R.; Kooch, Y. Effects of micro-climatic conditions on soil properties along a climate gradient in oak forests, west of Iran: Emphasizing phosphatase and urease enzyme activity. Catena 2023, 224, 106960. [Google Scholar] [CrossRef]
- Mirhashemi, H.; Heydari, M.; Ahmadi, K.; Karami, O.; Kavgaci, A.; Matsui, T.; Heung, B. Species distribution models of Brant’s oak (Quercus brantii Lindl.): The impact of spatial database on predicting the impacts of climate change. Ecol. Eng. 2023, 194, 107038. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Fonti, P.; Cherubini, P.; Martín-Benito, D.; Chaar, H.; Cañellas, I. Xylem hydraulic adjustment and growth response of Quercus canariensis Willd. to climatic variability. Tree Physiol. 2012, 32, 401–413. [Google Scholar] [CrossRef]
- Asadi, H.; Jalilvand, H.; Tafazoli, M.; Hosseini, S.F. Modeling habitat suitability of Quercus castaneifolia in the Hyrcanian forest: A comprehensive integration of environmental factors for conservation insights. Biodivers. Conserv. 2025, 34, 315–334. [Google Scholar] [CrossRef]
- Mazza, G.; Monteverdi, M.C.; Altieri, S.; Battipaglia, G. Climate-driven growth dynamics and trend reversal of Fagus sylvatica L. and Quercus cerris L. in a low-elevation beech forest in Central Italy. Sci. Total Environ. 2024, 908, 168250. [Google Scholar] [CrossRef]
- Mészáros, I.; Adorján, B.; Nyitrai, B.; Kanalas, P.; Oláh, V.; Levanič, T. Long-term radial growth and climate-growth relationships of Quercus petraea (Matt.) Liebl. and Quercus cerris L. in a xeric low elevation site from Hungary. Dendrochronologia 2022, 76, 126014. [Google Scholar] [CrossRef]
- Kostić, S.; Levanič, T.; Orlović, S.; Matović, B.; Stojanović, D.B. Turkey oak (Quercus cerris L.) is more drought tolerant and better reflects climate variations compared to pedunculate oak (Quercus robur L.) in lowland mixed forests in northwestern Serbia: A stable carbon isotope ratio (δ13C) and radial growth approach. Ecol. Indic. 2022, 142, 109242. [Google Scholar] [CrossRef]
- Šimková, M.; Vacek, S.; Šimůnek, V.; Vacek, Z.; Cukor, J.; Hájek, V.; Lukáčik, I. Turkey oak (Quercus cerris L.) resilience to climate change: Insights from coppice forests in Southern and Central Europe. Forests 2023, 14, 2403. [Google Scholar] [CrossRef]
- Jiang, X.L.; Deng, M.; Li, Y. Evolutionary history of subtropical evergreen broad-leaved forest in Yunnan Plateau and adjacent areas: An insight from Quercus schottkyana (Fagaceae). Tree Genet. Genomes 2016, 12, 104. [Google Scholar] [CrossRef]
- Onosato, K.; Shitara, T.; Matsumoto, A.; Matsuo, A.; Suyama, Y.; Tsumura, Y. Contact zone of two different chloroplast lineages and genetic guidelines for seed transfer in Quercus serrata and Quercus crispula. Plant Species Biol. 2021, 36, 72–83. [Google Scholar] [CrossRef]
- Argüelles-Marrón, B.; Meave, J.A.; Luna-Vega, I.; Crispin-DelaCruz, D.B.; Szejner, P.; Ames-Martínez, F.N.; Rodríguez-Ramírez, E.C. Adaptation potential of Neotropical montane oaks to drought events: Wood anatomy sensitivity in Quercus delgadoana and Quercus meavei. Funct. Ecol. 2023, 37, 2040–2055. [Google Scholar] [CrossRef]
- González-Rodríguez, V.; Villar, R.; Navarro-Cerrillo, R.M. Maternal influences on seed mass effect and initial seedling growth in four Quercus species. Acta Oecol. 2011, 37, 1–9. [Google Scholar] [CrossRef]
- Thapliyal, S.; Sati, S.P.; Singh, B.; Rawat, D.; Khanduri, V.P.; Riyal, M.K.; Singh, N. Effect of altitudes and aspects on carbon sequestration potential of Quercus floribunda forests of Garhwal Himalayas. Trees For. People 2024, 18, 100690. [Google Scholar] [CrossRef]
- Pellatt, M.G.; Gedalof, Z.E. Environmental change in Garry oak (Quercus garryana) ecosystems: The evolution of an eco-cultural landscape. Biodivers. Conserv. 2014, 23, 2053–2067. [Google Scholar] [CrossRef]
- Gedalof, Z.E.; Franks, J.A. Stand structure and composition affect the drought sensitivity of Oregon white oak (Quercus garryana Douglas ex Hook.) and Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco). Forests 2019, 10, 381. [Google Scholar] [CrossRef]
- Zorrilla-Azcué, S.; González-Rodríguez, A.; Oyama, K.; González, M.A.; Rodríguez-Correa, H. The DNA history of a lonely oak: Quercus humboldtii phylogeography in the Colombian Andes. Ecol. Evol. 2021, 11, 6814–6828. [Google Scholar] [CrossRef]
- Brasier, C.M. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. In Annales des Sciences Forestières; EDP Sciences: Paris, France, 1996; Volume 53, pp. 347–358. [Google Scholar]
- Ruiz-Gómez, F.J.; Pérez-de-Luque, A.; Navarro-Cerrillo, R.M. The involvement of Phytophthora root rot and drought stress in holm oak decline: From ecophysiology to microbiome influence. Curr. For. Rep. 2019, 5, 251–266. [Google Scholar] [CrossRef]
- Le Roncé, I.; Gavinet, J.; Ourcival, J.M.; Mouillot, F.; Chuine, I.; Limousin, J.M. Holm oak fecundity does not acclimate to a drier world. New Phytol. 2021, 231, 631–645. [Google Scholar] [CrossRef]
- Asensio, D.; Zuccarini, P.; Sardans, J.; Marañón-Jiménez, S.; Mattana, S.; Ogaya, R.; Peñuelas, J. Soil biomass-related enzyme activity indicates minimal functional changes after 16 years of persistent drought treatment in a Mediterranean holm oak forest. Soil Biol. Biochem. 2024, 189, 109281. [Google Scholar] [CrossRef]
- Hama, A.A.; Khwarahm, N.R. Predictive mapping of two endemic oak tree species under climate change scenarios in a semiarid region: Range overlap and implications for conservation. Ecol. Inform. 2023, 73, 101930. [Google Scholar] [CrossRef]
- Bayar, E. Influence of drought stress and N addition on the gas exchange, biochemical and growth traits in Quercus ithaburensis. Dendrobiology 2022, 88, 94–104. [Google Scholar] [CrossRef]
- Keyimu, M.; Li, Z.; Jiao, L.; Chen, W.; Wu, X.; Fan, Z.; Fu, B. Radial growth response of Quercus liaotungensis to climate change—A case study on the central Loess Plateau, China. Trees 2022, 36, 1811–1822. [Google Scholar] [CrossRef]
- Koenig, W.D.; Knops, J.M.; Carmen, W.J. Intraspecific variation in the relationship between weather and masting behavior in valley oak, Quercus lobata. Can. J. For. Res. 2020, 50, 1299–1306. [Google Scholar] [CrossRef]
- Sun, P.W.; Chang, J.T.; Luo, M.X.; Liao, P.C. Genomic insights into local adaptation and vulnerability of Quercus longinux to climate change. BMC Plant Biol. 2024, 24, 279. [Google Scholar] [CrossRef]
- Foroozan, Z.; Pourtahmasi, K.; Bräuning, A. Climatic signals in stable carbon isotope ratios of Juniper and Oak tree rings from northern Iran. Glob. Planet. Change 2018, 165, 90–99. [Google Scholar] [CrossRef]
- Rogers, T.R.; Russell, F.L. Historical patterns of oak population expansion in the Chautauqua Hills, Kansas. J. Biogeogr. 2014, 41, 2105–2114. [Google Scholar] [CrossRef]
- Altman, J.; Treydte, K.; Pejcha, V.; Cerny, T.; Petrik, P.; Srutek, M.; Dolezal, J. Tree growth response to recent warming of two endemic species in Northeast Asia. Clim. Change 2020, 162, 1345–1364. [Google Scholar] [CrossRef]
- Center, A.; Etterson, J.R.; Deacon, N.J.; Cavender-Bares, J. Seed production timing influences seedling fitness in the tropical live oak Quercus oleoides of Costa Rican dry forests. Am. J. Bot. 2016, 103, 1407–1417. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Li, Q.; Pu, F.; Xia, X.; Chang, M.; Song, K. Influence of colder temperature on the axial and radial parenchyma fraction of Quercus ciliaris CC Huang & YT Chang wood and its relationship with carbohydrate reserve (NSC). Forests 2022, 13, 169. [Google Scholar]
- Vospernik, S.; Heym, M.; Pretzsch, H.; Pach, M.; Steckel, M.; Aldea, J.; Wolff, B. Tree species growth response to climate in mixtures of Quercus robur/Quercus petraea and Pinus sylvestris across Europe—A dynamic, sensitive equilibrium. For. Ecol. Manag. 2023, 530, 120753. [Google Scholar] [CrossRef]
- Nölte, A.; Yousefpour, R.; Hanewinkel, M. Changes in sessile oak (Quercus petraea) productivity under climate change by improved leaf phenology in the 3-PG model. Ecol. Model. 2020, 438, 109285. [Google Scholar] [CrossRef]
- Friedrichs, D.A.; Trouet, V.; Büntgen, U.; Frank, D.C.; Esper, J.; Neuwirth, B.; Löffler, J. Species-specific climate sensitivity of tree growth in Central-West Germany. Trees 2009, 23, 729–739. [Google Scholar] [CrossRef]
- Moreno-Fernandez, D.; Aldea, J.; Gea-Izquierdo, G.; Canellas, I.; Martin-Benito, D. Influence of climate and thinning on Quercus pyrenaica Willd. coppices growth dynamics. Eur. J. For. Res. 2021, 140, 187–197. [Google Scholar] [CrossRef]
- Santonja, M.; Baldy, V.; Fernandez, C.; Balesdent, J.; Gauquelin, T. Potential shift in plant communities with climate change: Outcome on litter decomposition and nutrient release in a Mediterranean oak forest. Ecosystems 2015, 18, 1253–1268. [Google Scholar] [CrossRef]
- Safonova, M.; Tabunshchik, V.; Gorbunov, R.; Gorbunova, T. Heat budget of sub-Mediterranean downy oak landscapes of southeastern Crimea. Forests 2023, 14, 1927. [Google Scholar] [CrossRef]
- Šestan, L. Simulation model of the effect of air temperature on the leaves phenophases of the pubescent oak on the island of Pag. Šum. List 2012, 136, 253–260. [Google Scholar]
- Nechita, C.; Popa, I. The relationship between climate and radial growth for the oak (Quercus robur L.) in the western plain of Romania. Carpath. J. Earth Environ. Sci. 2012, 7, 137–144. [Google Scholar]
- Vranckx, G.; Jacquemyn, H.; Mergeay, J.; Cox, K.; Janssens, P.; Gielen, B.A.; Muys, B.; Honnay, O. The effect of drought stress on heterozygosity-fitness correlations in pedunculate oak (Quercus robur). Ann. Bot. 2014, 113, 1057–1069. [Google Scholar] [CrossRef]
- Shupe, H.A.; Jensen, K.; Oldeland, J.; Ludewig, K. Droughts decrease and floods increase carbon sequestration rates of Quercus robur in hardwood floodplain forests. Trees For. People 2022, 9, 100294. [Google Scholar] [CrossRef]
- Bouwman, M.; Forrester, D.I.; Den Ouden, J.; Nabuurs, G.J.; Mohren, G.M.J. Species interactions under climate change in mixed stands of Scots pine and pedunculate oak. For. Ecol. Manag. 2021, 481, 118615. [Google Scholar] [CrossRef]
- Ugarković, D.; Tikvić, I.; Mikac, S.; Stankić, I.; Balta, D. The influence of changing climate extremes on the ecological niche of pedunculate oak in Croatia. South-East Eur. For. 2016, 7, 143–148. [Google Scholar] [CrossRef][Green Version]
- Askeyev, O.V.; Tischin, D.; Sparks, T.H.; Askeyev, I.V. The effect of climate on the phenology, acorn crop and radial increment of pedunculate oak (Quercus robur) in the middle Volga region, Tatarstan, Russia. Int. J. Biometeorol. 2005, 49, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Juice, S.M.; Templer, P.H.; Phillips, N.G.; Ellison, A.M.; Pelini, S.L. Ecosystem warming increases sap flow rates of northern red oak trees. Ecosphere 2016, 7, e01221. [Google Scholar] [CrossRef]
- Kormann, J.M.; van der Maaten-Theunissen, M.; Unterholzner, L.; Liesebach, M.; Liepe, K.J.; van der Maaten, E. Variation in vessel traits of northern red oak (Quercus rubra L.) provenances revealed high phenotypic plasticity to prevailing environmental conditions. Trees 2024, 38, 1283–1295. [Google Scholar] [CrossRef]
- Saran, S.; Joshi, R.; Sharma, S.; Padalia, H.; Dadhwal, V.K. Geospatial modeling of brown oak (Quercus semecarpifolia) habitats in the Kumaun Himalaya under climate change scenario. J. Indian Soc. Remote Sens. 2010, 38, 535–547. [Google Scholar] [CrossRef]
- Shekhar, C.; Ginwal, H.S.; Meena, R.K.; Shankhwar, R.; Martins-Ferreira, M.A.C.; Pandey, S.; Bhandari, M.S. Spatio-temporal distribution of broad-leaved Quercus semecarpifolia indicates altitudinal shift in northwestern Himalayas. Plant Ecol. 2022, 223, 671–697. [Google Scholar] [CrossRef]
- Bouzidi, M.; Nouri, M.; Nasr, Z.; Khlifi, S. Contribution to the assessment of climate change effects on water balance in forest soil: Application of the Biljou model to the cork oak forest of Ain Snoussi (north-western Tunisia). Arab. J. Geosci. 2020, 13, 1152. [Google Scholar] [CrossRef]
- Mechergui, K.; Jaouadi, W.; Altamimi, A.S.; Naghmouchi, S.; Ammari, Y. Effect of climate change on the spatial distribution and cork production of Quercus suber L., the risk of exclusion by the Aleppo pine expansion, and management practices to protect Q. suber habitat: A review. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12218. [Google Scholar] [CrossRef]
- Ribeiro, S.; Cerveira, A.; Soares, P.; Ribeiro, N.A.; Camilo-Alves, C.; Fonseca, T.F. Natural regeneration of cork oak forests under climate change: A case study in Portugal. Front. For. Glob. Change 2024, 7, 1332708. [Google Scholar] [CrossRef]
- Yu, W.; Fahey, T.J.; Kang, H.; Zhou, P. Surface soil organic carbon in temperate and subtropical oriental oak stands of East China. Can. J. For. Res. 2016, 46, 621–628. [Google Scholar] [CrossRef]
- Keyser, T.L.; Brown, P.M. Drought response of upland oak (Quercus L.) species in Appalachian hardwood forests of the southeastern USA. Ann. For. Sci. 2016, 73, 971–986. [Google Scholar] [CrossRef]
- Bose, A.K.; Scherrer, D.; Camarero, J.J.; Ziche, D.; Babst, F.; Bigler, C.; Rigling, A. Climate sensitivity and drought seasonality determine post-drought growth recovery of Quercus petraea and Quercus robur in Europe. Sci. Total Environ. 2021, 784, 147222. [Google Scholar] [CrossRef]
- Kostić, S.; Kesić, L.; Matović, B.; Orlović, S.; Stojnić, S.; Stojanović, D.B. Soil properties are significant modifiers of pedunculate oak (Quercus robur L.) radial increment variations and their sensitivity to drought. Dendrochronologia 2021, 67, 125838. [Google Scholar] [CrossRef]
- Tumajer, J.; Treml, V. Response of floodplain pedunculate oak (Quercus robur L.) tree-ring width and vessel anatomy to climatic trends and extreme hydroclimatic events. For. Ecol. Manag. 2016, 379, 185–194. [Google Scholar] [CrossRef]
- Touhami, I.; Chirino, E.; Fkiri, S.; Aouinti, H.; Moutahir, H.; Bellot, J.; Nasr, Z. An analysis of tree-ring width and the standardized precipitation-evapotranspiration index within the cork oak (Quercus suber L.) forests mortality framework in the Kroumirie Mountains, northwestern Tunisia. Euro-Mediterr. J. Environ. Integr. 2021, 6, 42. [Google Scholar] [CrossRef]
- Čejková, A.; Poláková, S. Growth responses of sessile oak to climate and hydrological regime in the Zbytka Nature Reserve, Czech Republic. Geochronometria 2012, 39, 285–294. [Google Scholar] [CrossRef]
- Byun, J.G.; Lee, W.K.; Kim, M.; Kwak, D.A.; Kwak, H.; Park, T.; Jung, J.H. Radial growth response of Pinus densiflora and Quercus spp. to topographic and climatic factors in South Korea. J. Plant Ecol. 2013, 6, 380–392. [Google Scholar] [CrossRef]
- Kim, M.; Lee, W.K.; Son, Y.; Yoo, S.; Choi, G.M.; Chung, D.J. Assessing the impacts of topographic and climatic factors on radial growth of major forest forming tree species of South Korea. For. Ecol. Manag. 2017, 404, 269–279. [Google Scholar] [CrossRef]
- Nagavciuc, V.; Mursa, A.; Ionita, M.; Sfeclă, V.; Popa, I.; Roibu, C.C. An overview of extreme years in Quercus sp. tree ring records from the Northern Moldavian Plateau. Forests 2023, 14, 894. [Google Scholar] [CrossRef]
- Sedmáková, D.; Sedmák, R.; Saniga, M.; Parobeková, Z.; Potterf, M.; Lukáčik, I. Response of oaks to extreme climate events in the transition zone of oak and beech forests. Zprávy Lesnického Výzkumu 2021, 66, 104–114. [Google Scholar]
- Rohner, B.; Bigler, C.; Wunder, J.; Brang, P.; Bugmann, H. Fifty years of natural succession in Swiss forest reserves: Changes in stand structure and mortality rates of oak and beech. J. Veg. Sci. 2012, 23, 892–905. [Google Scholar] [CrossRef]
- Stimm, K.; Heym, M.; Uhl, E.; Tretter, S.; Pretzsch, H. Height growth-related competitiveness of oak (Quercus petraea (Matt.) Liebl. and Quercus robur L.) under climate change in Central Europe. Is silvicultural assistance still required in mixed-species stands? For. Ecol. Manag. 2021, 482, 118780. [Google Scholar] [CrossRef]
- Marques, M.; Bugalho, M.N.; Acácio, V.; Catry, F.X. Disentangling research on oak decline factors in Mediterranean-type climate regions: A systematic review. Trees For. People 2025, 1, 100803. [Google Scholar] [CrossRef]
- Hasnaoui, B. Régénération naturelle du chêne-liège: Difficultés et proposition de solutions. Ann. l’ENRGREF 1998, Numéro Spécial, 126–147. [Google Scholar]
- Selmi, M. Différenciation des sols et fonctionnement des écosystèmes sur grès numidiens de Kroumirie (Tunisie). Ph.D. Thesis, Écologie de la Subéraie Zénaie, Université de Nancy, Nancy, France, 1985; p. 200. [Google Scholar]
- Costa, D.; Tavares, R.M.; Baptista, P.; Lino-Neto, T. The influence of endophytes on cork oak forests under a changing climate. In Endophytes for a Growing World; Hodkinson, T., Doohan, F., Saunders, M., Murphy, B., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 250–274. [Google Scholar]
- Siwkcki, R.; Ufnalski, K. Review of oak stand decline with special reference to the role of drought in Poland. Eur. J. For. Pathol. 1998, 28, 99–112. [Google Scholar] [CrossRef]
- Popa, I.; Leca, S.; Crăciunescu, A.; Sidor, C.; Badea, O. Dendroclimatic response variability of Quercus species in the Romanian intensive forest monitoring network. Not. Bot. Horti Agrobo. Cluj-Napoca 2013, 41, 326–332. [Google Scholar] [CrossRef]
- Sohar, K.; Helama, S.; Läänelaid, A.; Raisio, J.; Tuomenvirta, H. Oak decline in a southern Finnish forest as affected by a drought sequence. Geochronometria 2014, 41, 92–103. [Google Scholar] [CrossRef]
- Dinca, L.; Constandache, C.; Postolache, R.; Murariu, G.; Tupu, E. Timber Harvesting in Mountainous Regions: A Comprehensive Review. Forests 2025, 16, 495. [Google Scholar] [CrossRef]
- Boisvenue, C.; Running, S.W. Impacts of climate change on natural forest productivity–evidence since the middle of the 20th century. Glob. Change Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Chen, G.; Tian, H.; Zhang, C.; Liu, M.; Ren, W.; Zhu, W.; Lockaby, G.B. Drought in the Southern United States over the 20th century: Variability and its impacts on terrestrial ecosystem productivity and carbon storage. Clim. Change 2012, 114, 379–397. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Cobb, N. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Anderegg, W.R.; Kane, J.M.; Anderegg, L.D. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Change 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Marçais, B.; Nageleisen, L.M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Tessier, L.; Nola, P.; Serre-Bachet, F. Deciduous Quercus in the Mediterranean region: Tree-ring/climate relationships. New Phytol. 1994, 126, 355–367. [Google Scholar] [CrossRef]
- Cooke, J.E.; Eriksson, M.E.; Junttila, O. The dynamic nature of bud dormancy in trees: Environmental control and molecular mechanisms. Plant Cell Environ. 2012, 35, 1707–1728. [Google Scholar] [CrossRef]
- Trontin, J.F.; Raschke, J.; Rupps, A. Tree ‘memory’: New insights on temperature-induced priming effects during early embryogenesis. Tree Physiol. 2021, 41, 906–911. [Google Scholar] [CrossRef]
- Becker, M.; Nieminen, T.M.; Gérémia, F. Short-term variations and long-term changes in oak productivity in north-eastern France: The role of climate and atmospheric CO2. Ann. For. Sci. 1994, 51, 477–492. [Google Scholar] [CrossRef]
- Bergès, L.; Dupouey, J.L.; Franc, A. Long-term changes in wood density and radial growth of Quercus petraea Liebl. in northern France since the middle of the nineteenth century. Trees 2000, 14, 398–408. [Google Scholar] [CrossRef]
- Patón, D.; García Herrera, R.; Guenca, J.; Galavis, M.; Roig, F.A. Influence of climate on radial growth of holm oaks (Quercus ilex subsp. ballota Desf.) from SW Spain. Geochronometria 2014, 41, 92–103. [Google Scholar] [CrossRef]
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe drought effects on Mediterranean woody flora in Spain. For. Sci. 2001, 47, 214–218. [Google Scholar] [CrossRef]
- Landmann, G.; Dreyer, E. Impacts of Drought and Heat on Forests. Synthesis of Available Knowledge, with Emphasis on the 2003 Event in Europe. Ann. For. Sci. 2006, 63, 567–652. [Google Scholar] [CrossRef][Green Version]
- Faber-Langendoen, D.; Tester, J.R. Oak mortality in sand savannas following drought in east-central Minnesota. Bull. Torrey Bot. Club 1993, 120, 248–256. [Google Scholar] [CrossRef]
- Catton, H.A.; George, S.S.; Remphrey, W.R. An evaluation of bur oak (Quercus macrocarpa) decline in the urban forest of Winnipeg, Manitoba, Canada. Arboric. Urban For. 2007, 33, 22–30. [Google Scholar] [CrossRef]
- Arend, M.; Kuster, T.; Günthardt-Goerg, M.S.; Dobbertin, M. Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol. 2011, 31, 287–297. [Google Scholar] [CrossRef]
- Li, M.H.; Cherubini, P.; Dobbertin, M.; Arend, M.; Xiao, W.F.; Rigling, A. Responses of leaf nitrogen and mobile carbohydrates in different Quercus species/provenances to moderate climate changes. Plant Biol. 2013, 15, 177–184. [Google Scholar] [CrossRef]
- Luciano, P.; Prota, R. Insect pests in Sardinian cork-oak forests. Bull. OILB/SROP 1995, 18, 1–7. [Google Scholar]
- Graf, P.; Basri, E.; Bakri, M. Cork oak decline in Morocco. In Proceedings of the International Congress “Recent Advances in Studies on Oak Decline”, Brindisi, Italy, 13–18 September 1992. [Google Scholar]
- Budeanu, M.; Besliu, E.; Pepelea, D. Testing the Radial Increment and Climate–Growth Relationship Between Swiss Stone Pine European Provenances in the Romanian Carpathians. Forests 2025, 16, 391. [Google Scholar] [CrossRef]
- Besliu, E.; Curtu, A.L.; Budeanu, M.; Apostol, E.N.; Ciocîrlan, M.I. Exploring the effects of the assisted transfer of European beech (Fagus sylvatica L.) provenances in the Romanian Carpathians. Not. Bot. Horti Agrobo. Cluj-Napoca 2024, 52, 13968. [Google Scholar] [CrossRef]
- Budeanu, M.; Apostol, E.N.; Radu, R.G.; Ioniță, L. Genetic variability and juvenile–adult correlations of Norway spruce (Picea abies) provenances, tested in multisite comparative trials. Ann. For. Res. 2021, 64, 105–122. [Google Scholar] [CrossRef]
- Huesca, M.; Ustin, S.L.; Shapiro, K.D.; Boynton, R.; Thorne, J.H. Detection of drought-induced blue oak mortality in the Sierra Nevada Mountains, California. Ecosphere 2021, 12, e03558. [Google Scholar] [CrossRef]
- Apostol, E.N.; Dinu, C.G.; Apostol, B.O.; Ciuvăț, A.L.; Lorenț, A.D.; Pleșca, I.; Postolache, D.R.; Leca, Ș.; Enescu, C.M. Importance of pubescent oak (Quercus pubescens Willd.) for Romanian forests in the context of climate change. Rev. De Silvic. și Cinegetică 2016, 21, 29–33. [Google Scholar]
- Gentilesca, T.; Camarero, J.J.; Colangelo, M.; Nolã, A.; Ripullone, F.; Nole, A. Drought-induced oak decline in the western Mediterranean region: An overview on current evidences, mechanisms and management options to improve forest resilience. IForest 2017, 10, 796–806. [Google Scholar] [CrossRef]
- Dănescu, F.; Ungurean, C.; Chira, F.; Horga, D.; Jung, T. Oak decline in Livada Forest District. Rev. De Silvic. si Cineg. 2015, 37, 66–78. [Google Scholar]
- Chira, D.; Borlea, F.G.; Chira, F.; Mantale, C.Ș.; Ciocîrlan, M.I.; Turcu, D.O.; Cadar, N.; Trotta, V.; Camele, I.; Marcone, C.; et al. Selection of Elms Tolerant to Dutch Elm Disease in South-West Romania. Diversity 2022, 14, 980. [Google Scholar] [CrossRef]
- Kaproth, M.A.; Fredericksen, B.W.; González-Rodríguez, A.; Hipp, A.L.; Cavender-Bares, J. Drought response strategies are coupled with leaf habit in 35 evergreen and deciduous oak (Quercus) species across a climatic gradient in the Americas. New Phytol. 2023, 239, 888–904. [Google Scholar] [CrossRef]
- Tăut, I.; Moldovan, M.; Șimonca, V.; Varga, M.I.; Rob, M.; Chira, F.; Chira, D. Control of Pathogen Erysiphe alphitoides Present in Forest Crops in Current Climatic Conditions. Microbiol. Res. 2024, 15, 1441–1458. [Google Scholar] [CrossRef]
- Buzatu, A.; Nețoiu, C.; Apostol, B.; Badea, O. The use of remote sensing indices derived from Sentinel 2 satellite images for the defoliation damage assessment of Lymantria dispar. Ann. For. Res. 2023, 66, 123–138. [Google Scholar] [CrossRef]
- Forzieri, G.; Dutrieux, L.P.; Elia, A.; Eckhardt, B.; Caudullo, G.; Taboada, F.Á.; Andriolo, A.; Bălăcenoiu, F.; Bastos, A.; Buzatu, A.; et al. The Database of European Forest Insect and Disease Disturbances: DEFID2. Glob. Change Biol. 2023, 29, 6040–6065. [Google Scholar] [CrossRef]
- Balacenoiu, F.; Netoiu, C.; Tomescu, R.; Simon, D.C.; Buzatu, A.; Toma, D.; Petritan, I.C. Chemical Control of Corythucha arcuata (Say, 1832), an Invasive Alien Species, in Oak Forests. Forests 2021, 12, 770. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).