Large-Scale Effects of Aridity on Leaf Nitrogen and Phosphorus Concentrations of Terrestrial Plants
Abstract
1. Introduction
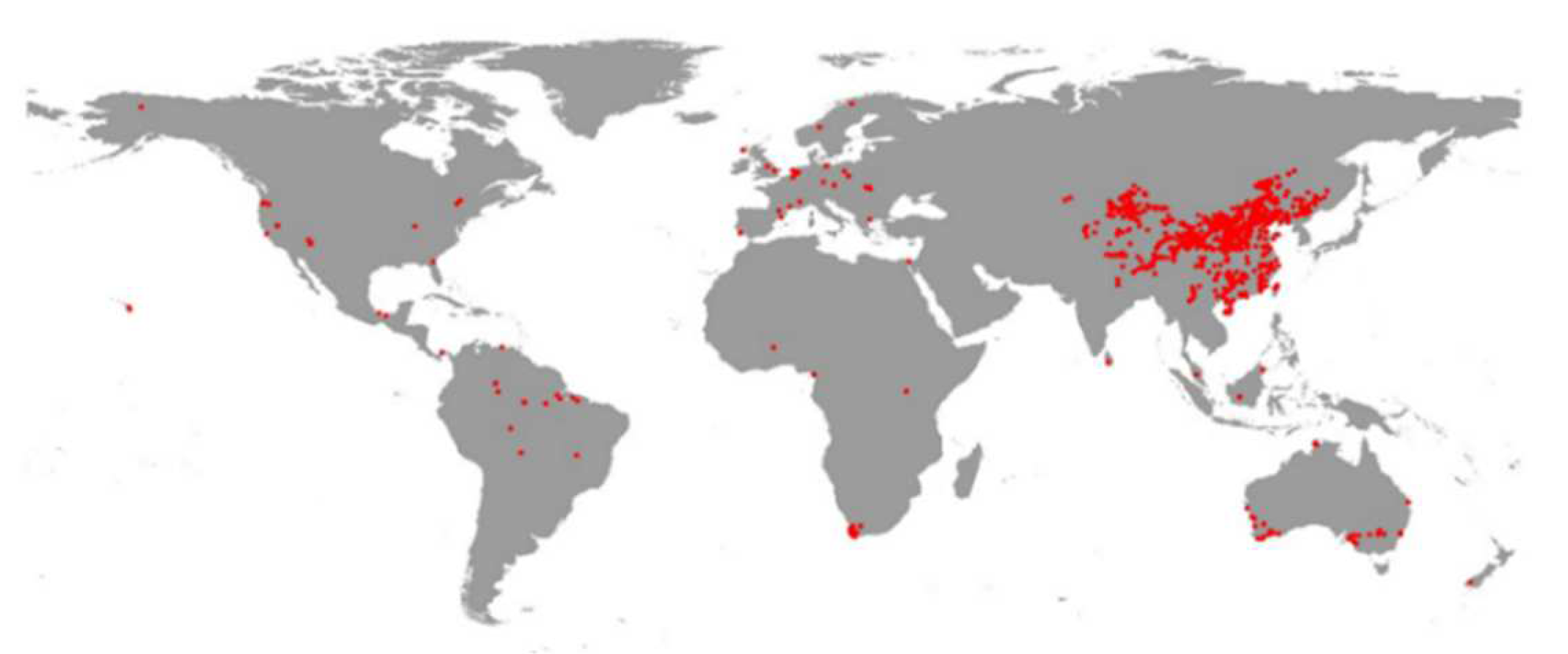
2. Materials and Methods
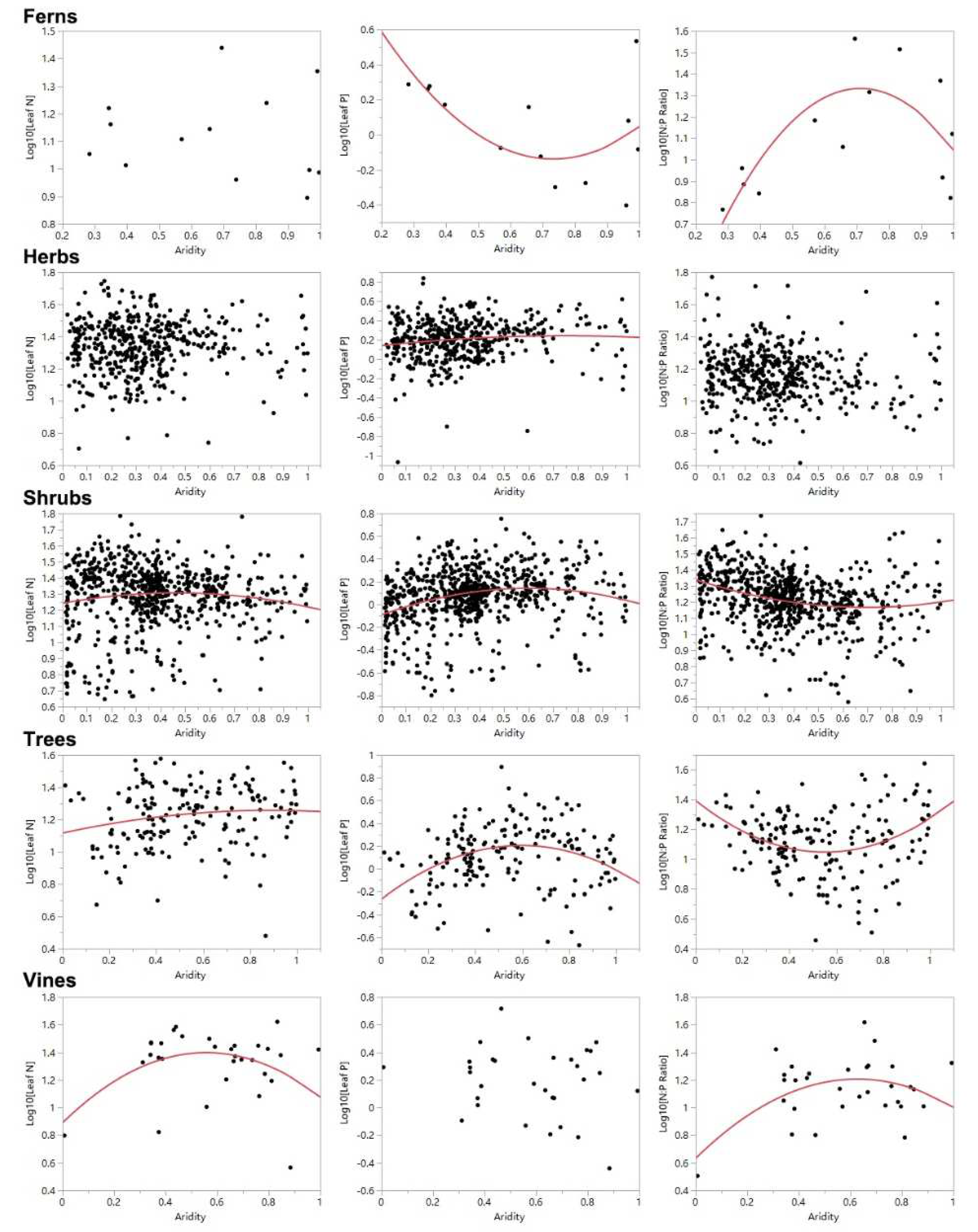
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niklas, K.J.; Owens, T.; Reich, P.B.; Cobb, E.D. Nitrogen/phosphorus leaf stoichiometry and the scaling of plant growth. Ecol. Lett. 2005, 8, 636–642. [Google Scholar] [CrossRef]
- Li, Y.; Niu, S.; Yu, G. Aggravated phosphorus limitation on biomass production under increasing nitrogen loading: A meta-analysis. Glob. Chang. Biol. 2015, 22, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Xu, W.; Zhou, G.; Bai, Y.; Li, J.; Tang, X.; Chen, D.; Liu, Q.; Ma, W.; Xiong, G.; et al. Patterns of plant carbon, nitrogen, and phosphorus concentration in relation to productivity in China’s terrestrial ecosystems. Proc. Natl. Acad. Sci. USA 2018, 115, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Kattge, J.; Chen, Y.; Han, W.; Luo, Y.; He, J.; Hu, H.; Tang, Z.; Ma, S.; Yan, Z.; et al. A global database of paired leaf nitrogen and phosphorus concentrations of terrestrial plants. Ecology 2019, 100, e02812. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; Van Der Velde, M.; Bopp, L.; Boucher, O.; Godderis, Y.; Hinsinger, P.; Llusia, J.; et al. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 4, 2934. [Google Scholar] [CrossRef]
- Bakker, M.A.; Carreño-Rocabado, G.; Poorter, L. Leaf economics traits predict litter decomposition of tropical plants and differ among land use types. Funct. Ecol. 2010, 25, 473–483. [Google Scholar] [CrossRef]
- Adams, M.A.; Turnbull, T.L.; Sprent, J.I.; Buchmann, N. Legumes are different: Leaf nitrogen, photosynthesis, and water use efficiency. Proc. Natl. Acad. Sci. USA 2016, 113, 4098–4103. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Q.; Chen, F.; Yuan, L.; Mi, G. Dynamic remobilization of leaf nitrogen components in relation to photosynthetic rate during grain filling in maize. Plant Physiol. Biochem. 2018, 129, 27–34. [Google Scholar] [CrossRef]
- Liu, C.; Muchhal, U.S.; Uthappa, M.; Kononowicz, A.K.; Raghothama, K.G. Tomato Phosphate Transporter Genes Are Differentially Regulated in Plant Tissues by Phosphorus. Plant Physiol. 1998, 116, 91–99. [Google Scholar] [CrossRef]
- Chen, Y.; Han, W.; Tang, L.; Tang, Z.; Fang, J. Leaf nitrogen and phosphorus concentrations of woody plants differ in responses to climate, soil and plant growth form. Ecography 2011, 36, 178–184. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The Impacts of Phosphorus Deficiency on the Photosynthetic Electron Transport Chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef]
- Gusewell, S.; Freeman, C. Nutrient limitation and enzyme activities during litter decomposition of nine wetland species in relation to litter N: P ratios. Funct. Ecol. 2005, 19, 582–593. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.; Ahlström, A.; Van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Tieleman, B.I.; Williams, J.B.; Bloomer, P. Adaptation of metabolism and evaporative water loss along an aridity gradient. Proc. R. Soc. B Boil. Sci. 2003, 270, 207–214. [Google Scholar] [CrossRef]
- Petrů, M.; Tielbörger, K.; Belkin, R.; Sternberg, M.; Jeltsch, F. Life history variation in an annual plant under two opposing environmental constraints along an aridity gradient. Ecography 2005, 29, 66–74. [Google Scholar] [CrossRef]
- Martínez-Cabrera, H.; Jones, C.S.; Espino, S.; Schenk, H.J. Wood anatomy and wood density in shrubs: Responses to varying aridity along transcontinental transects. Am. J. Bot. 2009, 96, 1388–1398. [Google Scholar] [CrossRef]
- Carlson, J.E.; Adams, C.A.; Holsinger, K. Intraspecific variation in stomatal traits, leaf traits and physiology reflects adaptation along aridity gradients in a South African shrub. Ann. Bot. 2015, 117, 195–207. [Google Scholar] [CrossRef]
- Bull-Hereñu, K.; Arroyo, M.T.K. Phenological and morphological differentiation in annual Chaetanthera moenchioides (Asteraceae) over an aridity gradient. Oesterreichische Bot. Z. 2009, 278, 159–167. [Google Scholar] [CrossRef]
- Quiroga, R.E.; Golluscio, R.A.; Blanco, L.J.; Fernández, R.J. Aridity and grazing as convergent selective forces: An experiment with an Arid Chaco bunchgrass. Ecol. Appl. 2010, 20, 1876–1889. [Google Scholar] [CrossRef]
- Armas, C.; Rodríguez-Echeverría, S.; Pugnaire, F.I. A field test of the stress-gradient hypothesis along an aridity gradient. J. Veg. Sci. 2011, 22, 818–827. [Google Scholar] [CrossRef]
- Quiroga, R.E.; Fernandez, R.; Golluscio, R.A.; Blanco, L.J. Differential water-use strategies and drought resistance in Trichloris crinita plants from contrasting aridity origins. Plant Ecol. 2013, 214, 1027–1035. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardo, A.; Quero, J.L.; Ochoa, V.; García-Gómez, M.; Escolar, C.; García-Palacios, P.; Berdugo, M.; Valencia, E.; et al. Aridity Modulates N Availability in Arid and Semiarid Mediterranean Grasslands. PLoS ONE 2013, 8, e59807. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Köbel, M.; Pinho, P.; Matos, P.; de Bello, F.; Correia, O.; Branquinho, C. Which plant traits respond to aridity? A critical step to assess functional diversity in Mediterranean drylands. Agric. For. Meteorol. 2017, 239, 176–184. [Google Scholar] [CrossRef]
- Gross, N.; Le Bagousse-Pinguet, Y.; Liancourt, P.; Berdugo, M.; Gotelli, N.J.; Maestre, F.T. Functional trait diversity maximizes ecosystem multifunctionality. Nat. Ecol. Evol. 2017, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A.; Zhang, K.; Li, X.; Tan, H.; Gao, Y.; Li, G. Leaf nitrogen and phosphorus of temperate desert plants in response to climate and soil nutrient availability. Sci. Rep. 2014, 4, 6932. [Google Scholar] [CrossRef]
- Blanke, J.H.; Lindeskog, M.; Lindström, J.; Lehsten, V. Effect of climate data on simulated carbon and nitrogen balances for Europe. J. Geophys. Res. Biogeosci. 2016, 121, 1352–1371. [Google Scholar] [CrossRef]
- Fan, J.; Harris, W.; Zhong, H. Stoichiometry of leaf nitrogen and phosphorus of grasslands of the Inner Mongolian and Qinghai-Tibet Plateaus in relation to climatic variables and vegetation organization levels. Ecol. Res. 2016, 31, 821–829. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef]
- Zomer, R.J.; Xu, J.; Trabucco, A. Version 3 of the Global Aridity Index and Potential Evapotranspiration Database. Sci. Data 2022, 9, 409. [Google Scholar] [CrossRef]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef]
- Berdugo, M.; Delgado-Baquerizo, M.; Soliveres, S.; Hernández-Clemente, R.; Zhao, Y.; Gaitán, J.J.; Gross, N.; Saiz, H.; Maire, V.; Lehmann, A.; et al. Global ecosystem thresholds driven by aridity. Science 2020, 367, 787–790. [Google Scholar] [CrossRef]
- Soussana, J.-F.; Lemaire, G. Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agric. Ecosyst. Environ. 2014, 190, 9–17. [Google Scholar] [CrossRef]
- Tully, K.; Ryals, R. Nutrient cycling in agroecosystems: Balancing food and environmental objectives. Agroecol. Sustain. Food Syst. 2017, 41, 761–798. [Google Scholar] [CrossRef]
- He, J.-S.; Wang, L.; Flynn, D.; Wang, X.; Ma, W.; Fang, J. Leaf nitrogen:phosphorus stoichiometry across Chinese grassland biomes. Oecologia 2007, 155, 301–310. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Wright, I.J. Leaf phosphorus influences the photosynthesis–nitrogen relation: A cross-biome analysis of 314 species. Oecologia 2009, 160, 207–212. [Google Scholar] [CrossRef]
- Gago, J.; Coopman, R.E.; Cabrera, H.M.; Hermida-Carrera, C.; Molins, A.; Conesa, M.; Galmés, J.; Ribas-Carbó, M.; Flexas, J. Photosynthesis limitations in three fern species. Physiol. Plant. 2013, 149, 599–611. [Google Scholar] [CrossRef]
- Tosens, T.; Nishida, K.; Gago, J.; Coopman, R.E.; Cabrera, H.M.; Carriquí, M.; Laanisto, L.; Morales, L.; Nadal, M.; Rojas, R.; et al. The photosynthetic capacity in 35 ferns and fern allies: Mesophyll CO2 diffusion as a key trait. New Phytol. 2016, 209, 1576–1590. [Google Scholar] [CrossRef]
- Sihvonen, M.; Jussi, M.; Elena, L.; Kari Hyytiäinen, V. Management of legacy nutrient stores through nitrogen and phosphorus fertilization, catch crops, and gypsum treatment. Nat. Resour. Model. 2020, 33, e12289. [Google Scholar] [CrossRef]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Menge, D.; Lichstein, J.W.; Ángeles-Pérez, G. Global climate change will increase the abundance of symbiotic nitrogen-fixing trees in much of North America. Glob. Chang. Biol. 2017, 23, 4777–4787. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, I.; Zak, D.R.; Burton, A.J.; Pregitzer, K.S. Anthropogenic nitrogen deposition ameliorates the decline in tree growth caused by a drier climate. Ecology 2018, 99, 411–420. [Google Scholar] [CrossRef]
- Reich, P.B.; Sendall, K.M.; Stefanski, A.; Rich, R.L.; Hobbie, S.E.; Montgomery, R.A. Effects of climate warming on photosynthesis in boreal tree species depend on soil moisture. Nature 2018, 562, 263–267. [Google Scholar] [CrossRef]
- Wang, C.J.; Zhang, Z.X.; Wan, J.Z. Relationship between gross primary productivity and plant species richness at geographical scales: Evidence from protected area data in China. Environ. Earth Sci. 2021, 80, 189. [Google Scholar] [CrossRef]
| Life Form | Leaf N | Leaf P | N:P Ratio | |||
|---|---|---|---|---|---|---|
| R2 | p-Value | R2 | p-Value | R2 | p-Value | |
| Fern | 0.062 | 0.725 | 0.371 | 0.099 | 0.562 | 0.016 |
| Herb | 0.004 | 0.427 | 0.016 | 0.026 | 0.008 | 0.140 |
| Shrub | 0.010 | 0.032 | 0.071 | 0.000 | 0.082 | 0.000 |
| Tree | 0.030 | 0.065 | 0.110 | 0.000 | 0.117 | 0.000 |
| Vine | 0.173 | 0.071 | 0.031 | 0.648 | 0.218 | 0.032 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, D.-J.; Wang, C.-J.; Wan, J.-Z. Large-Scale Effects of Aridity on Leaf Nitrogen and Phosphorus Concentrations of Terrestrial Plants. Climate 2022, 10, 171. https://doi.org/10.3390/cli10110171
Xie D-J, Wang C-J, Wan J-Z. Large-Scale Effects of Aridity on Leaf Nitrogen and Phosphorus Concentrations of Terrestrial Plants. Climate. 2022; 10(11):171. https://doi.org/10.3390/cli10110171
Chicago/Turabian StyleXie, De-Juan, Chun-Jing Wang, and Ji-Zhong Wan. 2022. "Large-Scale Effects of Aridity on Leaf Nitrogen and Phosphorus Concentrations of Terrestrial Plants" Climate 10, no. 11: 171. https://doi.org/10.3390/cli10110171
APA StyleXie, D.-J., Wang, C.-J., & Wan, J.-Z. (2022). Large-Scale Effects of Aridity on Leaf Nitrogen and Phosphorus Concentrations of Terrestrial Plants. Climate, 10(11), 171. https://doi.org/10.3390/cli10110171






