Are Vaccinations Alone Enough to Curb the Dynamics of the COVID-19 Pandemic in the European Union?
Abstract
:1. Introduction
2. Model
- RQ1—Is the pandemic dynamics homogeneous , for all ) or heterogeneous (, for at least some ?
- RQ2—Is the speed of convergence to the long-run equilibrium relationship the same in the whole EU ( for all ) or it is country specific ( for at least some )?
- RQ3—Whether the same applies to the long-run marginal death effects with respect to the number of new infections and vaccinations (equal marginal effects: , for all ; country specific marginal effects: , for at least some )?
- RQ4—What the intensity of vaccination shall be () to prevent the number of fatal cases from being large () in the case the number of infections reaches a level (?
3. Results
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | The middle- and lower-income countries have a limited access to vaccine (da Fonseca et al. 2021; OECD 2021). |
| 2 | Fiscon et al. (2021) demonstrated that increasing the complexity of the models in order to find how the infection dynamics depended on specific factors was useless if not supported by a high-quality data used to calibrate them. |
| 3 | Post et al. (2021) used the panel approach to modelling the surveillance of the Second Wave of COVID-19 in Europe but as-summed that variables in question were stationary. |
| 4 | Sweden was excluded from the analysis due to a different from the remaining EU countries attitude to combating the COVID-19 pandemic. In the case of the Netherlands the exclusion resulted from missing data points in the number of daily new vac-cinations. |
| 5 | Please note that the averaged country specific effect in the PMG model is found insignificant at 5% level. |
| 6 | The results of that and the next procedures are available on request. |
References
- Bhouri, Mohamed A., Francisco S. Costabal, Hanwen Wang, Kevin Linka, Mathias Peirlinck, Ellen Kuhl, and Paris Perdikaris. 2021. COVID-19 dynamics across the US: A deep learning study of human mobility and social behavior. Computer Methods in Applied Mechanics and Engineering 382: 113891. [Google Scholar] [CrossRef]
- Blackburne, Edward F., III, and Mark W. Frank. 2007. Estimation of nonstationary heterogeneous panels. Stata Journal 7: 197–208. [Google Scholar] [CrossRef]
- Burke, Paul F., Daniel Masters, and Graham Massey. 2021. Enablers and barriers to COVID-19 vaccine uptake: An international study of perceptions and intentions. Vaccine 39: 5116–28. [Google Scholar] [CrossRef] [PubMed]
- Cot, Correntin, Giacomo Cacciapaglia, Anna Sigridur Islind, Maria Óskarsdóttir, and Francesco Sannino. 2021. Impact of US vaccination strategy on COVID-19 wave dynamics. Scientific Reports 11: 10960. [Google Scholar] [CrossRef]
- da Fonseca, Elize Massard, Kenneth C. Shadlen, and Francisco I. Bastos. 2021. The politics of COVID-19 vaccination in middle-income countries: Lessons from Brazil. Social Science & Medicine 281: 114093. [Google Scholar] [CrossRef]
- Demetrescu, Matei, and Christoph Hanck. 2012. A simple nonstationary-volatility robust panel unit root test. Economics Letters 117: 10–13. [Google Scholar] [CrossRef]
- Fiscon, Giulia, Francesco Salvadore, Valerio Guarrasi, Anna Rosa Garbuglia, and Paola Paci. 2021. Assessing the impact of data-driven limitations on tracing and forecasting the outbreak dynamics of COVID-19. Computers in Biology and Medicine 135: 104657. [Google Scholar] [CrossRef]
- Gumel, Abba B., Enahoro A. Iboi, Calistus N. Ngonghala, and Elamin H. Elbasha. 2021. A primer on using mathematics to understand COVID-19 dynamics: Modeling, analysis and simulations. Infectious Disease Modelling 6: 148–68. [Google Scholar] [CrossRef]
- Hale, Thomas, Jessica Anania, Noam Angrist, Thomas Boby, Emily Cameron-Blake, Martina Di Folco, Lucy Ellen, Rafael Goldszmidt, Laura Hallas, Beatriz Kira, and et al. 2021. Variation in government responses to COVID-19. Version 12.0. Blavatnik School of Government Working Paper. June 11. Available online: https://www.bsg.ox.ac.uk/sites/default/files/2021-06/BSG-WP-2020-032-v12_0.pdf (accessed on 20 July 2021).
- Herwartz, Helmut, and Florian Siedenburg. 2008. Homogenous panel unit root tests under cross sectional dependence: Finite sample modifications and the wild bootstrap. Computational Statistics and Data Analysis 53: 137–50. [Google Scholar] [CrossRef]
- Herwartz, Helmut, Simone Maxand, Fabian H. C. Raters, and Yabibal M. Walle. 2018. Panel unit-root tests for heteroskedastic panels. Stata Journal 18: 184–96. [Google Scholar] [CrossRef]
- Herwartz, Helmut, Simone Maxand, and Yabibal M. Walle. 2019. Heteroskedasticity-robust unit root testing for trending panels. Journal of Time Series Analysis 40: 649–64. [Google Scholar] [CrossRef]
- Hyland, Philip, Frédérique Vallières, Mark Shevlin, Richard P. Bentall, Ryan McKay, Todd K. Hartman, Orla McBride, and Jamie Murphy. 2021. Resistance to COVID-19 vaccination has increased in Ireland and the United Kingdom during the pandemic. Public Health 195: 54–56. [Google Scholar] [CrossRef] [PubMed]
- Kessels, Roselinde, Jeroen Luyten, and Sandy Tubeuf. 2021. Willingness to get vaccinated against COVID-19 and attitudes toward vaccination in general. Vaccine 39: 4716–22. [Google Scholar] [CrossRef]
- Liu, Yang, Julian W. Tang, and Tommy T. Y. Lam. 2021. Transmission dynamics of the COVID-19 epidemic in England. International Journal of Infectious Diseases 104: 132–38. [Google Scholar] [CrossRef] [PubMed]
- Mondal, Pritish, Ankita Sinharoy, and Lilly Su. 2021. Sociodemographic predictors of COVID-19 vaccine acceptance: A nationwide US-based survey study. Public Health 198: 252–59. [Google Scholar] [CrossRef]
- Moore, Sam, Edward M. Hill, Michael J. Tildesley, Louise Dyson, and Matt J. Keeling. 2021. Vaccination and nonpharmaceutical interventions for COVID-19: A mathematical modelling study. Lancet Infectious Diseases 21: 793–802. [Google Scholar] [CrossRef]
- Musa, Salihu Sabiu, Sania Qureshi, Shi Zhao, Abdullahi Yusuf, Umar Tasiu Mustapha, and Daihai He. 2021. Mathematical modeling of COVID-19 epidemic with effect of awareness programs. Infectious Disease Modelling 6: 448–60. [Google Scholar] [CrossRef]
- OECD. 2021. Access to COVID-19 Vaccines: Global Approaches in a Global Crisis. March 18. Available online: https://www.oecd.org/coronavirus/policy-responses/access-to-covid-19-vaccines-global-approaches-in-a-global-crisis-c6a18370/ (accessed on 20 July 2021).
- Persyn, Damiaan, and Joakim Westerlund. 2008. Error-correction–based cointegration tests for panel data. Stata Journal 8: 232–41. [Google Scholar] [CrossRef] [Green Version]
- Pesaran, M. Hashem, Yongcheol Shin, and Ron P. Smith. 1999. Pooled Mean Group Estimation of Dynamic Heterogeneous Panels. Journal of the American Statistical Association 94: 621–34. [Google Scholar] [CrossRef]
- Post, Lori, Kasen Culler, Charles B. Moss, Robert L. Murphy, Chad J. Achenbach, Michael G. Ison, Danielle Resnick, Lauren Nadya Singh, Janine White, Michael J. Boctor, and et al. 2021. Surveillance of the Second Wave of COVID-19 in Europe: Longitudinal Trend Analyses. JMIR Public Health and Surveillance 7: e25695. [Google Scholar] [CrossRef]
- Rossman, Hagai, Smadar Shilo, Tomer Meir, Malka Gorfine, Uri Shalit, and Eran Segal. 2021. COVID-19 dynamics after a national immunization program in Israel. Nature Medicine 27: 1055–61. [Google Scholar] [CrossRef] [PubMed]
- Saad-Roy, Chadi M., Caroline E. Wagner, Rachel E. Baker, Sinead E. Morris, Jeremy Farrar, Andrea L. Graham, Simon A. Levin, Michael J. Mina, C. Jessica E. Metcalf, and Bryan T. Grenfell. 2020. Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science 370: 811–18. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, Katrin, and Samuel Bowles. 2021. Overcoming COVID-19 vaccination resistance when alternative policies affect the dynamics of conformism, social norms, and crowding out. Proceedings of the National Academy of Sciences of the United States of America 118: e2104912118. [Google Scholar] [CrossRef] [PubMed]
- Shayak, B., Mohit M. Sharma, Manas Gaur, and Anand Kumar Mishra. 2021. Impact of reproduction number on the multiwave spreading dynamics of COVID-19 with temporary immunity: A mathematical model. International Journal of Infectious Diseases 104: 649–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, Chao, Bingfeng Han, Tianshuo Zhao, Hanyu Liu, Bei Liu, Linyi Chen, Mingzhu Xie, Jiang Liu, Hui Zheng, Sihui Zhang, and et al. 2021. Vaccination willingness, vaccine hesitancy, and estimated coverage at the first round of COVID-19 vaccination in China: A national crosssectional study. Vaccine 39: 2833–42. [Google Scholar] [CrossRef]
- Westerlund, Joakim. 2007. Testing for error correction in panel data. Oxford Bulletin of Economics and Statistics 69: 709–48. [Google Scholar] [CrossRef] [Green Version]
- Worldometer. 2021. COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 20 October 2021).
- Xu, Zhe, Bo Wu, and Ufuk Topcu. 2021. Control strategies for COVID-19 epidemic with vaccination, shield immunity and quarantine: A metric temporal logic approach. PLoS ONE 16: e0247660. [Google Scholar] [CrossRef]
- Zhong, Ling. 2021. A dynamic pandemic model evaluating reopening strategies amid COVID-19. PLoS ONE 16: e0248302. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0248302 (accessed on 20 October 2021). [CrossRef]
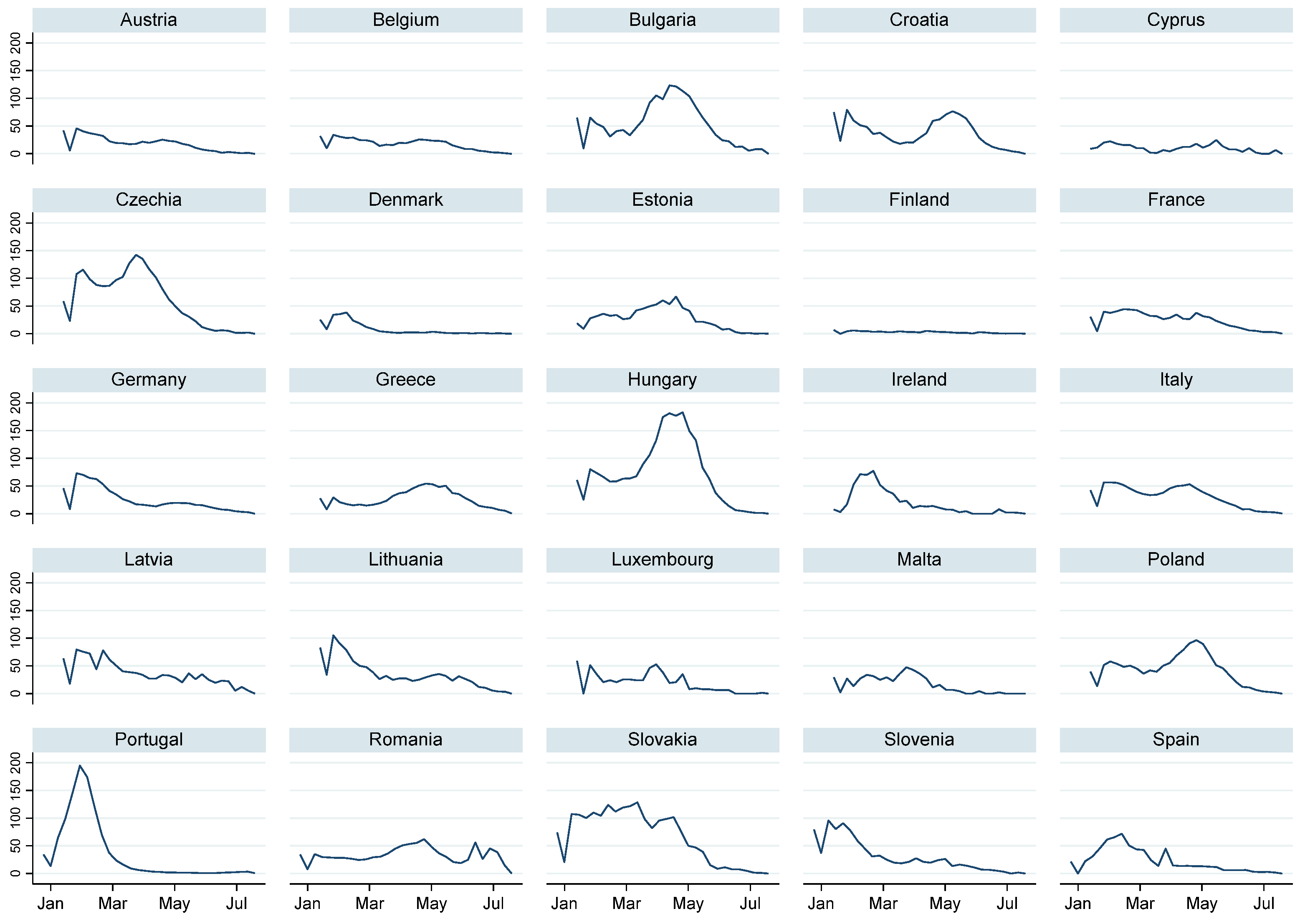
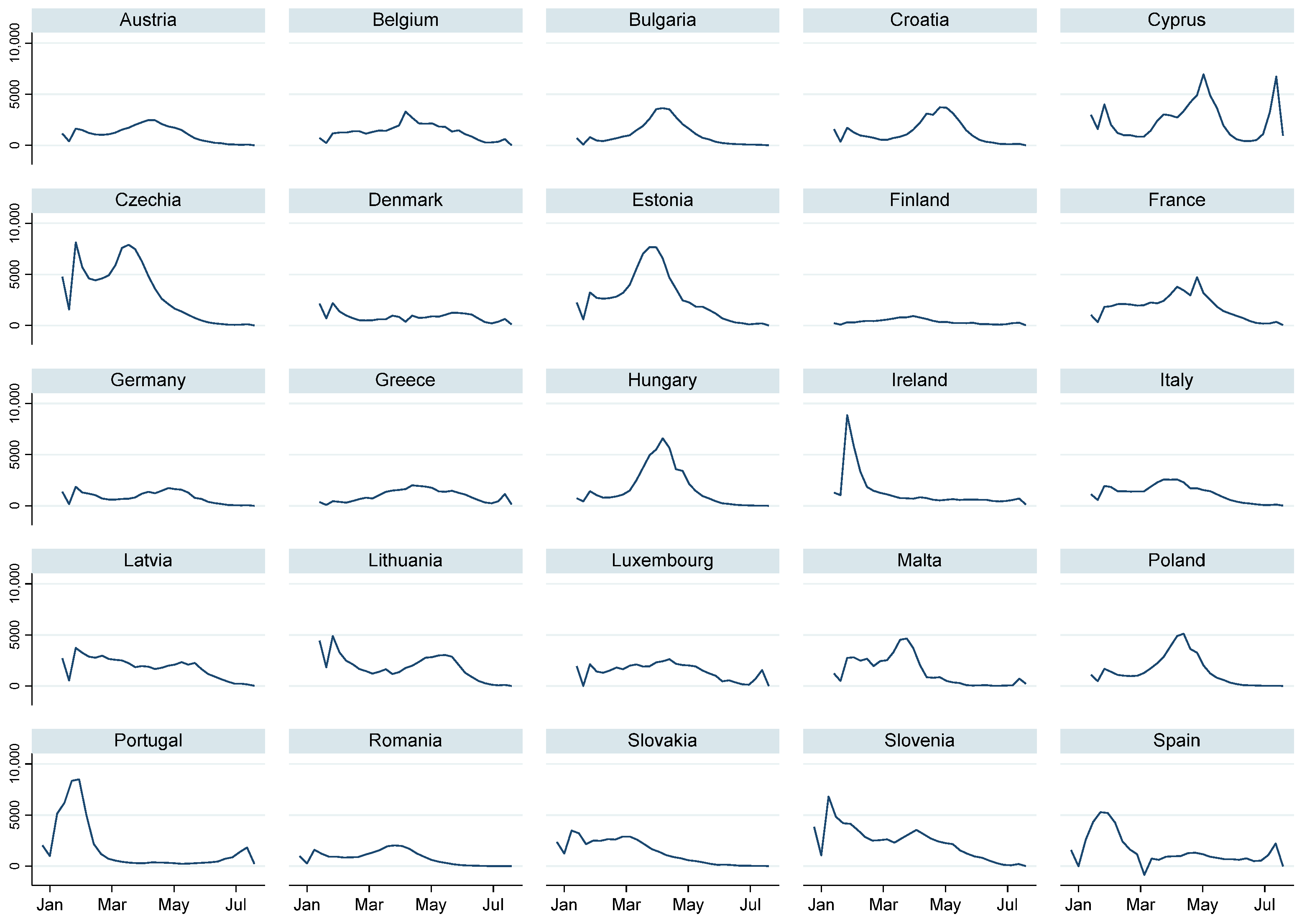
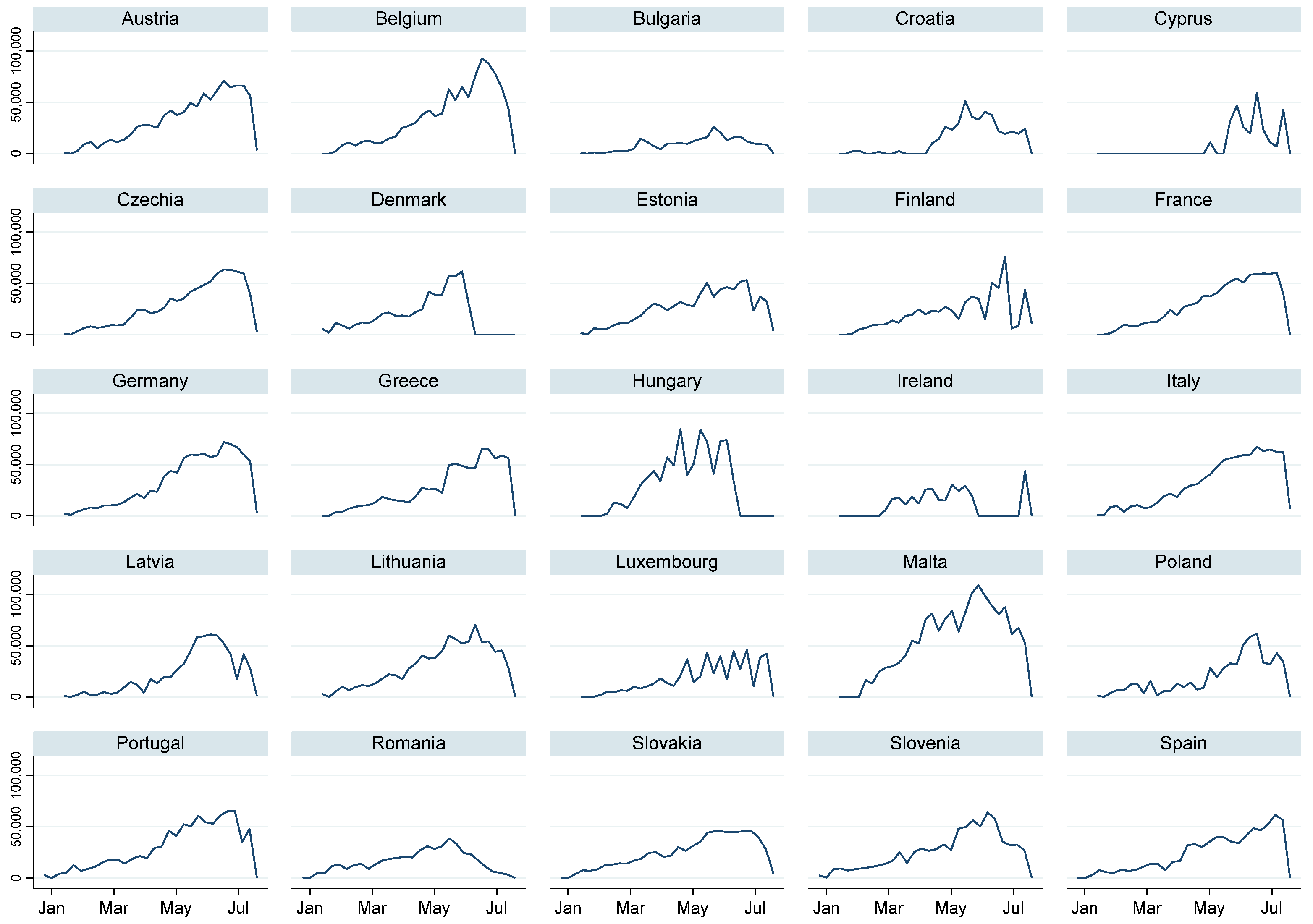
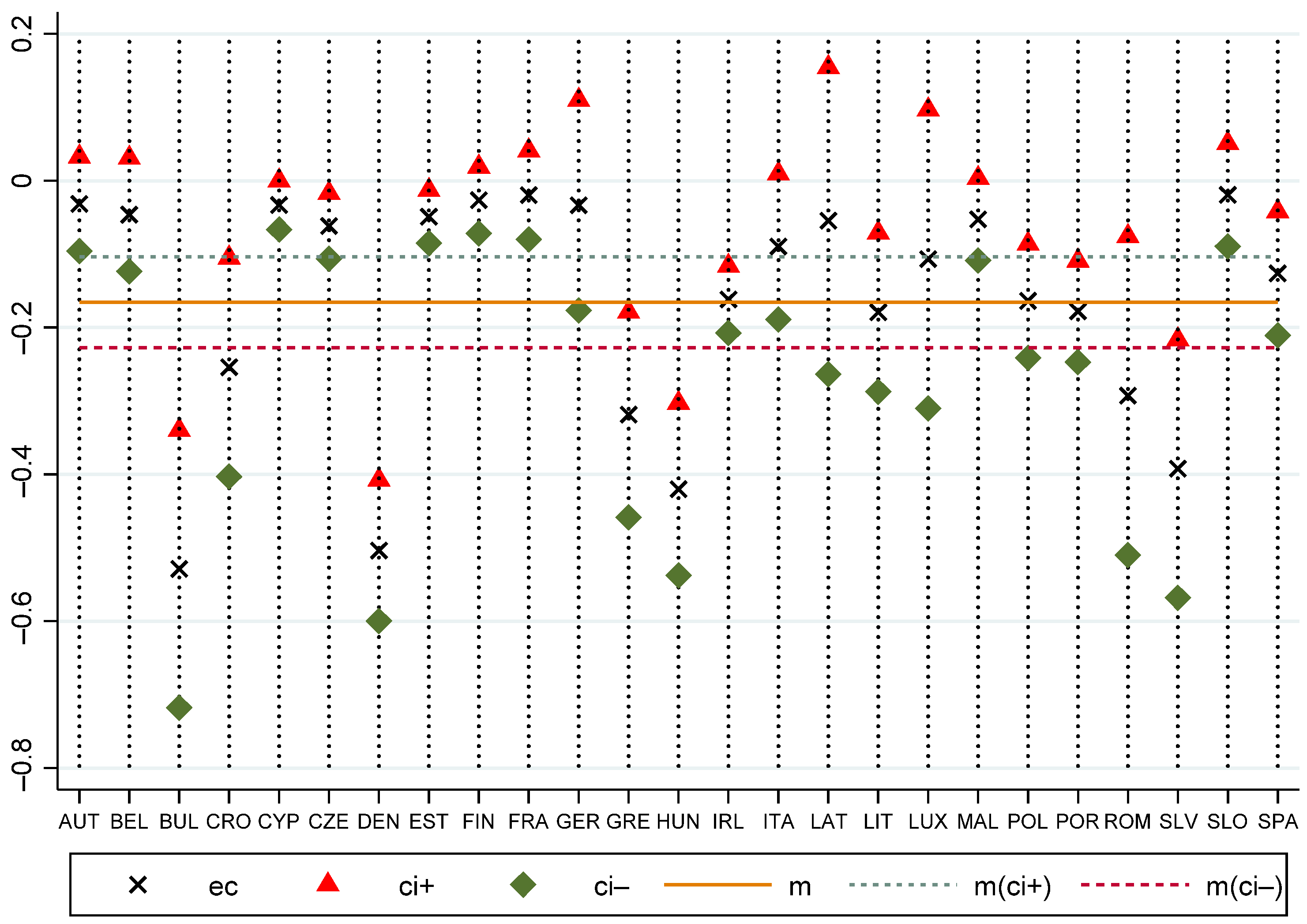
| Variable | Test | |||||
|---|---|---|---|---|---|---|
| HS | DH | HMW | ||||
| 2.2015 | 0.9862 | 1.8514 | 0.9679 | 4.9722 | 1.0000 | |
| −0.4447 | 0.3283 | 0.7841 | 0.7835 | 0.9002 | 0.8160 | |
| −1.2598 | 0.1039 | −0.4875 | 0.3129 | 1.8952 | 0.9710 | |
| −2.8223 | 0.0024 | −2.4767 | 0.0066 | −0.4312 | 0.3332 | |
| −1.8154 | 0.0347 | −2.1283 | 0.0167 | −1.5431 | 0.0614 | |
| −1.7507 | 0.0400 | −2.2255 | 0.0130 | −1.0741 | 0.1414 |
| Variable | Group Mean Tests | Panel Tests | ||||||
|---|---|---|---|---|---|---|---|---|
| a | a | a | a | |||||
| No constant in the cointegration relationship | ||||||||
| −3.097 | 0.000 | −10.699 | 0.000 | −14.915 | 0.000 | −9.259 | 0.000 | |
| With constant in the cointegration relationship | ||||||||
| −2.514 | 0.000 | −9.474 | 0.040 | −12.198 | 0.000 | −9.168 | 0.010 | |
| AIC | AIC | AIC | ||||||
|---|---|---|---|---|---|---|---|---|
| 1, 0, 0 | 5132.936 | 100 | 1, 2, 0 | 4686.905 | 150 | 2, 1, 0 | 4708.890 | 150 |
| 1, 0, 1 | 5142.862 | 125 | 1, 2, 1 | 4647.197 | 175 | 2, 1, 1 | 4683.162 | 175 |
| 1, 1, 0 | 5009.580 | 125 | 1, 2, 2 | 4641.517 | 200 | 2, 1, 2 | 4696.016 | 200 |
| 1, 1, 1 | 5020.774 | 150 | 2, 0, 0 | 4773.425 | 125 | 2, 2, 1 | 4649.488 | 200 |
| 1, 1, 2 | 4741.771 | 175 | 2, 0, 1 | 4749.394 | 150 | 2, 2, 2 | 4644.836 | 225 |
| Regressor | Estimator | ||
|---|---|---|---|
| MG | PMG | DFE | |
| Long-run | |||
| 0.0198 *** | 0.0371 *** | 0.0228 *** | |
| (4.78) | (23.48) | (6.19) | |
| −0.000368 ** | −0.000518 *** | −0.000266 * | |
| (−3.81) | (−10.58) | (−2.16) | |
| Short-run | |||
| −0.4914 *** | −0.1657 *** | −0.275 *** | |
| (−8.72) | (−5.24) | (−7.49) | |
| 0.002 | 0.00603 *** | 0.00179 | |
| (1.00) | (3.58) | (0.95) | |
| −0.00572 *** | −0.00462 *** | −0.00205 * | |
| (−5.14) | (−3.81) | (−2.32) | |
| 0.000133 * | 0.0000202 | 0.0000227 | |
| (2.10) | (0.63) | (0.61) | |
| 0.0000526 | −0.0000182 | 0.0000135 | |
| (1.11) | (−0.38) | (0.47) | |
| const. | 5.024 * | −0.165 | 0.426 |
| (1.98) | (−0.20) | (0.19) | |
| Infections | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 5 | 10 | 20 | 30 | 40 | 50 | 100 | 150 | 200 | 250 | |
| 20 | ||||||||||||
| 50 | 2 | |||||||||||
| 100 | 5 | 3 | ||||||||||
| 200 | 12 | 10 | 5 | |||||||||
| 500 | 36 | 32 | 26 | 17 | 3 | |||||||
| 1000 | 72 | 68 | 62 | 52 | 33 | 14 | ||||||
| 2000 | 143 | 139 | 134 | 124 | 105 | 85 | 66 | 47 | ||||
| 5000 | 358 | 354 | 348 | 339 | 319 | 300 | 281 | 262 | 165 | 69 | ||
| 10,000 | 716 | 712 | 707 | 697 | 678 | 658 | 639 | 620 | 523 | 427 | 330 | 234 |
| 20,000 | 1432 | 1429 | 1423 | 1413 | 1394 | 1374 | 1355 | 1336 | 1239 | 1143 | 1046 | 950 |
| 50,000 | 3581 | 3577 | 3571 | 3562 | 3542 | 3523 | 3504 | 3484 | 3388 | 3291 | 3195 | 3098 |
| Country | Estimate | Correction | |
|---|---|---|---|
| Bulgaria | 8.2068 | 0.5288 | 29,961 |
| Cyprus | −2.7628 | 0.0332 | −160,650 |
| Denmark | −7.0520 | 0.5036 | −27,033 |
| Estonia | −3.2903 | 0.0490 | −129,631 |
| Hungary | 9.3993 | 0.4203 | 43,172 |
| Ireland | −3.9769 | 0.1618 | −47,450 |
| Lithuania | −3.1295 | 0.1792 | −33,714 |
| Portugal | −2.5767 | 0.1781 | −27,930 |
| Slovakia | 11.5474 | 0.3922 | 56,839 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miłobędzki, P. Are Vaccinations Alone Enough to Curb the Dynamics of the COVID-19 Pandemic in the European Union? Econometrics 2022, 10, 25. https://doi.org/10.3390/econometrics10020025
Miłobędzki P. Are Vaccinations Alone Enough to Curb the Dynamics of the COVID-19 Pandemic in the European Union? Econometrics. 2022; 10(2):25. https://doi.org/10.3390/econometrics10020025
Chicago/Turabian StyleMiłobędzki, Paweł. 2022. "Are Vaccinations Alone Enough to Curb the Dynamics of the COVID-19 Pandemic in the European Union?" Econometrics 10, no. 2: 25. https://doi.org/10.3390/econometrics10020025
APA StyleMiłobędzki, P. (2022). Are Vaccinations Alone Enough to Curb the Dynamics of the COVID-19 Pandemic in the European Union? Econometrics, 10(2), 25. https://doi.org/10.3390/econometrics10020025






