Abstract
Tree rings provide an invaluable insight into how trees adapt to changes in climate. This study presents aggregated results, from our research on tree rings, climatic response and the insect Choristoneura murinana infestations from three studies on Greek fir, located in stands across Central Greece and Giona Mountain on three different altitudes. In our studies, was found that extreme droughts and wet events had a negative or positive effect on fir growth, respectively. April’s precipitation had a positive correlation with growth for all the stands, which supports other authors’ findings. Moreover, the average maximum temperature of the growing season and the maximum temperature of April, July and August were also linked to growth. Evapotranspiration during the growing season was seen to be inversely proportional to the growth of fir. An apparent decline in tree ring growth more severe in stand 3 (Average Tree Ring Width Index, ARWI < 0.6) has been observed, particularly in recent years. The data suggests that temperature is having a detrimental effect on fir growth in the area, with a significant decreasing tendency in growth from 1993 for the high altitude stands and from 1998 for the lower altitude stand. To ensure successful and sustainable forest management in the future, more research into tree rings and their relationship with climate must be carried out.
1. Introduction
In recent decades, extreme drought events with high temperatures and low precipitation have caused intense forest dieback across Europe [1,2]. Mediterranean regions are particularly vulnerable to tree species loss due to increased frequency and intensity of drought events [3,4]. The forests of Southern Europe have experienced direct abiotic disturbances, such as droughts and other climatic factors [5,6,7,8] resulting in decreased forest productivity [9] and increased tree mortality [10,11,12]. More specifically, it has been observed that many fir species have experienced a loss of radial growth and damage from pests and pathogens, which are linked to drought severity. Suggestively, Navarro-Cerrillo et al. [13] proved that the endangered and relict Abies pinsapo tree species in Andalusia, south-eastern Spain, is increasingly exposed to the combined pressures of climate change, pests, and pathogens. This study used a monitoring network dataset from 2001–2017 to quantify the spatiotemporal patterns of abiotic (drought, climate warming) and biotic (pathogen) risks and the resulting loss of vitality. Results showed that defoliation and mortality rates were higher in the eastern part of the study area and linked to long and severe droughts, beetle attacks, and root rot fungi. Moreover, Gasol et al. [14] evaluated multiple features of Abies alba, forests in Spain to determine whether previous growth rates and trends are valid predictors of dieback. The defoliation degree was strongly related with radial growth, and growth trends differed between moderately to highly defoliated trees and non-defoliated trees. In addition, low elevation and less rainfall in the summer were associated with dieback. At the same time, seems that climate warming is modifying growth patterns and responses to climate across the species’ European distribution area, with a decline in growth of many populations in south-western Europe due to increased aridity, and growth in more temperate areas due to warming [15]. Dendroclimatic analysis of 23 large size Abies alba trees in Southern Italy, revealed that late spring/summer precipitation is the most important factor affecting growth in the area [16]. Finally, results of a study regarding Abies alba in five mixed European beech-silver fir forests of Central Italy (Tuscany and Marches) revealed that beech-silver fir forests, may respond to climate variability at different altitudes by increasing water use in summer, in response to decreased spring precipitation and general temperature increase [17].
In Greece, water scarcity is likely the primary climatic factor limiting forest growth. Studies have shown a strong correlation between available water and tree ring width and tree growth in different Mediterranean forests and tree species [18,19,20,21,22]. Weakening or necrosis of Mediterranean fir forests in Greece, particularly in southern and central Greece, has been attributed primarily to drought-related extreme periods and high spring or low winter temperatures [23,24,25,26]. Tree-ring width relations with precipitation and temperature have also been studied on a local scale for A. cephalonica [21,26,27,28].
Tree growth can be directly affected by species-specific sensitivity to warmer or drier climates. Predicted increases in the severity and frequency of drought events globally [4,29] could have major impacts on tree growth [2]. Tree rings can be used as a research tool to explore these topics, as they record a wide range of variables related to environmental change which can be measured and accurately dated with sub-annual precision [30,31,32,33,34,35]. Fritts [31] found that tree ring variance is generally controlled by factors influencing growth. Tree-ring evidence from many different biophysical settings supports the idea that tree growth is limited by water in some ecosystems and by energy (growing season length, degree days, or mean temperature) in other ecosystems [36]. Littell et al. [37] observed that it is necessary to consider multiple scales of factors affecting the local climate of a forest stand in order to make successful comparisons among stands of the same species across their niche. Local topography, for example, can mediate the climate of a forest stand and moderate or exacerbate the influences of regional climate [38,39]. In this study, we reviewed our recent research on Greek fir growth in Giona Mt in Central Greece, incorporating new analyses from a newly measured fir stand at the mountain aiming to understand temperature sensitivities of growth and their temporal trends, including precipitation impact, at a local scale. Finally, all the aggregated results are briefly presented.
2. Materials and Methods
In 2021, two study sites of Greek fir with different altitudes (998 m and 1274 m) located in Central Greece on Mt Giona were studied regarding the impact of precipitation and air temperature on its radial growth using tree ring analysis after the calculation of the Average Tree Ring Width Index (ARWI) [21]. The forests in this region mostly consisted of natural Greek fir (Abies cephalonica) stands. The fir forest is affected by both natural and anthropogenic disturbances, such as insects, logging, and visitors. More specifically, the most important insect observed is the defoliator Choristoneura murinana Hb. (Lep., Tortricidae, Archipini), European fir budworm (hereafter as EFB), while there is no reference in the last 60 years of severe attacks of Scolytidaes. Moreover, defoliations by the insect European fir budworm (EFB), (Choristoneura murinana) were observed, described and published [40] in several areas across the mountains of Giona and Parnassus. However, the selected trees of the stands were relatively healthy and showed no severe damage or logging impacts. The two study sites (stand 1 and stand 2), both face the Southeast, were located in Central Greece, on Mt Giona, near the village of Kaloskopi. The third one (stand 3) is located almost 18 km north south of the village Kaloskopi, near the village Mavrolithari and also faces Southeast (Figure 1). All the characteristics of the case studies are outlined in Table 1.
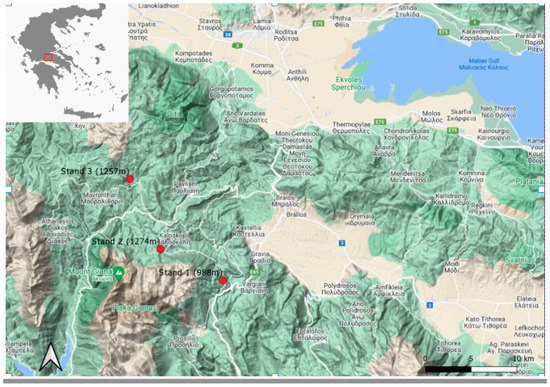
Figure 1.
Location of study areas on Giona Mountain, Central Greece. Giona Mt is located between the mountains of Parnassus to the east, Vardousia to the west, and Oeta to the north, and the nenearest town is Amfissa, to the southeast.

Table 1.
Coordinates and case studies characteristics.
The elevation of the three sample stands is 988 m (stand 1), 1.274 m (stand 2) and 1257 m (Stand 3) above sea level, respectively. The measurements concerning the third stand were taken at the end of the summer of 2022 and are presented in this study for the first time. The data is intended to be compared with the published analysis of stands 1 and 2 [21] in order to further our research in the area in order to understand more the nature of growth–climate relationships for the Greek fir across the Mt of Giona.
The third site was chosen for its unique climate. Mavrolithari, is renowned for its cold winters and often experiences the lowest temperatures across the country. Examining the growth of firs in this environment can provide insight into how trees respond to increasing temperatures due to climate warming. Regarding the sampling, we followed the same procedure as our last work [21], receiving two wood cores per tree at breast height above ground, total 20 dominant or co-dominant trees, separated at least 5 m from each other using a 400 mm long and 5.15 mm wide increment borer Mora three-threaded auger by Haglof (Haglof Inc., Långsele, Sweden). After their preparation (air drying and hand sanding), tree rings measurements were performed using the LignoVision software (version 1.40). Tree ages ranged from 61 to 136 years in Stand 1, from 50 to 83 years in stand 2 and 35 to 68 in stand 3. Fritts [31] suggested that various biological growth functions have been used for the curve fitting process, such as parabolas, hyperbolas, logarithmic, and polynomial functions. The literature referred that this kind of plots are typical if ring width data derived from many coniferous species growing on drought-subjected sites (ibid). Following that, common nonlinear function for determining an appropriate tree growth model against time were used. In our prior research [21], the hyperbola two-parameter function performed better, and we maintained these results. For stand 3, an exponential decay function was preferred while the evaluation of the ring width models was based on the adjusted coefficient of determination (Radj), and the significance (p < 0.05).
The precipitation data used on the already published study (daily values converted to monthly values) [21], were derived from the Kaloskopi hydrometeorological station (coordinates: 38.68932° N, 22.32333° E, at 1052.80 m above sea level), owned by the Ministry of Environment and Energy (Hellenic Ministry of Environment and Energy, 2020) and from the Mavrolithari meteorological station (coordinates: 38.70706° N, 22.30461° E, at 1.220 m), (Figure 1) owned by the National Observatory of Athens, Institute of Environmental Research and Sustainable Development (National Observatory of Athens, 2020). To extend our investigation in this study, we obtained climate time series data on temperature, precipitation and actual evapotranspiration using the online application Climateengine.com [41]. These data were sourced from TERRACLIMATE 4000 m (1/24-deg) monthly dataset (1965–2019) and CHIRPS 4800 m (1/20-deg) pentad dataset (UCSB/CHG) (1981–2019), as described in Huntington et al. [42].
To uncover any significant correlations between tree rings width and climate data, Pearson correlation was used, as with many other studies in the past.
To investigate significant changes in fundamental climate data and tree rings width over the years, we chose to implement the Pettitt Homogeneity test. In general, homogeneity tests involve a large number of tests for which the null hypothesis is that a time series is homogenous between two given times. The Pettitt’s test is a nonparametric test that requires no assumption about the distribution of data and actually is an adaptation of the tank-based Mann–Whitney test that allows identifying the time at which one statistical important (p value) in time series shift occurs. Inhomogeneity in time series can cause the incorrect interpretation of extreme events [43] and be misleading in the interpretation of tendencies in the time series. Abrupt changes in the mean are one of the normal outcomes of inhomogeneity in time series data (ibid). The importance of the homogeneity test is pointed out by Buffoni et al. [44], and Reiter et al. [45] and Bickici et al. [46]. This test detects shifts in the average and calculates their significance [47] in a hypothesis test.
Finally, the Mann–Kendall trend test was used to indicate a monotonic trend inside the timeseries of tree rigs width and climate data as well. The test was first proposed by Mann [48] then further studied by Kendall et al. [49] and improved by Hirsch et al. [50] who allowed to take into account a seasonality. The null hypothesis H0 for these tests is that there is no trend in the series. The three alternative hypotheses that there is a negative, non-null, or positive trend can be chosen. The Mann–Kendall test is based on the calculation of Kendall’s tau measure of association between two samples, which is itself based on the ranks with the samples.
3. Results and Discussion
3.1. Tree Growth
For stand 3 and each tree, the exponential decay function yielded satisfactory results. The adjusted coefficient of determination (Radj) had values between 0.399 and 0.763 and a significance level of p < 0.0001. The Durbin Watson statistic was used to test for autocorrelation, and the results were close to 2 (1.5964 to 1.6240), indicating no autocorrelation. After that the equation was solved for the expected yearly growth (Yt). The measured ring widths (Wt) were converted to average ring–width index (ARWI) for Stand 3 by dividing each width for year t by the expected growth (Yt).
This conversion both removes the trend in growth and scales the variance so that is approximately the same throughout the entire length of the time series. After constructing and solving all the above equations, concluding all rings measurements from all the stands, the ARWI values were calculated for Stand 3 and the new master averaged index for the particular stand was plotted and added to our previous results. (Figure 2). According to Fritts, [31] this standardization equalizes or brings all ring width curves to a uniform mean value, so that a tree record with a large average growth will not dominate other records. Figure 2 shows the expected year-to-year variation, as well as consistent annual fluctuations in the mean growth of each stand. Notably, stand 3 (ARWI < 0.6) has experienced a considerable decrease in tree ring growth, especially in the past decade. It appears that this particular fir forest is being affected by an external factor that the trees cannot easily cope with through their natural adaptive capabilities. It is well known, however, that forests’ adaptive capacity may be hampered by the rapid climate changes predicted for this century. Forest ecosystems are generally resilient, and many species and ecosystems have adapted to changing conditions in the past. However, the changes that may occur in the future might be too extreme or happen too quickly for some species and ecosystems to adapt, which could result in local extinctions and the loss of important functions and services, such as a decrease in forest carbon stocks and the ability to sequester carbon [51].
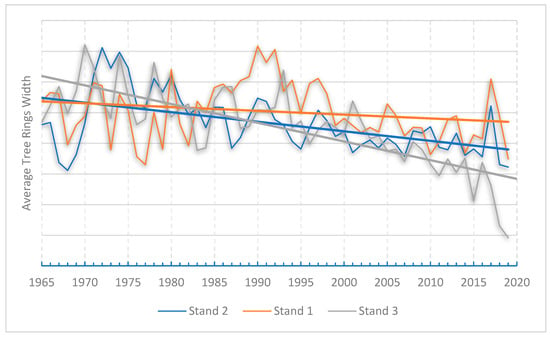
Figure 2.
Master Average Tree Rings Width indices for stands 1, 2, 3 against time.
3.2. Climatic Factors
We obtained more information about the growth relationship between climate conditions (from the previous and current year) utilizing climate data series from the noted international databases for the specified coordinates. The correlation results between the ARWI (Figure 1) and climate data are given in Table 2. The mean temperature and the mean maximum temperature of the previous year have a negative correlation with growth and is negatively associated with growth for stands 1, 2, and 3. As anticipated, the spring and summer of the current year have had an effect on tree rings width, across the three stands. Our previous research found a positive correlation with the average temperature of May, and negative correlations with the maximum average temperature of June and the minimum average temperature of August [21]. In this study, we extended our research to examine the climate conditions of the current year. We discovered negative correlations between the average maximum temperature of the Growing Season (April–October), as well as the maximum temperature of April, July, and August. A negative correlation was also observed between Growing Season (April–October) Actual Evapotranspiration for all the analyzed stands.

Table 2.
Climate-ring width Pearson correlation (r) statistics 1. Only the statisticallyimportant results are presented (* p < 0.05, ** p < 0.001).
It appears that both Papadopoulos et al. [19] and Koutavas et al. [28] reported that late spring and summer precipitation has the greatest impact on fir tree growth, which is positively correlated. Our data analysis, however, did not completely confirm this using correlation results. April’s precipitation had a positive correlation with growth for all the stands, which supports the authors’ findings. On the other hand, a negative correlation was found between tree ring growth and June precipitation across the stands as well. (Table 2). The graph in Figure 3 indicates a decrease in Average Ring Width Index (ARWI) as summer approaches, despite an increase in precipitation across the chosen stands in April and June. This suggests that, as summer arrives, precipitation has a lesser effect on fir growth. Moreover, the angle formed by the blue and black straight lines is also increasing as we move from stand 1 to stand 3, meaning that the latter stand is less dependable from precipitation than others, likely indicating wetter conditions and greater water availability.
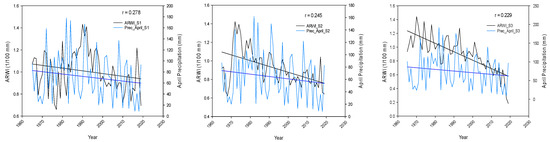
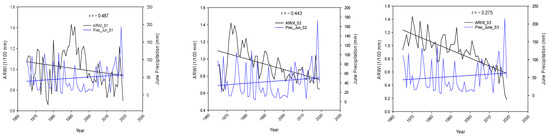
Figure 3.
Graphical representation of the Average Ring Width Index against April and June Total Precipitation across the stands S1, S2, S3.
Giving more attention to temperatures, the average maximum temperature of the previous year is negatively correlated to growth across all the stands. Additionally, the maximum temperature of the growth season (Tmax_Grose) is also negatively associated with growth, except for the low altitude (Stand 1), where no significant results were found. It appears that the higher altitudes on Giona Mountain are more susceptible to extreme temperatures of the growing season, but further data and analysis are needed to confirm this. As expected, the expected high temperatures of spring (March, April, May) and summer (June, July, August) were found to have a pronounced negative effect on fir growth, particularly on higher altitudes for July and August. Finally, the actual evapotranspiration of the previous year (ET actual _12M) and the actual evapotranspiration of the growing season of the current year (ETactual_Grose) are negatively correlated to growth for all the stands and altitudes, observation that makes sense if we consider that tree rings can be used to measure the amount of water a tree has absorbed over the course of a year via evapotranspiration. During times of drought, trees may not be able to access enough water to sustain healthy growth, resulting in narrower tree rings. It is appreciated that temperature always interacts with other factors in producing an effect. So, possible increasing temperature of the region might enhance the potential evapotranspiration which creates the scarcity of moisture available for trees over the area Moreover, especially during the growing season, increasing air temperature, is expected to worsen the impact of drought on tree water loss by elevating the vapor pressure deficit (VPD) in the atmosphere, thereby increasing evaporative demand at a regional scale [52]. This leads to a negative correlation between evapotranspiration and tree ring width.
Our findings demonstrate a variation in the behavior of the three stands when exposed to the highest temperatures during the Spring and Summer months. Notably, we observed statistically significant correlations for March, April, July, and August. The correlation between maximum temperatures in March and April with ARWI is weaker, as indicated by a Pearson’s correlation coefficient of less than 0.3. By contrast, the maximum temperatures in July and August could have a substantial negative impact on tree growth in stands 2 and 3 at higher altitudes, with a Pearson’s coefficient of up to 0.6, concluding that the ability of these stands to tolerate high temperatures is reduced at higher altitudes, and this varies according to altitude. However, when looking into how a species responds to climate across its distribution, various factors such as photoperiod, precipitation, and genetic variation and divergence become notably different depending on the altitude [53,54].
3.3. Break Years of Growth and Temperature Time Series
To assess the homogeneity in the mean of the growth and temperature time series across the three stands, the Pettitt’s test was performed with a two-tailed test. The null hypothesis for this test was that the mean of the dataset for the examined variables was not shifted. The alternative hypothesis was that there is a certain datum (year) at which a change point can be detected, and the mean of the dataset shifts at this break point (Figure 4). The empirical significance level (p value) is shown in Table 3. A statistically significant change in years can be detected in 1993 for the high altitude stands (2 and 3) and 1998 for stand 1. The datasets were therefore divided at the break point and the separate parts were examined from the perspective of the monotonic trend using the Mann–Kendall trend test and the results of these tests are shown in Table 4.
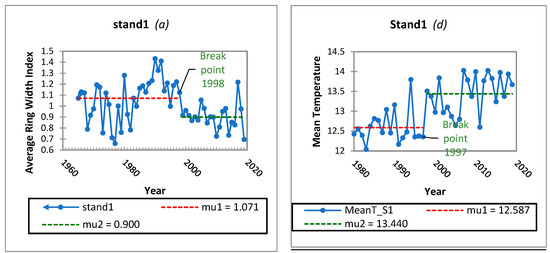
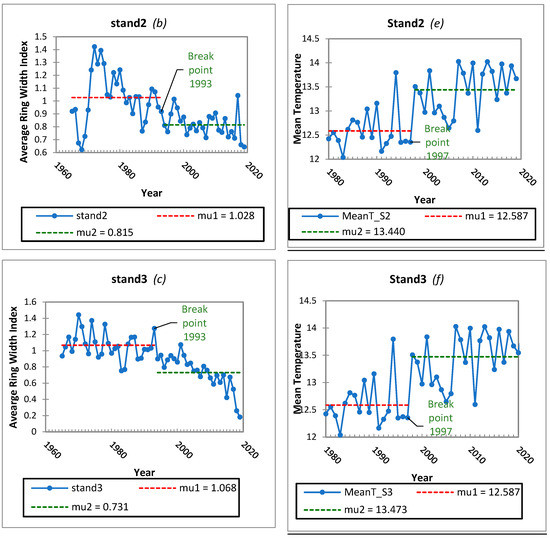
Figure 4.
Significant break points and downward shifts (a–c) in the ARWI and upward shifts (d–f) in the annual Mean T for Stands 1, 2, 3 during the last decades after the Pettitt homogeneity test (p < 0.001).

Table 3.
Results of the Pettitt homogeneity test and the Statistically significant change years regarding ARWI.

Table 4.
Results of the simple Mann–Kendall (MK) trend tests (the p value of the significant monotonic tendency).
A significant decreasing tendency was statistically demonstrated for stands 2, 3 the high altitude stands after the statistically significant change year of 1993 (Table 4). On the other hand, no important trend was revealed before the change year. The same analysis on climate variables could not confirm change years or decreasing and increasing tendencies in yearly and growth season precipitation across all the stands meaning that there were no significant Pettitt and Mann–Kendall results. The same tests were conducted on the temperature data, and the results are shown in Table 5.

Table 5.
Results of the Pettitt homogeneity test for Mean T °C and the statistically significant change years.
Statistically significant change points were detected in 1997 across all the stands. The datasets were therefore divided at the break point and the separate parts of temperature time series were examined from the perspective of the monotonic trend using the Mann–Kendall trend test, but not significant monotonic tendencies were found. Finally, the application of the homogeneity test to precipitation data for the same period did not yield any statistically significant results. Since 1997, there has been a noticeable increase in temperature which raises the question of whether this change has had any effect on the growth of the observed fir stands. If we compare this change point with the years of change in tree growth, we can understand that stand 1 has shown an important downward shift one year after, meaning 1998 and stands 2 and 3 have already shown important downward shift 5 years earlier. The data indicates that temperature has been having a detrimental effect on fir growth in the area, more obviously in lower altitude stand. Higher altitude stands from 1993 onwards have shown a statistically significant decline in growth. However, Mann–Kendall test could not confirm, that this shift was followed by a statistical important tendency (p value 0.264). At the same time, there were no significant trends in precipitation observed on the trees examined, making it difficult to identify a factor that could have caused the earlier decline of stands in higher altitudes. Some references are underlining, that in ecosystems where precipitation is more abundant or energy is more limiting, usually subalpine or high latitude areas, factors affecting the length of the growing season explain most of the variability in tree growth [37,55,56,57,58]. This particular issue requires more attention and more research on stands 2 and 3. The statistically significant decrease in growth observed five years prior suggests that rising temperatures may have a more severe impact at higher altitude, as other factors appear to have been ruled out as a cause. On the same track, we must take into account the possibility of prior infestations from insects or pathogens that are not now observable, even if our observations confirm that fir trees above 1.100 m have not been affected by the EFB. It is complicated to accurately interpret and explain all of these results. The same year of change in stands 2 and stand 3, in terms of growth, suggesting that fir trees in that altitude range respond similarly to climate, or may be affected by a biological factor, or both. At high elevations, fir trees have limited abilities to adapt to extreme climates such as extreme high temperatures. It is reported that, high-altitude ecosystems are extremely sensitive and vulnerable to the most rapid climate warming seen in the past decades, e.g., [3,5]. It is anticipated that a rapid increase in temperature will raise the temperature sensitivity of tree growth, thus causing a major shift in forest ecosystem composition and function due to the low temperature restriction in higher altitudes [59].They are also vulnerable to external and internal factors such as microclimatic conditions caused by local topography, soil conditions, a particular pathogen or insect, and competition, which can all cause additional stress to the trees after already enduring environmental stress. Despite some findings suggesting a decrease in tree growth [60,61,62] tree dieback [53] and even forest mortality [63] due to a warming climate, there are still conflicting results. The effects of a water deficit caused by warming may be the cause of these results. More work and data on different species or climate conditions are needed to clarify the uncertainty in accessing the effects of climate warming on forest growth [64]. Therefore, it is crucial to understand temperature sensitivities of tree growth and their temporal trends under different precipitation regimes firstly at local and secondly at a regional scale.
3.4. European Fir Budworm Infestations
Choristoneura murinana (Hubner), otherwise European Fir Budworm (EFB), is a west Palearctic species that has severe out breaks mostly on silver fir, Abies alba [65,66]. However, for all three fir species in Greece, the primary insect responsible for needles loss is EFB [40]. The pest is capable of causing extensive defoliation and damage and can lead to tree death. The budworm has one generation per year (univoltine). Imago’s fly period is in July and early August. After this period the insects lay eggs. Eggs are laid in batches on the needles and the larvae hatch in late summer, dispersing on silken threads. The L1 larvae spin overwintering hibernacula under bark scales at the base of twigs in the upper crown of the fir trees. In the hibernacula, the L1 molt to L2 stage before entering diapause. The larvae exit the hibernacula in late March and April and feed on the new shoots through to the penultimate instar (prepupa) which do not feed. Pupation occurs in rolled shoots at the larval feeding sites in June. In our previous measurements we reported a reduced growth index for the first two different altitude stands (Stands 1 and 2) after 1999 indicating unfavorable growth conditions for almost the two last decades [21,40].
We found that EFB mostly prefers Abies cephalonica and less Juniperus oxycedrus in sunny areas and/or near the country roads and forest openings where severe infestations of different scales were observed (Figure 5) and the size of the opening is moderate—i.e., not more than 10 m. No infestations were observed or reported by local populations regarding stands 2 and 3. This indicates that the infestation has not yet spread westward due to the lack of large openings and human-induced disturbances such as roads, forest roads, forest paths, and homebuilding. All these comprise the reason why fir trees above 1.100 m have not been affected by the EFB and confirm our previous observation. This is likely due to the fact that the EFB prefers areas which receive a lot of sunlight and new fir needle tissue [40]. Even though eruptive outbreaks are likely to spread over large areas, to last for a long time, and to cause more serious damage to the forest resources. Insects can have a considerable economic impact on forest trees and their products, ranging from the death of the tree to growth reduction, lower grade timber, stem deformity, reduced seed crops, as well as more subtle effects on recreation, wildlife, aesthetics, and even the potential for a fire hazard. Accurate diagnosis of insect problems in forests, relies on effective monitoring techniques and data on the extent of the damage. It appears essential to invest in more research and funding, in order to enable continuous monitoring of populations and the potential introduction of control measures when predetermined levels are reached.

Figure 5.
Giona Mt photos EFB infestations in altitudes lower than 1100 m, in mature trees but in nature regeneration as well.
4. Conclusions
In our studies, the implementation of specific methodologies on field measurements brought to light circumstantial fir’s growth decline in lower and higher altitudes. The latter sometimes is connected to climate parameters or seems to be associated with past insect infestations, especially regarding the lower altitudes. The analysis of the tree-ring width in relationship to precipitation and temperature in this study has revealed that the growth of Greek fir in both high and low altitude stands has significantly decreased, following a downward shift. This shift is likely due to the increasing temperatures, as a statistically significant upward shift in the annual mean temperatures across the three stands was detected. This observation confirms the hypothesis that increasing temperatures have a detrimental effect on fir growth in the area. Further research is needed, by using more tree rings and climate data, to determine the full impact of this effect locally.
Understanding how trees grow in different locations, species, origins, climates and management plans is an essential part of forestry for foresters, landowners, forest administrators and the public. Even in natural forests, climate variability has a huge impact on forest management, which can include higher mortality rates, decreased productivity, and changes to the composition of the forest. Therefore, strategies should be considered to reduce these effects, such as managing forests to achieve multiple objectives. Monitoring changes is a classic and essential aspect of forestry research.
Author Contributions
Conceptualization, P.P.K. and P.V.P.; methodology, P.P.K.; software, P.P.K.; validation, P.P.K. and P.V.P.; investigation, P.P.K. and P.V.P.; resources, P.P.K.; writing—original draft preparation, P.P.K. and P.V.P.; writing—review and editing, P.P.K. and P.V.P.; visualization P.P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data used in this study can be found in the Institute of Mediterranean Forest Ecosystems and the Laboratories of Forest Management and Economics and Forest Entomology, ELGO DIMITRA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peñuelas, J.; Lloret, F.; Montoya, R. Severe Drought Effects on Mediterranean Woody Flora in Spain. For. Sci. 2001, 47, 214–218. [Google Scholar]
- Dobbertin, M. Tree Growth as Indicator of Tree Vitality and of Tree Reaction to Environmental Stress: A Review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Parry, M.; Canziani, O.; Palutikof, J.; Linden, P.; Hanson, C. Climate Change 2007: Impact, Adaptation and Vulnerability by Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- IPCC; Field, C.B.; Barros, V.; Stocker, T.F.; Qin, D.; Dokken, D.J.; Ebi, K.L.; Mastrandrea, M.D.; Mach, K.J.; Plattner, G.-K.; et al. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation—SREX Summary for Policymakers; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012. [Google Scholar]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive While Others Succumb to Drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A Multi-Species Synthesis of Physiological Mechanisms in Drought-Induced Tree Mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of Tree Mortality under Drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-Wide Reduction in Primary Productivity Caused by the Heat and Drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.J.; Ripullone, F.; Gazol, A.; Sánchez-Salguero, R.; Oliva, J.; Redondo, M.A. Drought Decreases Growth and Increases Mortality of Coexisting Native and Introduced Tree Species in a Temperate Floodplain Forest. Forests 2018, 9, 205. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Rodriguez-Vallejo, C.; Silveiro, E.; Hortal, A.; Palacios-Rodríguez, G.; Duque-Lazo, J.; Camarero, J.J. Cumulative Drought Stress Leads to a Loss of Growth Resilience and Explains Higher Mortality in Planted than in Naturally Regenerated Pinus Pinaster Stands. Forests 2018, 9, 358. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; Gazol, A.; Rodríguez-Vallejo, C.; Manzanedo, R.D.; Palacios-Rodríguez, G.; Camarero, J.J. Linkages between Climate, Radial Growth and Defoliation in Abies Pinsapo Forests from Southern Spain. Forests 2020, 11, 1002. [Google Scholar] [CrossRef]
- Navarro-Cerrillo, R.M.; González-Moreno, P.; Ruiz-Gómez, F.J.; Sánchez-Cuesta, R.; Gazol, A.; Camarero, J.J. Drought Stress and Pests Increase Defoliation and Mortality Rates in Vulnerable Abies Pinsapo Forests. For. Ecol. Manag. 2022, 504, 119824. [Google Scholar] [CrossRef]
- Gazol, A.; Sangüesa-Barreda, G.; Camarero, J.J. Forecasting Forest Vulnerability to Drought in Pyrenean Silver Fir Forests Showing Dieback. Front. For. Glob. Chang. 2020, 3, 36. [Google Scholar] [CrossRef]
- Gazol, A.; Camarero, J.J.; Gutiérrez, E.; Popa, I.; Andreu-Hayles, L.; Motta, R.; Nola, P.; Ribas, M.; Sangüesa-Barreda, G.; Urbinati, C.; et al. Distinct Effects of Climate Warming on Populations of Silver Fir (Abies Alba) across Europe. J. Biogeogr. 2015, 42, 1150–1162. [Google Scholar] [CrossRef]
- Gentilesca, T.; Todaro, L. Tree-Ring Growth and Climate Response of Silver Fir (Abies Alba Mill.) in Basilicata (Southern Italy). For. J. Silvic. For. Ecol. 2008, 5, 47–56. [Google Scholar] [CrossRef]
- Mazza, G.; Gallucci, V.; Manetti, M.C.; Urbinati, C. Climate-Growth Relationships of Silver Fir (Abies Alba Mill.) in Marginal Populations of Central Italy. Dendrochronologia 2014, 32, 181–190. [Google Scholar] [CrossRef]
- Sarris, D.; Christodoulakis, D.; Körner, C. Recent Decline in Precipitation and Tree Growth in the Eastern Mediterranean. Glob. Change Biol. 2007, 13, 1187–1200. [Google Scholar] [CrossRef]
- Papadopoulos, A. Tree-Ring Patterns and Climate Response of Mediterranean Fir Populations in Central Greece. Dendrochronologia 2016, 40, 17–25. [Google Scholar] [CrossRef]
- Koulelis, P.P.; Daskalakou, E.N.; Ioannidis, K.E. Impact of Regional Climatic Conditions on Tree Growth on Mainland Greece. Folia Oecologica 2019, 46, 127–136. [Google Scholar] [CrossRef]
- Koulelis, P.P.; Fassouli, V.P.; Petrakis, P.V.; Ioannidis, K.D. The Impact of Selected Climatic Factors on the Growth of Greek Fir on Mount Giona in Mainland Greece Based on Tree Ring Analysis. Austrian J. For. Sci. 2022, 1, 1–30. [Google Scholar]
- Koutavas, A. Late 20th Century Growth Acceleration in Greek Firs (Abies Cephalonica) from Cephalonia Island, Greece: A CO2 Fertilization Effect? Dendrochronologia 2008, 26, 13–19. [Google Scholar] [CrossRef]
- Markalas, S. Site and Stand Factors Related to Mortality Rate in a Fir Forest after a Combined Incidence of Drought and Insect Attack. For. Ecol. Manag. 1992, 47, 367–374. [Google Scholar] [CrossRef]
- Brofas, G.; Economidou, E. Le dépérissement du Sapin du Mont Parnasse. Lerôle des conditions climatiques et écologiques. Ecol. Mediterr. 1994, 20, 1–8. [Google Scholar] [CrossRef]
- Tsopelas, P.; Angelopoulos, A.; Economou, A.; Soulioti, N. Mistletoe (Viscum Album) in the Fir Forest of Mount Parnis, Greece. For. Ecol. Manag. 2004, 202, 59–65. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Raftoyannis, Y.; Pantera, A. Fir decline in Greece: A dendroclimatological approach. In Proceedings of the 10th International Conference on Environmental Science and Technology (CEST-2007), Kos, Greece, 5–7 September 2007; pp. 571–578. [Google Scholar]
- Papadopoulos, A.M. Investigations dendroclimatologiques du Sapin deCéphalonie en Grèce Centrale. Geogr. Tech. 2009, 2, 34–38. [Google Scholar]
- Koutavas, A. CO2 fertilization and enhanced drought resistance in Greek firsfrom Cephalonia Island, Greece. Glob. Change Biol. 2013, 19, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, F.; Im, E.S.; Coppola, E.; Diffenbaugh, N.S.; Gao, X.J.; Mariotti, L.; Shi, Y. Higher Hydroclimatic Intensity with Global Warming. J. Clim. 2011, 24, 5309–5324. [Google Scholar] [CrossRef]
- Baillie, M.G. Tree-Ring Dating and Archaeology; Croom Helm: Dundee, UK, 1982. [Google Scholar]
- Fritts, H.C. Tree-Rings and Climate; Academic Press: London, UK, 1976; 567p. [Google Scholar]
- Schweingruber, F.H. Tree Rings: Basics and Applications of Dendrochronology; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Speer, J.H. Fundamentals of Tree-Ring Research; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar]
- Stokes, M.A. An Introduction to Tree-Ring Dating; University of Arizona Press: Tucson, AZ, USA, 1996. [Google Scholar]
- Pearl, J.K.; Keck, J.R.; Tintor, W.; Siekacz, L.; Herrick, H.M.; Meko, M.D.; Pearson, C.L. New Frontiers in Tree-Ring Research. Holocene 2020, 30, 923–941. [Google Scholar] [CrossRef]
- Waring, R.H.; Running, S.W. Forest Ecosystems: Analysis at Multiple Scales; Academic Press: San Diego, CA, USA, 1998; 370p. [Google Scholar]
- Littell, J.S.; Peterson, D.L.; Tjoelker, M. Douglas-Fir Growth in Mountain Ecosystems: Water Limits Tree Growth from Stand to Region. Ecol. Monogr. 2008, 78, 349–368. [Google Scholar] [CrossRef]
- Bunn, A.G.; Waggoner, L.A.; Graumlich, L.J. Topographic Mediation of Growth in High Elevation Foxtail Pine (Pinus Balfouriana Grev. et Balf.) Forests in the Sierra Nevada, USA. Glob. Ecol. Biogeogr. 2005, 14, 103–114. [Google Scholar] [CrossRef]
- Holman, M.L.; Peterson, D.L. Spatial and Temporal Variability in Forest Growth in the Olympic Mountains, Washington: Sensitivity to Climatic Variability. Can. J. For. Res. 2006, 36, 92–104. [Google Scholar] [CrossRef]
- Petrakis, P.V.; Koulelis, P.P.; Fassouli, V.P.; Solomou, A.D. Preliminary Results of European Budworm Choristoneura Murinana (Hubner) Impact on Greek Fir Radial Growth at Mts Parnassus and Giona. Folia Oecologica 2022, 49, 102–109. [Google Scholar] [CrossRef]
- Climate Engine. Desert Research Institute and University of Idaho. 2023. Available online: http://climateengine.org (accessed on 20 November 2022).
- Huntington, J.L.; Hegewisch, K.C.; Daudert, B.; Morton, C.G.; Abatzoglou, J.T.; McEvoy, D.J.; Erickson, T. Climate Engine: Cloud Computing and Visualization of Climate and Remote Sensing Data for Advanced Natural Resource Monitoring and Process Understanding. Bull. Am. Meteorol. Soc. 2017, 98, 2397–2410. [Google Scholar] [CrossRef]
- Rahman, M.A.; Yunsheng, L.; Sultana, N. Analysis and Prediction of Rainfall Trends over Bangladesh Using Mann–Kendall, Spearman’s Rho Tests and ARIMA Model. Meteorol. Atmos. Phys. 2017, 129, 409–424. [Google Scholar] [CrossRef]
- Buffoni, L.; Maugeri, M.; Nanni, T. Precipitation in Italy from 1833 to 1996. Theor. Appl. Climatol. 1999, 63, 33–40. [Google Scholar] [CrossRef]
- Reiter, A.; Weidinger, R.; Mauser, W. Recent Climate Change at the Upper Danube-A Temporal and Spatial Analysis of Temperature and Precipitation Time Series. Clim. Change 2012, 111, 665–696. [Google Scholar] [CrossRef]
- Bickici Arikan, B.; Kahya, E. Homogeneity Revisited: Analysis of Updated Precipitation Series in Turkey. Theor. Appl. Climatol. 2019, 135, 211–220. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Z.X.; Huang, J.X. Spatio-Temporal Variation and Abrupt Changes for Major Climate Variables in the Taihu Basin, China. Stoch. Environ. Res. Risk Assess. 2012, 26, 777–791. [Google Scholar] [CrossRef]
- Mann, H.B. Non-Parametric Test against Trend. Econometrica 1945, 13, 245–259. [Google Scholar] [CrossRef]
- Kendall, M.G. Rank Correlation Methods, 4th ed.; Charles Griffin: London, UK, 1975. [Google Scholar]
- Hirsch, R.M.; Slack, J.R. A nonparametric trend test for seasonal data with serial dependence. Water Resour. Res. 1984, 20, 727–732. [Google Scholar] [CrossRef]
- Seppälä, R.; Buck, A.; Katila, P. Adaptation of Forests & People to Climate Change. A Global Assessment Report, Prepared by the Global Forest Expert Panel on Adaptation of Forests to Climate Change; IUFRO World Series; International Union of Forest Research Organizations (IUFRO): Vienna, Austria, 2009; Volume 22. [Google Scholar]
- Williams, A.P.; Allen, C.D.; Macalady, A.K.; Griffin, D.; Woodhouse, C.A.; Meko, D.M.; Swetnam, T.W.; Rauscher, S.A.; Seager, R.; Grissino-Mayer, H.D.; et al. Temperature as a Potent Driver of Regional Forest Drought Stress and Tree Mortality. Nat. Clim. Change 2013, 3, 292–297. [Google Scholar] [CrossRef]
- Jump, A.S.; Mátyás, C.; Peñuelas, J. The Altitude-for-Latitude Disparity in the Range Retractions of Woody Species. Trends Ecol. Evol. 2009, 24, 694–701. [Google Scholar] [CrossRef] [PubMed]
- King, G.M.; Gugerli, F.; Fonti, P.; Frank, D.C. Tree Growth Response along an Elevational Gradient: Climate or Genetics? Oecologia 2013, 173, 1587–1600. [Google Scholar] [CrossRef]
- Graumlich, L.J.; Brubaker, L.B. Reconstruction of Annual Temperature (1590-1979) for Longmire, Washington, Derived from Tree Rings. Quat. Res. 1986, 25, 223–234. [Google Scholar] [CrossRef]
- Peterson, D.W.; Peterson, D.L. Effects of Climate on Radial Growth of Subalpine Conifers in the North Cascade Mountains. Can. J. For. Res. 1994, 24, 1921–1932. [Google Scholar] [CrossRef]
- Peterson, D.W.; Peterson, D.L.; Ettl, G.J. Growth Responses of Subalpine Fir to Climatic Variability in the Pacific Northwest. Can. J. For. Res. 2002, 32, 1503–1517. [Google Scholar] [CrossRef]
- Nakawatase, J.M.; Peterson, D.L. Spatial Variability in Forest Growth—Climate Relationships in the Olympic Mountains, Washington. Can. J. For. Res. 2006, 36, 77–91. [Google Scholar] [CrossRef]
- Schickhoff, U.; Bobrowski, M.; Böhner, J.; Bürzle, B.; Chaudhary, R.P.; Gerlitz, L.; Heyken, H.; Lange, J.; Müller, M.; Scholten, T.; et al. Do Himalayan Treelines Respond to Recent Climate Change? An Evaluation of Sensitivity Indicators. Earth Syst. Dynamis 2015, 6, 245–265. [Google Scholar] [CrossRef]
- Köorner, C. Alpine Treelines: Functional Ecology of the Global High Elevation Tree Limits; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.G.; Alam, S.A.; Zhai, L.; Dawson, A.; Stadt, K.J.; Comeau, P.G. Drought Causes Reduced Growth of Trembling Aspen in Western Canada. Glob. Change Biol. 2017, 23, 2887–2902. [Google Scholar] [CrossRef]
- Zheng, L.; Gaire, N.P.; Shi, P. High-Altitude Tree Growth Responses to Climate Change across the Hindu Kush Himalaya. J. Plant Ecol. 2021, 14, 829–842. [Google Scholar] [CrossRef]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On Underestimation of Global Vulnerability to Tree Mortality and Forest Die-off from Hotter Drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D. Darcy’s Law Predicts Widespread Forest Mortality under Climate Warming. Nat. Clim. Change 2015, 5, 669–672. [Google Scholar] [CrossRef]
- Bogenschütz, H. Tortricinae. Die Eur. 1978, 3, 55–89. [Google Scholar]
- Pschorn-Walcher, H. Biological control of forest insects. Annu. Rev. Entomol. 1977, 22, 1–22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).