Abstract
Metal oxide semiconductor (MOX) sensors are emerging as a groundbreaking technology due to their remarkable features: high sensitivity, rapid response time, low cost, and potential for miniaturization. Their ability to detect volatile organic compounds (VOCs) in real time makes them ideal tools for applications across various fields, including environmental monitoring, medicine, and the food industry. This paper explores the evolution and growing utilization of MOX sensors, with a particular focus on atmospheric pollution monitoring, non-invasive disease diagnostics through the analysis of volatile compounds emitted by the human body, and food quality assessment. The crucial role of MOX sensors in monitoring the freshness of food and water, detecting chemical and biological contamination, and identifying food fraud is specifically examined. The rapid advancement of this technology offers new opportunities to improve quality of life, food safety, and public health, positioning MOX sensors as a key tool to address future challenges in these vital sectors.
1. Introduction
In recent decades, technological innovation has revolutionized various sectors, ranging from environmental monitoring to medical diagnostics and food safety [1]. While traditional methodologies remain a reference point, sensor-based technologies are gaining increasing relevance, offering faster, more efficient solutions that can be easily integrated into modern monitoring systems.
Among these emerging technologies, metal oxide (MOX) sensors stand out for their ability to detect gases and volatile organic compounds (VOCs) in real time. Their operation is based on variations in electrical conductivity in response to redox reactions occurring on the sensor surface: the presence of specific analytes in the air induces changes in the electronic properties of the metal oxide, leading to measurable variations in electrical resistance [2]. To enhance their sensitivity and reliability, it is crucial to optimize their chemical composition—such as through doping—and to control their operating temperature. Based on their conduction type, these semiconductors can be classified into two main categories: n-type, where electrical conduction is governed by free electrons, and p-type, where charge transport depends on holes, i.e., the absence of electrons. The choice between these two types depends on the specific application and environmental conditions [3,4].
Due to their characteristics, MOX sensors are employed in a wide range of applications [5]. Their compatibility with silicon-based technology facilitates seamless integration into IoT devices, wearable systems, and mobile platforms. Furthermore, large-scale production is enabled by well-established industrial techniques, such as sputtering and evaporation–condensation methods, ensuring an efficient and sustainable manufacturing process [2].
In environmental monitoring, MOX sensors are emerging as innovative tools for detecting atmospheric pollutants and hazardous substances, significantly contributing to public health protection [6]. Currently, the analysis of hazardous gases such as nitrogen oxides (NOx), carbon monoxide (CO), and toxic VOCs relies on laboratory methodologies, which are often slow and impractical for widespread monitoring [7]. However, thanks to advances in MOX technology, real-time air quality control is becoming a tangible reality, with compact devices that can be seamlessly integrated into urban networks [8,9].
In the medical field, MOX sensors are opening new perspectives for non-invasive diagnostics based on exhaled breath analysis [10]. Volatile biomarkers, such as acetone for diabetes and ammonia for kidney dysfunction, can provide valuable insights into a patient’s health status [11]. The ability of these sensors to detect such compounds enables rapid and non-invasive screening, offering new possibilities in telemedicine: wearable devices equipped with MOX sensors could continuously monitor patients’ breath, promptly detecting potential anomalies. Although they do not yet fully replace laboratory analyses, their use as a preliminary screening tool is gaining increasing interest within the scientific community [12].
In food safety, MOX sensors are also emerging as complementary tools to laboratory analyses for quality control [13]. They are particularly useful for assessing product freshness, detecting chemical or biological contamination, and verifying the authenticity of high-value food products [14]. With ongoing research advancements, the integration of these sensors into quality control processes is becoming increasingly feasible, enhancing safety and transparency in the agri-food sector [13] (Figure 1).
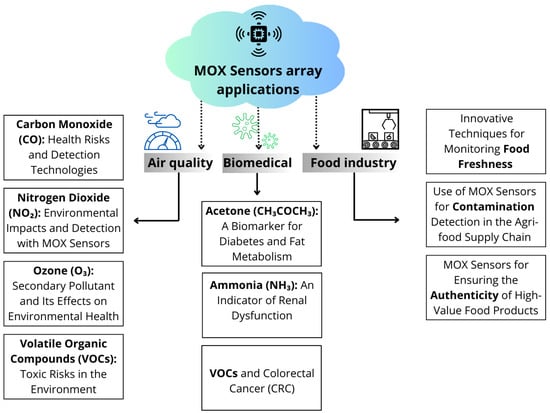
Figure 1.
Representation of a MOX sensor and its applications in three key sectors: air quality, biomedicine, and food industry.
Thanks to their versatility, MOX sensors are transforming environmental, healthcare, and industrial monitoring, providing real-time data, and improving resource management and public health protection. In this review, we will analyze the latest developments in MOX technology, examining its applications in air quality control, biomedical diagnostics, and food safety.
2. Advancements in Air Quality Monitoring Using MOX Sensors
MOX sensors represent an emerging technology in environmental monitoring, offering innovative solutions for real-time detection of atmospheric pollutants [6]. Recent studies highlight the potential of these sensors in detecting hazardous gases, such as carbon monoxide (CO), nitrogen dioxide (NO2), and ozone (O3), presenting a viable alternative to traditional methods [1,15,16,17].
Despite their versatility, metal oxide (MOX) sensors exhibit several limitations, including low chemical selectivity, signal drift over time, and susceptibility to environmental conditions such as temperature and humidity. To ensure measurement reliability, MOX sensors are commonly used in conjunction with well-established analytical techniques, such as gas chromatography coupled with mass spectrometry (GC-MS), which is widely regarded as the reference method for the identification and quantification of volatile organic compounds (VOCs). Dynamic olfactometry (UNI EN 13725:2004) is also frequently employed for the sensory assessment of odor intensity, particularly in industrial contexts [7]. In numerous studies, data obtained from GC-MS and olfactometry have been integrated with MOX sensor outputs to enhance calibration and improve the characterization of odor profiles. Emerging techniques such as UV-vis and FTIR spectroscopy provide additional tools to support sensor validation. The integration of these technologies with advanced data analysis algorithms is contributing to the development of increasingly accurate and intelligent environmental monitoring systems [8].
2.1. Carbon Monoxide (CO): Health Risks and Detection Technologies
Carbon monoxide (CO) is a colorless, odorless, and highly toxic gas that binds to hemoglobin with a higher affinity than oxygen, leading to hypoxia and severe health damage. It originates from the incomplete combustion of fossil fuels and can be produced by both natural and anthropogenic sources, such as vehicles, heating systems, and industrial processes [18,19]. The detection of CO requires highly sensitive and selective sensors, which are crucial for ensuring a rapid response in residential, industrial, and urban environments [20].
Recent studies have focused on n-type semiconductor materials, such as tin oxide (SnO2), titanium dioxide (TiO2), and zinc oxide (ZnO), for CO detection. However, certain metal oxides, including indium oxides (In2O3 and In3O4), cerium oxide (CeO2), and tungsten oxide (WO3), have also garnered significant interest due to their potential to enhance detection performance [21]. Among these, SnO2 is one of the most extensively studied materials due to its high sensitivity, which can be further improved through doping with metals such as copper (Cu), palladium (Pd), and platinum (Pt) [22]. Metal doping enhances oxygen adsorption and enables a faster response to variations in the target gas concentration. Moreover, the utilization of nanowires and nanoparticles further optimizes gas adsorption, thereby improving sensor performance. For instance, nanowires with diameters of approximately 60 nm and thin films with thicknesses ranging from 1.59 nm to 5.87 nm—particularly at 2.62 nm—exhibit optimal performance within the temperature range of 300 to 325 °C [23,24,25].
Additionally, the integration of palladium oxide (PdO) nanoparticles into SnO2 enhances the detection of CO at 300 °C for concentrations of 200 ppm [26]. The addition of gold (Au) further enhances sensitivity, reducing response times from 16 to 1 s at 500 ppm CO, particularly at an Au concentration of 2.86% [27,28]. Calcium (Ca) and zinc oxide (ZnO) have also demonstrated effective CO detection capabilities. Ca doping enables linear responses at low CO concentrations (30 ppm at 350 °C), while ZnO integration optimizes response at 300 ppm at lower temperatures [29].
Hybrid CuO-SnO2 sensors enriched with graphene and silver (Ag) nanoparticles have shown significant improvements in sensitivity at 100 ppm CO at 400 °C, surpassing the performance of pure SnO2 sensors [20,30]. Like tin oxide, TiO2 also exhibits good sensitivity for CO detection. Studies on TiO2 nanofibers obtained via electrospinning with PVP identified optimal calcination at 600 °C and peak response at 200 °C for 25 ppm CO. Due to their high density of adsorption sites, these nanofibers have proven particularly effective in detecting low CO concentrations [31].
Parallel research has explored hollow hemispherical TiO2 nanostructures (NTHH), which exhibit a resistance variation of up to 20 times higher than traditional thin films, significantly improving CO sensitivity [32]. Furthermore, increased porosity in TiO2 films enhances sensitivity and recovery times, with an optimal response observed in films with 62% porosity and 3.3 nm pores [33]. Other approaches have involved the development of miniaturized MEMS sensors with TiO2 and molybdenum (Mo)-based micro-heaters, achieving high CO sensitivity (84%) at optimal operating temperatures of 500 °C [34].
Finally, the integration of multi-walled carbon nanotubes (MWCNTs) with TiO2 has resulted in a sevenfold increase in sensitivity compared to pure films, with rapid response times (4 s at 50 ppm CO) and overall good sensor stability [35].
Several studies have analyzed ZnO and its doped variants for CO detection. It has been observed that in thin films of aluminum-doped ZnO, sensitivity decreases with increasing thickness, with an optimal operating temperature of 400 °C and a faster response in thinner films [36]. Similarly, aluminum nanoparticles have exhibited high sensitivity and optimal performance in the 0–80 ppm CO range, even at lower temperatures (300 °C) [37]. Copper-doped ZnO has shown a high response at 350 °C for 20 ppm of CO [38], while palladium-doped ZnO nanofibers demonstrated strong sensitivity to low CO concentrations (1–20 ppm) as early as 220 °C, exhibiting greater selectivity compared to gases such as NO2 and methane [39]. In the case of indium-doped ZnO, the optimal response was recorded for indium concentrations between 1% and 2% at 300 °C [40]. Gallium-doped ZnO nanowires exhibited the best response at a gallium concentration of 3 wt% [41]. Finally, the integration of cerium oxide (CeO2) with ZnO resulted in maximum sensitivity at 10,000 ppm of CO at 380 °C, with particularly fast response and recovery times [42].
P-type semiconductors, such as tricobalt tetraoxide (Co3O4), nickel oxide (NiO), and cupric oxide (CuO), have been investigated for their reactivity with CO, demonstrating rapid responses at low concentrations. In general, these materials are less sensitive to CO than n-type semiconductors, but Co3O4 is widely used for gas detection [20]. Specifically, Co3O4 nanorods, due to their high surface-to-volume ratio, offer a fast and highly sensitive response to CO concentrations between 5 and 50 ppm, with an optimal operating temperature of approximately 250 °C. Other materials, such as AuPdPt-doped CoO, enhance selectivity, while n-p heterostructured sensors, such as SnO2-Co3O4, ensure optimal performance at low CO concentrations (1–10 ppm) at 350 °C [43]. NiO, particularly in combination with activated carbon (NiO-AC), exhibits superior performance compared to pure NiO due to an increased surface area that enhances CO adsorption. CuO nanowires, obtained via thermal oxidation of Cu microstructures, provide a stable response even in humid environments. Additionally, a 1:1 mixture of CeO2 and CuO has demonstrated greater CO sensitivity than individual oxides, even at room temperature. Overall, the use of dopants or material combinations enhances the selectivity and response of these semiconductors to CO [44].
2.2. Nitrogen Dioxide (NO2): Environmental Impacts and Detection with MOX Sensors
Nitrogen dioxide (NO2) is a highly relevant atmospheric pollutant with detrimental effects on respiratory health and air quality. It is one of the primary contributors to respiratory diseases and plays a significant role in the formation of smog and acid rain. Major sources of NO2 include vehicular traffic, industrial activities, and combustion processes. The detection of NO2 is crucial for environmental protection and public health [45].
Although nitrogen dioxide (NO2) does not have a distinctive odor at low concentrations, it can significantly affect overall odor perception in polluted environments. This is primarily due to its reactivity with other atmospheric pollutants, such as ozone (O3), leading to the formation of secondary compounds with pungent odors, including acrolein (acrylic aldehyde), which is known to contribute to unpleasant odors in urban areas.
The standard technique for quantifying NO2 is chemiluminescence, which relies on the reaction between nitric oxide (NO) and ozone, producing light in proportion to the NO concentration. Since NO2 does not directly participate in this reaction, it must first be catalytically reduced to NO, allowing for indirect yet highly sensitive and precise measurements. This method is widely adopted in environmental monitoring networks [46].
To enable real-time measurements and enhance spatial coverage, chemiluminescence can be complemented by metal oxide (MOX) sensors, which offer a cost-effective and easily deployable alternative. However, the accuracy of MOX sensors strongly depends on the calibration strategy employed. Recent studies have demonstrated that field calibration—performed directly under real-world environmental conditions—provides significantly better performance than laboratory-based calibration [47]. The analysis of semiconductor materials such as TiO2, SnO2, and ZnO has shown that doping SnO2 with vanadium (V) or calcium (Ca) can enhance its sensitivity. Additionally, the use of nanostructures, such as nanowires and mesoporous films, facilitates increased gas adsorption, thereby improving material performance. However, environmental variability necessitates the application of advanced calibration algorithms and hydrophobic materials to ensure greater operational stability [48].
Beyond SnO2 doping, other studies have investigated the enhancement of MOX sensors through ZnO modification. A particularly effective approach is the functionalization of ZnO with self-assembled monolayers (SAMs), which improves sensitivity and selectivity towards NO2. Specifically, the use of organic molecules such as tris(hydroxymethyl)aminomethane (THMA) and dodecanethiol (DT) has been shown to optimize sensor performance, making it more efficient in detecting atmospheric pollutants [49]. Three sensor configurations were tested: pure ZnO nanowires, ZnO nanowires coated with an organic SAM, and ZnO nanowires covered with SAM-functionalized ZnO nanoparticles [50].
The electrical response was measured by applying a constant voltage of 0.2 V and recording the resistance variation during exposure to target gases (NH3, N2O, and NO2) at an operating temperature of 190 °C. This temperature was selected based on thermogravimetric analysis (TGA), which confirmed the stability of the organic coatings up to approximately 225 °C. The results indicated a significant increase in NO2 response, with a doubling of the ΔR/R ratio in sensors coated with THMA-functionalized nanoparticles compared to pure nanowires. This improvement is attributed to the modulation of the depletion layer width induced by the binding of THMA ligands to the ZnO nanoparticle surface [51].
X-ray photoelectron spectroscopy (XPS) analysis confirmed the formation of Zn–S bonds for DT and N–Zn bonds for THMA, indicating strong interaction with the semiconductor surface, while Fourier transform infrared (FTIR) measurements detected the presence of characteristic functional groups on the treated surfaces. From a performance perspective, functionalization with THMA-ZnO nanoparticles led to an increase in baseline conductivity and enhanced NO2 sensitivity, with no evidence of sensor poisoning. These findings demonstrate that the chemical modification of ZnO surfaces with organic SAMs represents an effective strategy for optimizing MOX sensor response and selectivity, opening new perspectives for the development of low-power, high-efficiency devices for air quality monitoring [52].
Some studies have instead focused on field-effect transistor (FET) sensors, comparing their performance in NO2 detection with that of MOX sensors [53]. Due to their lower operating temperatures and reduced power consumption, FET sensors offer significant advantages in applications requiring efficiency and energy savings [54]. In particular, FET sensors based on indium oxide (In2O3) films modified with hydroxyl groups exhibit superior performance compared to traditional MOX sensors. This configuration enhances sensitivity and reactivity, especially at lower temperatures, enabling NO2 detection at approximately 100 °C. Studies conducted via diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) have shown that NO2 initially adsorbs onto the sensor surface as nitrite (NO2−) and, with increasing temperature, converts into nitrate (NO3−) [55].
However, the highest sensor efficiency is achieved when NO2 is present as nitrite, whereas its reactivity decreases upon nitrate formation. An additional advantage of FET sensors is their low power consumption, measured at 1.03 mW, and their high response, with a 2460% signal increase at an NO2 concentration of 500 ppb. Although MOX sensors remain viable for various applications, FET sensors are emerging as a promising solution, particularly in contexts that demand high sensitivity and low energy consumption [53].
2.3. Ozone (O3): Secondary Pollutant and Its Effects on Environmental Health
Another important environmental pollutant is ozone (O3), known for its harmful effects on human health and ecosystems. Conventional monitoring, based on chemiluminescence and/or UV spectrophotometry, is generally expensive and impractical for large-scale applications. As an alternative, innovative techniques are emerging as particularly advantageous in this field [56].
In particular, commercially available MOX sensors such as the O3 Sens 3000, NanoEnvi, MiCS 2610, Oz-47, and SP-61 have been analyzed and compared with a prototype developed by IMN2P based on WO3. Research has evaluated their performance under various operating conditions. These sensors, which operate at temperatures ranging from 300 °C to 400 °C, exhibit ozone sensitivity due to the presence of SnO2 in the semiconductor material [57,58,59]. Response times vary significantly: while the MiCS 2610 and the WO3-IMN2P prototype reach equilibrium within 5–10 min, models such as the O3 Sens 3000(AlphaStrumenti Srl, Melzo, Milan Italy) and SP-61(Nissha FIS, Inc. 2-4-28, Tagawa Yodogawa, Osaka 532-0027 Japan) require up to 90 min. Sensor calibration follows sigmoidal models, with the WO3-IMN2P prototype offering a more linear response compared to the others. Despite good sensitivity to O3 concentrations at the ppb level and repeatability, temporal drift and sensitivity to temperature and humidity variations remain significant challenges. Notably, drift can reach up to 30 ppb over 150 days, and environmental variations markedly influence measurements [60].
Additional studies have examined the use of SGX Sensortech MICS 2614 MOX sensors(Switzerland, Courtils 1, 2035 Corcelles-Cormondreche) in an active environmental monitoring network across Spain, Italy, and Austria, where they have been utilized to collect air quality data. These sensors, operating at temperatures between 300 °C and 400 °C, have highlighted the importance of continuous calibration to achieve accurate measurements. Since MOX sensors provide data in the form of electrical resistance [61], precise conversion processes are necessary [62,63]. The adoption of advanced calibration models, compensation for environmental variations, and the integration of machine learning techniques are promising strategies to enhance the stability and reliability of these sensors for ozone monitoring [64].
Other studies have investigated ozone detection in urban environments, testing sensors under different conditions and employing three calibration methodologies: laboratory calibration, field calibration, and the Environmental Adaptive Sensor Evaluation (EASE) method. The MiCS-6814 sensors manufactured by SGX Sensortech(Switzerland, Courtils 1, 2035 Corcelles-Cormondreche), which operate using a thermal cycle technique (TCO), exhibited good correlation with reference instruments for O3, with R2 values of 0.96 for field calibration and 0.93 for EASE, whereas laboratory calibration performed less effectively (R2 = 0.82). Field calibrations proved more reliable; however, sensor variability was influenced by the complexity of the sampling system and the fragility of MOX chips, with 41% of the devices excluded due to instability. Moreover, low temperatures (<0 °C) compromised sensor responses, likely due to alterations in the thermal cycle. Despite these limitations, MOX sensors have demonstrated a strong capability for O3 detection, although frequent calibrations are required to compensate for drift and individual variations [47,65].
2.4. Volatile Organic Compounds (VOCs): Sources, Toxic Risks, and Carcinogenic Potential in the Environment
Odor analysis of environmental samples is traditionally performed through olfactometric tests conducted by trained panelists. These tests provide reliable and detailed data on odor perception, leveraging the human ability to detect and evaluate different odor intensities and qualities with high sensitivity and discernment [66]. However, to complement and support such assessments, appropriately trained sensor systems can be employed. When properly calibrated, these devices are capable of continuous real-time monitoring, contributing to a more comprehensive and objective characterization of odor-emitting environments. MOX sensors have been extensively studied for the monitoring of volatile organic compounds (VOCs), including methane and hydrogen sulfide (H2S) [67]. Methane (CH4) is a greenhouse gas with a significant impact on global warming, being approximately 25–34 times more potent than CO2 over a 100-year period. Its emissions originate from both natural sources, such as wetlands, and anthropogenic activities, including agriculture and waste management. Specifically, enteric fermentation in ruminants represents one of the primary emission sources, making the development of effective monitoring techniques essential for both reducing environmental impact and enhancing agricultural productivity [68]. Various methods have been developed to measure methane emissions in ruminants [69]. Respiratory chambers provide accurate measurements but are costly and impractical for real-world farming conditions. Masks and portable devices offer a more agile solution but can influence animal behavior. Sulfur hexafluoride (SF6)-based tracers enable more flexible monitoring, although SF6 itself is a potent greenhouse gas. A recent innovation involves capsules with gas sensors capable of providing real-time data on CH4, CO2, and H2 production directly from the rumen. Finally, predictive models based on physiological and dietary parameters are used to estimate global emissions but require reliable experimental data to refine predictions [70,71]. Meanwhile, chemoresistive sensors based on metal oxide semiconductors have been developed for environmental methane monitoring [72,73]. A recent study analyzed the effectiveness of sensors based on copper oxide (CuO) and cobalt oxide (CoO) obtained through sol–gel deposition on alumina substrates with gold and platinum interdigital electrodes. These devices operate at relatively low temperatures (210–220 °C) and stand out for their high sensitivity, capable of detecting CH4 concentrations as low as 5 ppm with response and recovery times of approximately 250 s. Morphological analysis of the surfaces, conducted via atomic force microscopy (AFM) and scanning electron microscopy (SEM), revealed a porous and rough structure that enhances gas adsorption and sensor performance. Furthermore, the CoO-based sensor demonstrated a more pronounced response and greater stability over time, with performance remaining unchanged after six months of use, confirming its reliability in environmental monitoring [74].
Regarding hydrogen sulfide, H2S is a toxic gas formed through both natural processes and industrial activities. It is considered an environmental pollutant, as it contributes to soil and water acidification, damaging vegetation. Additionally, it poses a risk to human health: at low concentrations, it can irritate the respiratory system, while at higher levels, it can cause severe neurological damage or even be lethal. For this reason, H2S emissions are subject to regulation to limit their environmental and health impact. In recent years, several studies have aimed to improve the performance of sensors for H2S detection, focusing on aspects such as material morphology, chemical composition, and optimal operating conditions [75]. Among the solutions developed, sensors based on CuO microspheres enriched with CuFe2O4 nanoparticles stand out. This configuration provides a porous and rough surface, ideal for promoting gas adsorption [76]. Performance analysis of these devices has highlighted the importance of operating temperature. At low temperatures, the interaction between H2S molecules and adsorbed oxygen species is weak, reducing sensor sensitivity. Conversely, at high temperatures, gas diffusion on the sensor surface becomes a limiting factor. To achieve the best balance between sensitivity and response time, the operating temperature was optimized to 240 °C, ensuring a response time of 31 s and a recovery time of 40 s [77]. Further advances have been made through the use of mesoporous materials. Specifically, it has been demonstrated that Co3O4 nano-chains can be employed to detect H2S with high efficiency. Thermal treatment at 600 °C led to the formation of a rough and highly porous surface, improving chemical reactivity with the target gas. Temperature-dependent response analysis revealed a behavior similar to that observed in CuO/CuFe2O4 systems: below the optimal temperature (300 °C), chemical activity is reduced, while above it, desorbed H2S molecules inhibit the sensor’s response. Response and recovery times for H2S concentrations ranging from 1 to 100 ppm were 46 and 24 s, respectively, demonstrating good selectivity towards other toxic gases [78]. Another advancement in H2S detection technology has been achieved by employing materials based on metal–organic frameworks (MOFs). Some studies have developed a sensor based on Zr(TBAPy)5(TCPP), characterized by a shuttle-like structure and particles with a diameter of approximately 100 nm. The interaction between sulfide ions (S2−) and the functional units of the MOF was confirmed by spectroscopic analyses (FTIR), which revealed a shift in the N-H and C=N peaks following gas adsorption. Additionally, the fluorescence intensity was found to be proportional to H2S concentration, suggesting a potential application of these materials for optical gas detection [79]. It is evident that the microstructure of materials plays a crucial role in detection performance. Factors such as the presence of active surface sites, the specific surface area of the material, and electronic interactions between different heterogeneous phases influence sensor sensitivity, selectivity, and response speed. A critical aspect concerns the operational stability of MOX sensors, as prolonged exposure to reducing gases like H2S can lead to aging and performance degradation. Therefore, future developments could focus on surface functionalization strategies and the integration of hybrid structures to improve selectivity and resistance to gas poisoning [77,80].
Figure 2 provides a graphical representation of the content discussed in the corresponding section, highlighting the key role that MOX sensors can play in the environmental field, particularly in the monitoring of volatile components.
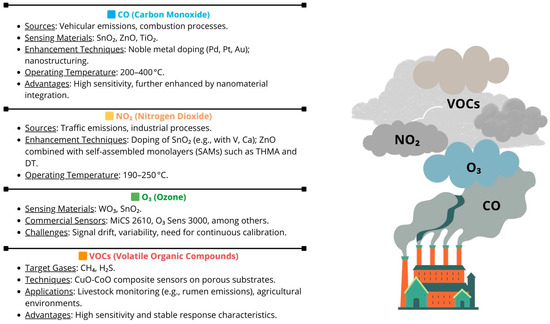
Figure 2.
Volatile compounds targeted for monitoring using MOX sensors.
A summary table (Table 1) has been provided, detailing the gas sources, semiconductor types, sensing materials, doping elements, operating conditions, performance characteristics, and corresponding references for each sensor discussed above in the context of air quality monitoring using MOX sensors.

Table 1.
A summary table for air quality monitoring using MOX sensors, highlighting key characteristics.
3. Biomedical Applications of MOX Sensors: Breath Analysis for Disease Diagnosis
MOX sensors, traditionally used in environmental monitoring, are gaining increasing attention in the medical field, particularly for exhaled breath analysis. This area, known as breathomics, focuses on the identification of endogenous volatile organic compounds (VOCs) that may be associated with specific pathological conditions, such as infections, metabolic disorders, or various forms of cancer [81,82]. The growing interest in MOX sensors lies in their low cost, compactness, and ability to perform real-time measurements. However, a major challenge in their medical application is the extremely low concentration of biomarkers in human breath, often in the parts-per-billion (ppb) or parts-per-trillion (ppt) range, which imposes stringent requirements on both sensitivity and selectivity [83]. To address the limitations of MOX sensors, particularly their limited selectivity and sensitivity, these devices are often integrated with well-established analytical techniques that offer high chemical resolution. In many studies, MOX sensors serve as rapid screening tools, while detailed and specific sample analyses are carried out using GC-MS. For instance, Dragonieri et al. (2012) demonstrated the combined use of MOX sensors and GC-MS to distinguish patients with chronic obstructive pulmonary disease (COPD) from healthy individuals based on breath analysis [84].
Additional support can be provided by spectroscopic methods such as Fourier transform infrared (FTIR) spectroscopy, Raman spectroscopy, and nuclear magnetic resonance (NMR). Although these techniques are not typically employed for direct VOC identification in exhaled air, they can yield valuable molecular insights into metabolites and cellular components linked to pathological conditions [85].
The synergistic integration of advanced analytical techniques with MOX-based systems, enhanced by data processing tools such as machine learning and pattern recognition algorithms, is paving the way for the development of non-invasive, rapid, and reliable diagnostic devices suitable for large-scale clinical use [86].
3.1. Acetone (CH3COCH3): A Biomarker for Diabetes and Fat Metabolism
Acetone present in exhaled air is considered a potential biomarker for monitoring diabetes, as its concentration correlates with blood glucose levels. In healthy individuals, the values range between 0.3 and 1.8 ppm [87], while in type 1 diabetes patients, it can exceed 25 ppm [88]. However, these ranges are not rigidly defined, as individual and methodological factors can influence their variability [89,90,91].
Numerous studies have developed sensors based on metal oxide semiconductors, with their detection mechanisms relying on the chemisorption of oxygen on the surface [92]. Under optimal operating conditions, acetone reacts with the adsorbed oxygen ions, leading to an increase in the semiconductor’s conductivity and a variation in electrical resistance, which is used as the detection signal. Optimizing the operating temperature and sensor composition is crucial to improving sensitivity and selectivity [93,94]. Tin oxide (SnO2) is one of the most commonly used materials for acetone detection [95]. Studies have shown that SnO2-based composite structures, combined with materials such as NiO and graphene oxide, improve sensitivity and reduce operating temperatures. Sensors based on ternary nanocomposites have demonstrated fast and high responses in the 0.25–30 ppm range at temperatures around 200 °C, making them suitable for acetone monitoring in breath [94]. Titanium oxide (TiO2) is another widely studied semiconductor due to its physical–chemical properties and ease of synthesis [96]. However, pure TiO2 shows low sensitivity to volatile organic compounds, including acetone [97,98,99]. To overcome this limitation, heterojunctions with other metal oxides have been developed to create new electron transport pathways and enhance sensitivity [100]. TiO2/Ag2V4O11 nanostructure sensors and TiO2-Fe2O3 nanofibers have shown significantly superior responses compared to pure TiO2 [101], while TiO2-SnO2 heterostructures have ensured high selectivity, long-term stability, and rapid response times (15 s) [102]. Tungsten oxide (WO3) is an n-type semiconductor with catalytic properties that promote oxidation and reduction reactions on its surface. WO3-based sensors have demonstrated good performance in acetone detection, with detection limits below ppm and improved sensitivity through modifications with Au or Pt nanoparticles [103]. Macroporous WO3-based structures doped with Au have reached detection limits in the ppb range at 410 °C, making them suitable for non-invasive diagnostic applications [104]. Iron oxides, particularly Fe2O3, exhibit high operating temperatures (450–1075 °C), limiting their practical application. However, the use of the sol–gel technique on pure γ-Fe2O3, appropriately doped with various amounts of gadolinium, has enabled improvements in both sensitivity and selectivity, allowing the detection of low acetone concentrations (~1 ppm) at lower temperatures (~200 °C) [105]. These sensors show good stability and reproducibility, making them promising for future applications. Indium oxide (In2O3) is known for its high conductivity and the presence of structural defects that modulate its electrical resistance [106,107]. The sensing strategy relies on the use of nanowires to maximize the electrical response at low gas concentrations. Sensors based on In2O3 nanowires have demonstrated excellent performance, featuring very fast response times (1–7 s) and good selectivity. However, their sensitivity in humid environments, typical of human breath, remains to be verified [108]. Finally, copper oxide (CuO) is a p-type semiconductor with low sensitivity to gases. To enhance performance, several studies have explored the use of Cu2O–CuO composites decorated with Ag nanoparticles, tested at various acetone concentrations (from 0.125 to 1000 ppm) at an operating temperature of 350 °C. The results showed superior performance compared to undoped structures (from 8.0 to 0.125 ppb and from 34 to 1000 ppm), with recovery times of 27 s for 125 ppb and 37.9 s for 1000 ppm acetone. However, the operating temperature of these sensors remains high, limiting their practical applications [109,110].
In recent years, several prototypes for acetone monitoring in breath have been developed. In 2014, a compact analyzer was designed to detect various target gases, including acetaldehyde, methane, and acetone, enabling the assessment of an individual’s health status and metabolic activity in a less invasive manner than blood and urine tests [111]. In 2020, the “Diabetomat” was introduced, a device capable of measuring ketone bodies in exhaled air and correlating these data with blood glucose levels. This tool employs a pre-concentration technique to reduce moisture in breath samples, thus improving measurement accuracy [112]. Finally, in 2022, an ultrasensitive sensor based on a porous p-Rh2O3-n-ZnO heterostructure was developed and tested by simulating a diabetic respiratory environment with acetone concentrations ranging from 1200 to 1800 ppb. Other studies have evaluated the use of composite materials, such as CdS/Co3O4, for acetone detection under the humid conditions typical of exhaled air. Despite the advancements made, most devices are still in laboratory validation or clinical testing stages [113]. Further studies are necessary to ensure their reliability before they are introduced to the market and adopted in clinical practice [112].
3.2. Ammonia (NH3): An Indicator of Renal Dysfunction
The presence of ammonia in the breath of patients with renal failure is directly correlated with elevated blood urea nitrogen (BUN) levels. Under normal conditions, the liver converts ammonia and ammonium ions into urea via the urea cycle. However, in patients with renal dysfunction, urea elimination is impaired, leading to ammonia accumulation in the body. Due to their small size, ammonia and ammonium ions can cross the blood–lung barrier and enter the breath. For this reason, ammonia in exhaled air could serve as a useful biomarker for monitoring renal diseases, particularly in patients undergoing hemodialysis [114].
Several techniques are available for diagnostic breath analysis, including proton transfer reaction mass spectrometry (PTR-MS), selected ion flow tube mass spectrometry (SIFT-MS), photoacoustic spectroscopy (PAS), surface acoustic wave (SAW) sensors, and quartz crystal microbalance (QCM). These advanced technologies rely on principles such as ionization, absorption, or resonance to detect and quantify ammonia at trace levels, often below 10 ppb. However, they are generally expensive and technically complex [115,116]. Recent research, however, has highlighted the significant potential of devices based on nanomaterials, such as polyaniline nanofibers, conductive polymer nanojunctions, and polypyrrole nanowires, for these specific applications [117,118]. These sensors exploit the variation in the electrical conductivity of polymeric materials in the presence of ammonia, providing high sensitivity and a rapid response. Numerous studies have explored the correlation between ammonia concentration in breath (breath-NH3) and blood urea nitrogen (BUN) levels in patients with chronic kidney disease (CKD) undergoing dialysis. Analysis using sensor-equipped devices has shown a positive correlation between breath-NH3, BUN, and salivary urea. Additionally, a significant reduction in ammonia concentration was observed after dialysis and water rinsing, suggesting that oral factors may influence NH3 levels in the breath. The sensors use a polymer semiconductor chip with a nanoporous structure, PTB7 (poly({4,8-bis[(2-ethylsiloxy)]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl}{3-fluoro-2-[(2-ethylsil)carbonyl]thieno[3,4-b]thiophenediyl})), which allows for the detection of NH3 concentration in breath in the range of 50 ppb to 8000 ppb. The nanoporous structure enhances the selective interaction between the target gas and the active material, allowing for a measurable electrical response. The sensor has a response time of 30 s and requires a 150 s interval between two consecutive measurements. A threshold value for breath-NH3 has been identified that could predict elevated BUN levels with high sensitivity and specificity [119]. Meanwhile, ammonia detection sensors based on innovative nanomaterials have been developed, such as conductive polyaniline nanojunctions (PANI) with gold electrodes and filters to reduce moisture interference. These devices have shown excellent sensitivity to NH3 variations in breath, with measurement ranges between 16 and 575 ppb. Among the materials studied, Si-doped MoO3 has demonstrated high selectivity for ammonia, maintaining thermal stability and precision even under high relative humidity. The addition of dopants such as silicon improves the electronic response of the material, even under complex environmental conditions, while maintaining thermal stability and accuracy [120]. Other studies have focused on analyzing the MOX sensors used to examine the breath of dialysis patients, utilizing devices such as TGS 2444, MQ 135, MQ 137, and TGS 826 [121]. These sensors, using SnO2 as the sensing element, detect ammonia through an increase in its electrical conductivity, allowing for the quantification of its concentration in breath [122]. Among the analyzed devices, TGS 2444 showed the highest sensitivity, with a detection range between 10 and 300 ppm and reduced interference from other gases, due to the presence of an activated carbon filter and advanced thermal insulation. All the sensors are powered by 5 V, with TGS 2444 requiring a controlled heating cycle to maintain a stable operating temperature of 300 °C. The accuracy of the breath data classification, achieved through pattern recognition techniques, reached a maximum value of 88% [123]. Additionally, the use of these sensors enabled the monitoring of dialysis efficacy, highlighting a significant reduction in ammonia levels in the breath post-treatment. In recent years, portable sensors based on Si-doped WO3 nanoparticle films or quartz microbalances with porous films have also been developed [124]. These devices have shown high sensitivity and selectivity at 350 °C, with detection limits below 20 ppb and a rapid response, even in the presence of high relative humidity [125]. Finally, researchers have developed sensors for electronic noses (e-nose), enhancing them with quartz crystals coated with synthetic peptides [126], to detect volatile biomarkers such as uremia, dimethylamine, trimethylamine, ammonia, and monomethylamine, making significant progress in research in this field [114,127,128].
3.3. VOCs and Colorectal Cancer (CRC)
Colorectal cancer (CRC) is one of the leading causes of morbidity and mortality worldwide. Among the emerging methodologies for monitoring and diagnosing CRC, the use of metal oxide sensors has become a promising alternative to traditional invasive methods such as colonoscopy. The analysis of exhaled VOCs represents a non-invasive detection strategy based on the identification of volatile biomarkers associated with the presence of cancer. Relevant biomarkers include aldehydes, ketones, and hydrocarbons, which are formed due to increased oxidative stress and abnormal metabolic activity in cancer cells [129].
Numerous studies have focused on developing sensors based on semiconductor oxides, such as SnO2, TiO2, WO3, Nb2O5, and V2O5, often enhanced with Au nanoparticles to improve sensitivity [130]. These sensors operate at temperatures ranging from 350 to 450 °C, conditions that optimize their ability to discriminate against VOCs specific to CRC. The effectiveness of MOX sensors in diagnosing this condition has been evaluated through various experimental studies. For instance, one study used the SCENT B2 device(SCENT S.r.l., Ferrara, Italy), which employs a combination of MOX sensors to monitor changes in VOCs in blood samples from CRC patients before and after surgery. The strategy involves comparing the VOC profile before and after surgery to detect variations associated with the presence of cancer. SCENT B2 is equipped with four different sensors. The first sensor, ST25 + 1% Au (or ST25), is a mixture of SnO2 and TiO2 with 1% gold (Au) nanoparticles and is an n-type sensor. The second sensor, SmFeO3, consists of Sm and Fe2O3 and is a p-type sensor. The third sensor, STN, combines SnO2, TiO2, and Nb2O5 and is also an n-type sensor. Finally, the fourth sensor, TiTaV, is made of TiO2, Ta2O5, and V2O5 and is an n-type sensor. Each sensor has a different sensitivity to VOCs, allowing for a broader and more precise detection of the tumor profile. The results showed a significant reduction in sensor response after tumor removal. The discriminative ability of the sensors was confirmed through PCA analysis and ROC curves, with an area under the curve (AUC) greater than 0.9. This statistical analysis strategy allows for the identification of specific patterns in the data collected from the sensors that are useful for distinguishing between healthy and sick individuals. This confirmed the direct influence of CRC on the VOCs in the blood [11]. Another study evaluated the use of MOX sensors in breath analysis for CRC diagnosis. The approach is based on sampling exhaled breath and real-time analysis of VOC composition using high-temperature MOX sensors. The MOX sensors used in the study, consisting of semiconductor oxides such as SnO2 or WO3, operate at elevated temperatures (between 200 and 400 °C). The breath analysis system tested in the study showed promising results, with the machine learning method C4.5 achieving 77% accuracy, 63.3% sensitivity, and 84.2% specificity using the MOX sensors [131]. The use of machine learning algorithms allows for the classification of sensor data, improving diagnostic accuracy compared to manual analysis. Finally, a comparative analysis of various materials for MOX sensors, such as SnO2, TiO2, Ta2O5, V2O5, and Nb2O5, revealed that sensors made with SnO2 and TiO2, decorated with gold nanoparticles, offer the best performance. These sensors achieve up to 80% sensitivity and 70% specificity. Their optimal operation is achieved at temperatures around 450 °C, ensuring stable and reproducible responses. The optimization of materials and sensor configuration is a key strategy to enhance the selectivity and reproducibility of the system. Once perfected, these devices could be integrated into non-invasive screening protocols, enhancing the diagnostic efficacy of CRC and reducing the need for invasive procedures [130,132].
Figure 3 provides a graphical overview of the concepts discussed in the corresponding section, emphasizing the key role that MOX sensors can play in the medical field by enabling the detection or prevention of diseases through the analysis of volatile compounds emitted by the human body.
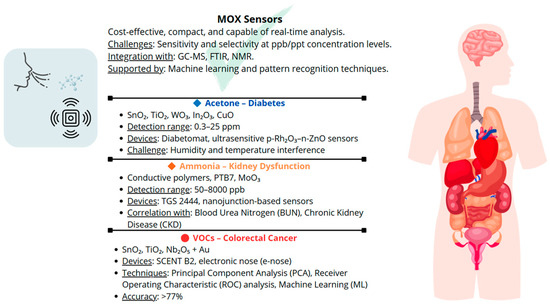
Figure 3.
Graphical overview emphasizing the key role that MOX sensors can play in the medical field.
A summary table (Table 2) has been provided, detailing the gas sources, semiconductor types, sensing materials, doping elements, operating conditions, performance characteristics, and corresponding references for each sensor discussed above in the context of the detection of gas markers for breath analysis for disease diagnosis using MOX sensors.

Table 2.
A summary table for breath analysis for disease diagnosis using MOX sensors, highlighting key characteristics.
4. Food Industry: Detection of Gas Markers for Quality Control Using MOX Sensors
The agri-food sector is particularly sensitive to numerous factors influencing product quality and safety. It is essential to continuously monitor food freshness, detect potential chemical or microbiological contamination, and prevent food fraud. Currently, quality control mainly relies on well-established analytical techniques, such as gas chromatography coupled with mass spectrometry (GC-MS) and microbiological laboratory analyses. However, these methods are applied to selected samples, and despite their high reliability, they can be costly and require relatively long analysis times. To enhance monitoring efficiency and ensure faster and more widespread control, a viable solution is represented by metal oxide sensors [2].
4.1. Innovative Techniques for Monitoring Food Freshness
The freshness of food is a crucial aspect of the food industry. To monitor it, MOX sensors detect the presence of key compounds. In particular, TMA and H2S are volatile nitrogen and sulfur compounds that are strongly correlated with microbial decay and protein degradation in animal products. C2H4, on the other hand, is an unsaturated hydrocarbon that acts as a plant hormone, with its concentration increasing during the ripening and senescence of fruits and vegetables. Other compounds, such as NH3, are produced by enzymatic deamination reactions, while alcohols (e.g., ethanol), aldehydes (e.g., hexanal), and ketones (e.g., 2-nonanone) result from oxidative and fermentative processes accompanying microbial growth or lipid degradation [133]. Fish deterioration is associated with the production of trimethylamine (TMA), a volatile compound derived from the degradation of trimethylamine oxide (TMAO) [134]. A recent study developed a molybdenum oxide (MoO3) sensor, tested at various temperatures (225 °C, 250 °C, 275 °C, and 325 °C). The results showed high sensitivity to TMA, detecting concentrations as low as 2 ppm even at room temperature (25 °C), enabling rapid recognition of fish spoilage [135]. Other research explored a room-temperature MOX sensor based on an MXene-MoO3 composite. The use of Ti3C2Tx, a material with a high surface area and good conductivity, improved performance, allowing the synthesis of a hybrid structure composed of MoO3 nanofibers and Ti3C2Tx nanoflakes. This composite exhibited enhanced TMA sensitivity and rapid response times due to the interaction of p-n junctions, which improved conductivity and increased surface area [136]. Regarding hydrogen sulfide, H2S is a characteristic gas formed during the deterioration of meat and dairy products, produced by enzymatic interactions with methionine (C5H11NO2S) or cysteine (C3H7NO2S). As spoilage progresses, H2S levels increase significantly [134]. To enhance the quality of poultry products, alternative feed formulations enriched with varying percentages of insect meal were tested, with the aim of reducing the formation of undesirable compounds such as hydrogen sulfide (H2S) while preserving beneficial volatile compounds. The analysis included both conventional and experimental feed samples, which were evaluated using gas chromatography–mass spectrometry (GC-MS) for the identification of volatile markers, as well as an electronic nose equipped with three types of metal oxide semiconductor (MOX) sensors (pure SnO2, SnO2 doped with Pd, and SnO2 doped with Au) operating at 500 °C. This combined analytical approach enabled clear discrimination among the samples, highlighting the impact of different feed formulations on the overall aromatic profile [137]. To enhance the sensitivity and selectivity of H2S detection, advanced sensors based on various nanostructured architectures have been developed. In particular, sensors utilizing CrO3 nanorods fabricated via glancing angle deposition (GLAD) have been produced [138]. Other innovative devices rely on hybrid organic–inorganic nanospheres incorporating Cu2+ ions to facilitate the dissociation of H2S into H+ and HS− (or S2−), leading to changes in electrical resistance through interactions with polypyrrole and SnO2 [139]. Additionally, room-temperature sensors with In2O3 nanofibers decorated with MoS2 have been created, demonstrating excellent performance due to enhanced electron transfer [140]. Ethylene, a crucial plant hormone for fruit ripening, is monitored using MOX sensors based on SnO2, WO3, and CuO, optimizing cold chain management [141]. However, ethylene detection remains challenging due to its nonpolar nature, which complicates identification. To enhance sensor effectiveness, some studies have proposed a gas filtration method that preferentially oxidizes interfering gases, increasing ethylene selectivity [142]. Other sensors based on hydrothermally synthesized Co3O4 nanorods and platinum-based catalysts have improved sensitivity and selectivity, while novel room-temperature operation modes enable real-time monitoring [143]. Additionally, ZnO nanoflowers embedded in Ag nanoparticles have been developed. The surface plasmon resonance and catalytic properties of silver enhance sensitivity, enabling ethylene detection at room temperature under UV light activation. Finally, several studies have analyzed the freshness of table grapes stored in a modified atmosphere using both MOX sensors and conventional GC-MS techniques to determine optimal storage conditions. SnO2 sensors operating at 500 °C, both in pure and Pd- and Au-doped forms, were tested. Although no ideal modified atmosphere was identified to prolong grape shelf life, the results confirmed the efficacy of MOX sensors in distinguishing samples based on ethylene content [144,145].
4.2. Use of MOX Sensors for Contamination Detection in the Agri-Food Supply Chain
In the agri-food sector, food quality control is not only concerned with freshness but also includes the detection of chemical and biological contaminants [2]. In this context, MOX sensors have been extensively studied for their ability to detect such contamination. A crucial aspect is the quality of water used in agriculture, as potential contaminants may compromise food safety [146]. Among the gas compounds most strongly correlated with microbial contamination are ammonia (NH3), which results from the degradation of nitrogenous compounds, and various volatile organic compounds (VOCs) such as ethanol, ethyl acetate, acetic acid, and 2,3-butanedione. These molecules are often released as a result of the metabolic activity of microorganisms like lactic acid bacteria, coliforms, yeasts, and molds, serving as volatile markers of microbial proliferation. MOX sensors detect anomalies in the volatilomic profile of the sample in real time, enabling the early identification of contamination linked to microbial activity [147].
Recent research has combined traditional GC-MS methods and microbiological analysis with innovative technologies based on the use of MOX sensors integrated into an electronic nose [148]. This device has been used to identify biological contaminants, including lactic acid bacteria and coliforms, using tin oxide (SnO2)-based sensors, both in their pure form and modified with palladium (Pd) and gold (Au). Operating at temperatures between 350 °C and 400 °C, the sensors demonstrated high sensitivity in detecting these contaminants. The main target volatile compounds emitted during bacterial growth include aldehydes (such as hexanal), ketones (like acetone), organic acids (acetic acid), and hydrogen sulfide (H2S), typically associated with microbial fermentation and sugar degradation in food substrates. Sample analysis showed a clear distinction between contaminated and healthy samples, as confirmed by principal component analysis (PCA). Notably, the electronic nose detected variations in the samples before GC-MS could identify the production of metabolites, demonstrating a high sensitivity for early microbial growth monitoring. Additionally, in the case of coliforms, a pattern corresponding to the logarithmic phase of bacterial proliferation was observed, confirming the device’s effectiveness in real-time monitoring [149].
Another study focused on the detection of biological contamination in maize, particularly the identification of toxigenic strains of the fungus Fusarium verticillioides [150]. This pathogen is known for its ability to produce fumonisins, mycotoxins associated with severe human and animal diseases, such as esophageal cancer and neurotoxic disorders in horses and pigs [151]. The analysis was conducted on two types of samples: fungal cultures grown on synthetic media to ensure controlled conditions, and contaminated maize grains, a more realistic system to test the electronic nose’s effectiveness in distinguishing between healthy and infected maize. Fumonisin identification was confirmed by enzyme-linked immunosorbent assay (CD-ELISA). To analyze the volatile compounds emitted by the fungus, the EOS835 electronic nose (Sacmi Imola) (SACMI IMOLA S.C Via Selice Provinciale, 17/A, Imola, Italy)was used, equipped with six thin-film MOX sensors based on metal oxides such as SnO2, WO3, and In2O3, with catalysts such as Au, Ag, and Mo. The sensors’ operating temperatures ranged from 350 °C to 475 °C, enabling optimal detection of volatile compounds produced during fungal growth. The target gas species associated with Fusarium contamination included alcohols (such as 1-octen-3-ol), volatile esters, aldehydes, and terpenes, which serve as volatile biomarkers of the presence of mycelium and fungal metabolic activity. The results demonstrated the device’s ability to distinguish between different contamination conditions. In the synthetic media experiment, the differentiation between contaminated and non-contaminated samples was evident from the early days, while the distinction between fumonisin-producing (FB1+) and non-producing (FB1−) strains became clear only on the seventh day, when PCA analysis highlighted differences in volatile compounds. In the contaminated maize experiment, the electronic nose exhibited high discriminative capability, not only distinguishing healthy maize from infected samples but also identifying toxigenic strains after just 48 h of growth. Classification accuracy was evaluated using two methods: k-nearest neighbor (kNN) and linear discriminant analysis (LDA). The 1NN method (k = 1) achieved the best result, with an accuracy of 98.8%, while LDA reached 94%, confirming the potential of the electronic nose, and thus MOX sensors, as an effective tool for early microbiological contamination monitoring [150]. Finally, MOX sensors were also applied to analyze jam and jelly samples [152]. The study developed a system based on MOX sensors for the early detection of mold contamination. Both uncontaminated samples and those artificially contaminated with mold spores (Fusarium spp., Alternaria spp., Botrytis spp., and Penicillium spp.) at a final concentration of 350 CFU/g were analyzed. The analysis was conducted using the S3+ device, an electronic nose equipped with MOX sensors optimized for the detection of volatile organic compounds, whose fingerprint is characteristic of microbial growth. The target VOCs included compounds such as methanol, ethanol, 2-methyl-1-propanol, and short-chain carbonyl compounds, all commonly associated with fungal degradation of sugar matrices. Two different sensor configurations were tested, operating at 500 °C and 400 °C, with different combinations of Pd- and Au-doped SnO2. The samples were analyzed under controlled conditions, kept at 35 °C to favor VOC release, and the data obtained were processed through multivariate analysis, particularly PCA, to identify distinctive patterns between contaminated and non-contaminated samples. The results demonstrated that the system was able to detect contamination from the first day, showing distinct clusters between the two groups. However, for products containing antimicrobial ingredients such as ginger, the discrimination was less pronounced due to reduced VOC production resulting from limited fungal growth [153]. The system’s effectiveness was confirmed by parallel microbiological analyses, which showed an increase in contamination over time, as in the case of raspberry jam, where contamination increased from 7 CFU/g to 31 CFU/g in two days. To automate the anomaly identification process, an algorithm was developed based on the comparison of sample aroma profiles: the system triggers an alert if at least three out of six sensors detect significant variations compared to the reference values. The integration of sensors with artificial intelligence proved to be an innovative and reliable strategy for monitoring food product quality, enabling more effective food safety control and reducing contamination risks [152].
4.3. MOX Sensors for Ensuring the Authenticity of High-Value Food Products
The identification and differentiation of food matrices through the analysis of their volatile profiles is a key element in the fight against food fraud [153]. Ensuring the authenticity of protected designation of origin (PDO) or controlled designation of origin (PGI) products is a matter not only of quality but also of economic protection and consumer safety. These products, in fact, adhere to strict production standards, making them highly vulnerable to adulteration aimed at reducing production costs and increasing illicit profit. In response to these threats, science has developed advanced analytical methods, including MOX sensors and gas chromatography-mass spectrometry (GC-MS), tools capable of accurately distinguishing authentic samples from adulterated ones [154]. In the food industry context, the chemical classes of greatest interest include numerous VOCs, such as alcohols (e.g., ethanol, hexanol), aldehydes (e.g., hexanal, nonanal), ketones (e.g., 2-butanone, acetophenone), organic acids (e.g., acetic acid, butyric acid), esters (e.g., ethyl acetate, butyl acetate), and hydrocarbons (e.g., limonene, toluene). These compounds, released during the processing, maturation, or spoilage of food products, serve as true volatile markers of the quality and authenticity of the analyzed matrices. The high sensitivity of MOX sensors allows for the detection of even minor variations in the chemical composition of aromatic profiles, making them particularly effective tools for real-time quality control.
One of the most innovative studies in this field concerned Umbria PDO extra virgin olive oil, a product of high economic and symbolic value [155,156]. The research employed GC-MS to characterize the distinctive volatile compounds and a system based on an electronic nose (S3+) equipped with three MOX sensors. These sensors, made of SnO2 and SnO2Au, operated at temperatures of 300 °C and 400 °C, respectively, and were integrated into a fluid dynamic system to channel volatile compounds (VOCs) towards the detection chamber. An advanced electronic module managed real-time data acquisition and transmission, connecting to a dedicated web application for analysis. The results demonstrated the system’s exceptional ability to accurately discriminate the aromatic characteristics of oils, suggesting that MOX technology could complement, and in some cases even replace, traditional organoleptic tests. Statistical analyses, including Pearson correlations, confirmed a strong association between chemical and sensory information, highlighting the potential applicability of MOX sensors in the quality control of extra virgin olive oil [14].
Another study focused on the authenticity of Parmigiano Reggiano PDO using a MOX sensor system to analyze the composition of the cheese. The device integrated six sensors: three nanowires and three thin films. The thin-film (TF) sensors were made of SnO2 layers catalyzed with Au and Ag, operating at a temperature of 350 °C. The nanowire (NW) sensors, made of ZnO and SnO2, worked within a temperature range of 350 °C to 450 °C. Thanks to RGTO and PVD technologies, these sensors ensured high stability and sensitivity in VOC analysis. In this case, the detected gases included aldehydes such as hexanal and heptanal, volatile esters, and sulfur compounds, which serve as significant markers for assessing maturation and the presence of anomalous fractions. The results showed that the system was able to distinguish samples with different crust percentages, even identifying those close to the legal limit of 18%. PCA analysis revealed a clear separation between the various mixtures, allowing accurate identification of non-compliant samples and offering a new tool for quality control of Parmigiano Reggiano [157].
Finally, another study addressed the problem of roasted coffee adulteration with roasted barley, a phenomenon that is difficult to detect with traditional methods [158]. The analysis was conducted using GC-MS, complemented by SnO2-based MOX sensors, both pure and doped with Au, operating at 400 °C. In this context, the main target gases identified include furans (e.g., 2-furancarbaldehyde), pyrazines (e.g., methylpyrazine), and volatile phenolic compounds, which form the aromatic fingerprint of coffee and reveal any potential adulterations. The combination of these technologies allowed the precise identification of differences in the volatile profiles between pure coffee, pure barley, and adulterated blends, with sensitivity sufficient to detect fraud even at low percentages. PCA highlighted that adulterations were particularly recognizable in dark roasted coffee samples, suggesting that roasting conditions significantly influence detection capabilities [159]. Figure 4 offers a graphical summary of the concepts addressed in the corresponding section, underscoring the significant potential of MOX sensors in the agri-food sector, particularly for assessing food freshness, detecting contaminants, and preventing as well as identifying food fraud, including adulteration.
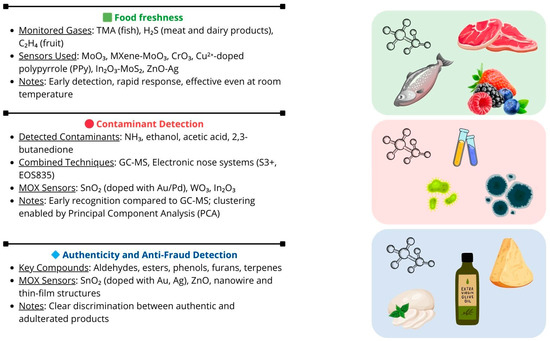
Figure 4.
Graphical overview emphasizing the key role that MOX sensors can play in the agri-food field.
A summary table (Table 3) has been provided, detailing the gas sources, semiconductor types, sensing materials, doping elements, operating conditions, performance characteristics, and corresponding references for each sensor discussed above in the context of the detection of gas markers for quality control in the food industry using MOX sensors.

Table 3.
A summary table for detection of gas markers for quality control in the food industry using MOX sensors, highlighting key characteristics.
5. Comparison of MOX, Electrochemical, and Optical Sensors: Performance and Applications
Within the environmental, biomedical, and agri-food domains, metal oxide sensors have emerged as a particularly promising technology due to their robustness, long operational lifetime, and broad VOC detection range. Compared to electrochemical sensors, which are highly sensitive and selective but hindered by shorter lifespans and environmental susceptibility, MOX sensors offer superior stability [77]. In environmental applications, MOX sensors provide a cost-effective and reliable alternative for air quality monitoring and gas leak detection.
In the medical field, optical sensors are often preferred for their precision and non-invasive operation, yet MOX sensors offer a more affordable and easily integrable option for breath analysis in early diagnostics. Similarly, in the agri-food sector, MOX sensors enable rapid and low-cost assessments of freshness and ripeness, in contrast to the greater complexity and cost of optical systems [8].
Overall, despite the targeted strengths of electrochemical and optical sensors, MOX technology distinguishes itself as a versatile, stable, and economical solution with strong applicability across all three domains. Table 4 provides a comparative overview of MOX, electrochemical, and optical sensing technologies, emphasizing their respective advantages and limitations in terms of sensitivity, cost, durability, and system integration in environmental, medical, and agri-food contexts.

Table 4.
Comparison of metal oxide (MOX), electrochemical, and optical sensors in terms of key advantages, limitations, and primary application domains in the environmental, medical, and agri-food sectors.
6. Conclusions
Metal oxide semiconductor (MOX) sensors are increasingly positioning themselves as a key technology for the detection of volatile organic compounds (VOCs) across a wide spectrum of applications, including environmental monitoring, medical diagnostics, and food safety. Their high sensitivity, low cost, miniaturization potential, and compatibility with portable devices make them particularly well suited for distributed and real-time monitoring systems.
Nonetheless, certain technological limitations still hinder their overall reliability, especially their susceptibility to environmental factors such as humidity, signal drift, and limited long-term stability. Despite these challenges, MOX sensors offer notable advantages over alternative technologies, such as electrochemical and optical sensors, in terms of operational lifetime, environmental robustness, mechanical durability, and fast response times.
Future prospects are highly promising: ongoing advancements in materials science, such as the use of nanostructures, composite materials, and advanced surface functionalization, combined with the integration of artificial intelligence algorithms and Internet of Things (IoT) frameworks, are paving the way for smart, adaptive, and energy-efficient MOX sensors. These developments will enable the deployment of highly reliable multisensor platforms capable of predictive and personalized analysis in critical domains such as indoor air quality monitoring, preventive medicine, and food traceability. MOX sensors represent a strategic technological solution to address emerging challenges in public health, environmental quality, and food safety, establishing themselves as a central pillar in the development of intelligent, sustainable, and pervasive monitoring systems.
Author Contributions
Conceptualization, E.P., E.N.-C., and V.S.; methodology, E.P., E.N.-C., and V.S.; software, E.P., E.N.-C., and V.S.; validation, E.P., E.N.-C., and V.S.; formal analysis, E.P., E.N.-C., and V.S.; investigation, E.P., E.N.-C., and V.S.; resources, E.P., E.N.-C., and V.S.; data curation, E.P., E.N.-C., and V.S.; writing—original draft preparation, E.P., E.N.-C., and V.S.; writing—review and editing, E.P., E.N.-C., and V.S.; visualization, E.P., E.N.-C., and V.S.; supervision, V.S.; project administration, V.S.; funding acquisition, V.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ON Foods—Research and Innovation Network on Food and Nutrition Sustainability, Safety, and Security—Working ON Foods, CUP B83C22004790001 Project Code PE00000003, Ministerial Decree MUR No. 1550 of 11 October 2022.
Data Availability Statement
No new data were created.
Conflicts of Interest
Author Veronica Sberveglieri was employed by the company Nano Sensor Systems SRL (NASYS). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Khorramifar, A.; Karami, H.; Lvova, L.; Kolouri, A.; Łazuka, E.; Piłat-Rożek, M.; Łagód, G.; Ramos, J.; Lozano, J.; Kaveh, M.; et al. Environmental Engineering Applications of Electronic Nose Systems Based on MOX Gas Sensors. Sensors 2023, 23, 5716. [Google Scholar] [CrossRef] [PubMed]
- Poeta, E.; Liboà, A.; Mistrali, S.; Núñez-Carmona, E.; Sberveglieri, V. Nanotechnology and E-Sensing for Food Chain Quality and Safety. Sensors 2023, 23, 8429. [Google Scholar] [CrossRef] [PubMed]
- Firooz, A.A.; Mahjoub, A.R.; Khodadadi, A.A. Highly Sensitive CO and Ethanol Nanoflower-like SnO2 Sensor Among Various Morphologies Obtained by Using Single and Mixed Ionic Surfactant Templates. Sens. Actuators B Chem. 2009, 141, 89–96. [Google Scholar] [CrossRef]
- Singh, N.; Ponzoni, A.; Gupta, R.K.; Lee, P.S.; Comini, E. Synthesis of In2O3–ZnO Core–Shell Nanowires and Their Application in Gas Sensing. Sens. Actuators B Chem. 2011, 160, 1346–1351. [Google Scholar] [CrossRef]
- Schütze, A.; Baur, T.; Leidinger, M.; Reimringer, W.; Jung, R.; Conrad, T.; Sauerwald, T. Highly Sensitive and Selective VOC Sensor Systems Based on Semiconductor Gas Sensors: How to? Environments 2017, 4, 20. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; Díaz de León-Martínez, L.; Gorocica-Rosete, P.; Padilla, R.P.; Thirión-Romero, I.; Ornelas-Rebolledo, O.; Flores-Ramírez, R. Identification of Breath-Prints for the COPD Detection Associated with Smoking and Household Air Pollution by Electronic Nose. Respir. Med. 2020, 163, 105901. [Google Scholar] [CrossRef] [PubMed]
- Kanan, S.; Obeideen, K.; Moyet, M.; Abed, H.; Khan, D.; Shabnam, A.; El-Sayed, Y.; Arooj, M.; Mohamed, A.A. Recent Advances on Metal Oxide-Based Sensors for Environmental Gas Pollutants Detection. Crit. Rev. Anal. Chem. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Isaac, N.A.; Pikaar, I.; Biskos, G. Metal Oxide Semiconducting Nanomaterials for Air Quality Gas Sensors: Operating Principles, Performance, and Synthesis Techniques. Mikrochim. Acta 2022, 189, 196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semiconductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tisch, U.; Haick, H. Chemical Sensors for Breath Gas Analysis: The Latest Developments at the Breath Analysis Summit 2013. J. Breath Res. 2014, 8, 027103. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, M.; Zonta, G.; Malagù, C.; Anania, G.; Rispoli, G. MOX Nanosensors to Detect Colorectal Cancer Relapses from Patient’s Blood at Three Years Follow-Up, and Gender Correlation. Biosensors 2025, 15, 56. [Google Scholar] [CrossRef]
- Wilson, A.D. Developments of Recent Applications for Early Diagnosis of Diseases Using Electronic-Nose and Other VOC-Detection Devices. Sensors 2023, 23, 7885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ponzoni, A.; Comini, E.; Concina, I.; Ferroni, M.; Falasconi, M.; Gobbi, E.; Sberveglieri, V.; Sberveglieri, G. Nanostructured Metal Oxide Gas Sensors, A Survey of Applications Carried Out at SENSOR Lab, Brescia (Italy) in the Security and Food Quality Fields. Sensors 2012, 12, 17023–17045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mariotti, R.; Núñez-Carmona, E.; Genzardi, D.; Pandolfi, S.; Sberveglieri, V.; Mousavi, S. Volatile Olfactory Profiles of Umbrian Extra Virgin Olive Oils and Their Discrimination through MOX Chemical Sensors. Sensors 2022, 22, 7164. [Google Scholar] [CrossRef]
- Rüffer, D.; Hoehne, F.; Bühler, J. New Digital Metal-Oxide (MOx) Sensor Platform. Sensors 2018, 18, 1052. [Google Scholar] [CrossRef]
- Collier-Oxandale, A.M.; Thorson, J.; Halliday, H.; Milford, J.; Hannigan, M. Understanding the ability of low-cost MOx sensors to quantify ambient VOCs. Atmos. Meas. Tech. 2019, 12, 1441–1460. [Google Scholar] [CrossRef]
- Patil, A.G.; Pramanick, B.; Madhukar, A. MOS Based Gas Sensors for Monitoring of Air Pollution: A Review. IEEE Sens. J. 2025, 25, 9250–9262. [Google Scholar] [CrossRef]
- Sun, Y.; Milando, C.W.; Spangler, K.R.; Wei, Y.; Schwartz, J.; Dominici, F.; Nori-Sarma, A.; Sun, S.; Wellenius, G.A. Short term exposure to low level ambient fine particulate matter and natural cause, cardiovascular, and respiratory morbidity among US adults with health insurance: Case time series study. BMJ 2024, 384, e076322. [Google Scholar] [CrossRef]
- Varon, J.; Marik, P.E.; Fromm, R.E., Jr.; Gueler, A. Carbon monoxide poisoning: A review for clinicians. J. Emerg. Med. 1999, 17, 87–93. [Google Scholar] [CrossRef]
- Nandy, T.; Coutu, R.A., Jr.; Ababei, C. Carbon Monoxide Sensing Technologies for Next-Generation Cyber-Physical Systems. Sensors 2018, 18, 3443. [Google Scholar] [CrossRef]
- Seo, M.H.; Yuasa, M.; Kida, T.; Huh, J.S.; Yamazoe, N.; Shimanoe, K. Microstructure control of TiO2 nanotubular films for improved VOC sensing. Sens. Actuators B Chem. 2011, 154, 251–256. [Google Scholar] [CrossRef]
- Menini, P.; Parret, F.; Guerrero, M.; Soulantica, K.; Erades, L.; Maisonnat, A.; Chaudret, B. CO response of a nanostructured SnO2 gas sensor doped with palladium and platinum. Sens. Actuators B Chem. 2004, 103, 111–114. [Google Scholar] [CrossRef]
- Kolmakov, A.; Zhang, Y.; Cheng, G.; Moskovits, M. Detection of CO and O2 using tin oxide nanowire sensors. Adv. Mater. 2003, 15, 997–1000. [Google Scholar] [CrossRef]
- Du, X.; George, S.M. Thickness dependence of sensor response for CO gas sensing by tin oxide films grown using atomic layer deposition. Sens. Actuators B Chem. 2008, 135, 152–160. [Google Scholar] [CrossRef]
- Tischner, A.; Maier, T.; Stepper, C.; Köck, A. Ultrathin SnO2 gas sensors fabricated by spray pyrolysis for the detection of humidity and carbon monoxide. Sens. Actuators B Chem. 2008, 134, 796–802. [Google Scholar] [CrossRef]
- Yuasa, M.; Masaki, T.; Kida, T.; Shimanoe, K.; Yamazoe, N. Nano-sized PdO loaded SnO2 nanoparticles by reverse micelle method for highly sensitive CO gas sensor. Sens. Actuators B Chem. 2009, 136, 99–104. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Huang, J.; Wang, Y.; Kong, F.; Wu, S.; Zhang, S.; Huang, W. Preparation and CO gas-sensing behavior of Au-doped SnO2 sensors. Vacuum 2006, 81, 394–397. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K.; Gulina, L.; Tolstoy, V. SnO2 thin films modified by the SnO2–Au nanocomposites: Response to reducing gases. Sens. Actuators B Chem. 2009, 141, 610–616. [Google Scholar] [CrossRef]
- Wang, C.T.; Chen, M.T. Vanadium-promoted tin oxide semiconductor carbon monoxide gas sensors. Sens. Actuators B Chem. 2010, 150, 360–366. [Google Scholar] [CrossRef]
- Kim, H.; Park, C.S.; Kang, K.M.; Hong, M.H.; Choi, Y.J.; Park, H.H. The CO gas sensing properties of direct-patternable SnO2 films containing graphene or Ag nanoparticles. New J. Chem. 2015, 39, 2256–2260. [Google Scholar] [CrossRef]
- Park, J.A.; Moon, J.; Lee, S.J.; Kim, S.H.; Zyung, T.; Chu, H.Y. Structure and CO gas sensing properties of electrospun TiO2 nanofibers. Mater. Lett. 2010, 64, 255–257. [Google Scholar] [CrossRef]
- Moon, H.G.; Shim, Y.S.; Jang, H.W.; Kim, J.S.; Choi, K.J.; Kang, C.Y.; Choi, J.W.; Park, H.H.; Yoon, S.J. Highly sensitive CO sensors based on cross-linked TiO2 hollow hemispheres. Sens. Actuators B Chem. 2010, 149, 116–121. [Google Scholar] [CrossRef]
- Lee, J.S.; Ha, T.J.; Hong, M.H.; Park, H.H. The effect of porosity on the CO sensing properties of TiO2 xerogel thin films. Thin Solid Films 2013, 529, 98–102. [Google Scholar] [CrossRef]
- Rao, L.L.R.; Singha, M.K.; Subramaniam, K.M.; Jampana, N.; Asokan, S. Molybdenum microheaters for MEMS-based gas sensor applications: Fabrication, electro-thermo-mechanical and response characterization. IEEE Sens. J. 2017, 17, 22–29. [Google Scholar]
- Lee, J.S.; Ha, T.J.; Hong, M.H.; Park, C.S.; Park, H.H. The effect of multiwalled carbon nanotube doping on the CO gas sensitivity of TiO2 xerogel composite film. Appl. Surf. Sci. 2013, 269, 125–128. [Google Scholar] [CrossRef]
- Chang, J.F.; Kuo, H.H.; Leu, I.C.; Hon, M.H. The effects of thickness and operation temperature on ZnO: Al thin film CO gas sensor. Sens. Actuators B Chem. 2002, 84, 258–264. [Google Scholar] [CrossRef]
- Hjiri, M.; El Mir, L.; Leonardi, S.G.; Pistone, A.; Mavilia, L.; Neri, G. Al-doped ZnO for highly sensitive CO gas sensors. Sens. Actuators B Chem. 2014, 196, 413–420. [Google Scholar] [CrossRef]
- Gong, H.; Hu, J.Q.; Wang, J.H.; Ong, C.H.; Zhu, F.R. Nano-crystalline Cu-doped ZnO thin film gas sensor for CO. Sens. Actuators B Chem. 2006, 115, 247–251. [Google Scholar] [CrossRef]
- Wei, S.; Yu, Y.; Zhou, M. CO gas sensing of Pd-doped ZnO nanofibers synthesized by electrospinning method. Mater. Lett. 2010, 64, 2284–2286. [Google Scholar] [CrossRef]
- Dhahri, R.; Hjiri, M.; El Mir, L.; Alamri, H.; Bonavita, A.; Iannazzo, D.; Leonardi, S.G.; Neri, G. CO Sensing Characteristics of In-Doped ZnO Semiconductor Nanoparticles. J. Sci. Adv. Mater. Devices 2017, 2, 34–40. [Google Scholar] [CrossRef]
- Kim, K.; Song, Y.W.; Chang, S.; Kim, I.H.; Kim, S.; Lee, S.Y. Fabrication and characterization of Ga-doped ZnO nanowire gas sensor for the detection of CO. Thin Solid Films 2009, 518, 1190–1193. [Google Scholar] [CrossRef]
- Kuhaili, M.F.; Durrani, S.M.A.; Bakhtiari, I.A. Carbon monoxide gas-sensing properties of CeO2–ZnO thin films. Appl. Surf. Sci. 2008, 255, 3033–3039. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Wen, Z.; Zhu, L.; Li, Y.; Zhang, Z.; Ye, Z. Mesoporous Co3O4 nanoneedle arrays for high-performance gas sensor. Sens. Actuators B Chem. 2014, 203, 873–879. [Google Scholar] [CrossRef]
- Ackermann-Liebrich, U. Respiratory and Cardiovascular Effects of NO2 in Epidemiological Studies. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 840–844. ISBN 9780444522726. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, K.K.; Yang, G.P.; Li, P.F.; Liu, C.Y.; Ingeniero, R.C.O.; Bange, H.W. Continuous Chemiluminescence Measurements of Dissolved Nitric Oxide (NO) and Nitrogen Dioxide (NO2) in the Ocean Surface Layer of the East China Sea. Environ. Sci. Technol. 2021, 55, 3668–3675. [Google Scholar] [CrossRef] [PubMed]
- Russell, H.S.; Frederickson, L.B.; Kwiatkowski, S.; Emygdio, A.P.M.; Kumar, P.; Schmidt, J.A.; Hertel, O.; Johnson, M.S. Enhanced Ambient Sensing Environment—A New Method for Calibrating Low-Cost Gas Sensors. Sensors 2022, 22, 7238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fazio, E.; Spadaro, S.; Corsaro, C.; Neri, G.; Leonardi, S.G.; Neri, F.; Lavanya, N.; Sekar, C.; Donato, N.; Neri, G. Metal-Oxide Based Nanomaterials: Synthesis, Characterization and Their Applications in Electrical and Electrochemical Sensors. Sensors 2021, 21, 2494. [Google Scholar] [CrossRef]
- Forleo, A.; Francioso, L.; Capone, S.; Siciliano, P.; Lommens, P.; Hens, Z. Synthesis and gas sensing properties of ZnO quantum dots. Sens. Actuators B 2010, 146, 111–115. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Baratto, C.; Comini, E.; Faglia, G.; Ferroni, M.; Ponzoni, A.; Vomiero, A. Synthesis and characterization of semiconducting nanowires for gas sensing. Sens. Actuators B 2007, 121, 208–213. [Google Scholar] [CrossRef]
- Dvorak, J.; Jirsak, T.; Rodriguez, J.A. Fundamental Studies of Desulfurization Processes: Reaction of Methanethiol on ZnO and Cs/ZnO. Surf. Sci. 2001, 479, 155–168. [Google Scholar] [CrossRef]
- Waclawik, E.R.; Chang, J.; Ponzoni, A.; Concina, I.; Zappa, D.; Comini, E.; Motta, N.; Faglia, G.; Sberveglieri, G. Functionalised Zinc Oxide Nanowire Gas Sensors: Enhanced NO2 Gas Sensor Response by Chemical Modification of Nanowire Surfaces. Beilstein J. Nanotechnol. 2012, 3, 368–377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, G.; Shin, H.; Jeon, S.W.; Lim, Y.H.; Hong, S.; Kim, D.H.; Lee, J.H. Transducer-Aware Hydroxy-Rich-Surface Indium Oxide Gas Sensor for Low-Power and High-Sensitivity NO2 Gas Sensing. ACS Appl. Mater. Interfaces 2023, 15, 22651–22661. [Google Scholar] [CrossRef]
- Spirito, P. Elettronica Digitale; McGraw-Hill Libri Italia SRL: Milano, Italy, 2006; ISBN 978-88-386-6323-9. [Google Scholar]
- Field Effect Transistor (JPG), in Elettronica 2000, n. 7; MK Periodici: Milano, Italy, 1979; pp. 72–76, OCLC 955598089.
- Sicard, P.; Agathokleous, E.; Anenberg, S.C.; De Marco, A.; Paoletti, E.; Calatayud, V. Trends in Urban Air Pollution Over the Last Two Decades: A Global Perspective. Sci. Total Environ. 2023, 858 Pt 2, 160064. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, M.; Gerboles, M. Review of Small Commercial Sensors for Indicative Monitoring of Ambient Gas. Chem. Eng. Trans. 2012, 30, 169–174. [Google Scholar] [CrossRef]
- Castell, N.; Viana, M.; Minguillón, M.C.; Guerreiro, C.; Querol, X. Real-World Application of New Sensor Technologies for Air Quality Monitoring. 2013. Available online: http://acm.eionet.europa.eu/docs/ETCACM_TP_2013_16_new_AQ_SensorTechn.pdf (accessed on 26 February 2025).
- Huda, R.K.; Kumar, P.; Gupta, R.; Sharma, A.K.; Toteja, G.S.; Babu, B.V. Air Quality Monitoring Using Low-Cost Sensors in Urban Areas of Jodhpur, Rajasthan. Int. J. Environ. Res. Public Health 2024, 21, 623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Spinelle, L.; Gerboles, M.; Aleixandre, M.; Bonavitacola, F. Evaluation of Metal Oxides Sensors for the Monitoring of O3 in Ambient Air at ppb Level. Chem. Eng. Trans. 2016, 54, 319–324. [Google Scholar] [CrossRef]
- Rusinek, R.; Żytek, A.; Stasiak, M.; Wiącek, J.; Gancarz, M. Application of MOX Sensors to Determine the Emission of Volatile Compounds in Corn Groats as a Function of Vertical Pressure in the Silo and Moisture Content of the Bed. Sensors 2024, 24, 2187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, H.; Othman, M.H.D.; Kek, H.Y.; Chong, W.T.; Nyakuma, B.B.; Wahab, R.A.; Teck, G.L.H.; Wong, K.Y. Revolutionizing Indoor Air Quality Monitoring through IoT Innovations: A Comprehensive Systematic Review and Bibliometric Analysis. Environ. Sci. Pollut. Res. Int. 2024, 31, 44463–44488. [Google Scholar] [CrossRef] [PubMed]
- Folifack Signing, V.R.; Mbarndouka Taamté, J.; Kountchou Noube, M.; Hamadou Yerima, A.; Azzopardi, J.; Tchuente Siaka, Y.F.; Saïdou. IoT-Based Monitoring System and Air Quality Prediction Using Machine Learning for a Healthy Environment in Cameroon. Environ. Monit. Assess. 2024, 196, 621. [Google Scholar] [CrossRef] [PubMed]
- Barcelo-Ordinas, J.M.; Ferrer-Cid, P.; Garcia-Vidal, J.; Viana, M.; Ripoll, A. H2020 Project CAPTOR Dataset: Raw Data Collected by Low-Cost MOX Ozone Sensors in a Real Air Pollution Monitoring Network. Data Brief 2021, 36, 107127. [Google Scholar] [CrossRef]
- Morawska, L.; Thai, P.K.; Liu, X.; Asumadu-Sakyi, A.; Ayoko, G.; Bartonova, A.; Bedini, A.; Chai, F.; Christensen, B.; Dunbabin, M.; et al. Applications of Low-Cost Sensing Technologies for Air Quality Monitoring and Exposure Assessment: How Far Have They Gone? Environ. Int. 2018, 116, 286–299. [Google Scholar] [CrossRef]
- Freeman, T.; Cudmore, R. Review of Odour Management in New Zealand; Air Quality Technical Report 24; New Zealand Ministry of Environment: Wellington, New Zealand, 2002; p. 163.
- Botticini, S.; Comini, E.; Dello Iacono, S.; Flammini, A.; Gaioni, L.; Galliani, A.; Ghislotti, L.; Lazzaroni, P.; Re, V.; Sisinni, E.; et al. Index Air Quality Monitoring for Light and Active Mobility. Sensors 2024, 24, 3170. [Google Scholar] [CrossRef]
- Rydosz, A. The Use of Copper Oxide Thin Films in Gas-Sensing Applications. Coatings 2018, 8, 425. [Google Scholar] [CrossRef]
- Kong, Y.; Teather, R.M.; Forster, R.J. Biodiversity and Composition of Methanogenic Populations in the Rumen of Cows Fed Alfalfa Hay or Triticale Straw. FEMS Microbiol. Ecol. 2013, 84, 302–315. [Google Scholar] [CrossRef]
- Knapp, J.R.; Laur, G.L.; Vadas, P.A.; Weiss, W.P.; Tricarico, J.M. Enteric Methane in Dairy Cattle Production: Quantifying the Opportunities and Impact of Reducing Emissions. J. Dairy Sci. 2014, 97, 3231–3261. [Google Scholar] [CrossRef]
- Hill, J.; McSweeney, C.; Wright, A.G.; Bishop-Hurley, G.; Kalantar-Zadeh, K. Measuring Methane Production from Ruminants. Trends Biotechnol. 2016, 34, 26–35. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Abdel-Rahim, M.A. CuO Nanoparticles Synthesized by Microwave-Assisted Method for Methane Sensing. Opt. Quantum Electron. 2016, 48, 531. [Google Scholar] [CrossRef]
- Lakhmi, R.; Fischer, M.; Darves-Blanc, Q.; Alrammouz, R.; Rieu, M.; Viricelle, J.P. Linear and Non-Linear Modelling Methods for a Gas Sensor Array Developed for Process Control Applications. Sensors 2024, 24, 3499. [Google Scholar] [CrossRef]
- Chesler, P.; Hornoiu, C.; Anastasescu, M.; Calderon-Moreno, J.M.; Gheorghe, M.; Gartner, M. Cobalt- and Copper-Based Chemiresistors for Low Concentration Methane Detection, a Comparison Study. Gels 2022, 8, 721. [Google Scholar] [CrossRef]
- Aroca, A.; Gotor, C.; Bassham, D.C. Hydrogen Sulfide: From a Toxic Molecule to a Key Molecule of Cell Life. Antioxidants 2020, 9, 621. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, Z.; Li, Z.; Xie, L.; Wu, Y.; Zheng, L. Heterostructure of CuO Microspheres Modified with CuFe2O4 Nanoparticles for Highly Sensitive H2S Gas Sensor. Sens. Actuators B Chem. 2018, 264, 139–149. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent Advances in Electrochemical Sensors for Detecting Toxic Gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef]
- Quang, P.L.; Cuong, N.D.; Hoa, T.T.; Long, H.T.; Hung, C.M.; Le, D.T.T.; Hieu, N.V. Simple Post-Synthesis of Mesoporous p-Type Co3O4 Nanochains for Enhanced H2S Gas Sensing Performance. Sens. Actuators B Chem. 2018, 270, 158–166. [Google Scholar] [CrossRef]
- Guo, L.; Wang, M.; Cao, D. A Novel Zr-MOF as Fluorescence Turn-On Probe for Real-Time Detecting H2S Gas and Fingerprint Identification. Small 2018, 14, 1703822. [Google Scholar] [CrossRef]
- Jo, S.B.; Kim, H.J.; Ahn, J.H.; Hwang, B.W.; Huh, J.S.; Ragupathy, D.; Lee, S.C.; Kim, J.C. Effects of Thin-Film Thickness on Sensing Properties of SnO2-Based Gas Sensors for the Detection of H2S Gas at ppm Levels. J. Nanosci. Nanotechnol. 2020, 20, 7169–7174. [Google Scholar] [CrossRef]
- Heranjal, S.; Maciel, M.; Kamalapally, S.N.R.; Ramrakhiani, I.; Schulz, E.; Cao, S.; Liu, X.; Relich, R.F.; Wek, R.; Woollam, M.; et al. Establishing Healthy Breath Baselines with Tin Oxide Sensors: Fundamental Building Blocks for Noninvasive Health Monitoring. Mil. Med. 2024, 189 (Suppl. S3), 221–229. [Google Scholar] [CrossRef]
- Glöckler, J.; Jaeschke, C.; Padilla, M.; Mitrovics, J.; Mizaikoff, B. Ultratrace eNose Sensing of VOCs toward Breath Analysis Applications Utilizing an eNose-Based Analyzer. ACS Meas. Sci. Au 2024, 4, 184–187. [Google Scholar] [CrossRef]
- Glöckler, J.; Jaeschke, C.; Kocaöz, Y.; Kokoric, V.; Tütüncü, E.; Mitrovics, J.; Mizaikoff, B. iHWG-MOX: A Hybrid Breath Analysis System via the Combination of Substrate-Integrated Hollow Waveguide Infrared Spectroscopy with Metal Oxide Gas Sensors. ACS Sens. 2020, 5, 1033–1039. [Google Scholar] [CrossRef]
- Dragonieri, S.; Annema, J.T.; Schot, R.; van der Schee, M.P.; Spanevello, A.; Carratú, P.; Resta, O.; Rabe, K.F.; Sterk, P.J. An Electronic Nose in the Discrimination of Patients with Non-Small Cell Lung Cancer and COPD. Lung Cancer 2009, 64, 166–170. [Google Scholar] [CrossRef]
- Santos, F.; Magalhães, S.; Henriques, M.C.; Fardilha, M.; Nunes, A. Spectroscopic Features of Cancer Cells: FTIR Spectroscopy as a Tool for Early Diagnosis. Curr. Metabolomics 2018, 6, 103–111. [Google Scholar] [CrossRef]
- Derveaux, E.; Van Brussel, T.; Van Weyenbergh, J.; Lievens, Y.; De Neve, W.; Derua, R.; Dhaene, T.; De Preter, K.; Bockstal, M.; Bosteels, C.; et al. NMR-Metabolomics Reveals a Metabolic Shift after Surgical Resection of Non-Small Cell Lung Cancer. Cancers 2023, 15, 2127. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of Acetone in Human Breath by Gas Chromatography–Mass Spectrometry and Solid-Phase Microextraction with on-Fiber Derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Si:WO3 Sensors for Highly Selective Detection of Acetone for Easy Diagnosis of Diabetes by Breath Analysis. Anal. Chem. 2010, 82, 3581–3587. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.Z. Diabetes: A Look to the Future. Lancet Diabetes Endocrinol. 2014, 2, e1–e2. [Google Scholar] [CrossRef]
- Rydosz, A. A Negative Correlation between Blood Glucose and Acetone Measured in Healthy and Type 1 Diabetes Mellitus Patient Breath. J. Diabetes Sci. Technol. 2015, 9, 881–884. [Google Scholar] [CrossRef]
- Jiang, L.; Lv, S.; Tang, W.; Zhao, L.; Wang, C.; Wang, J.; Wang, T.; Guo, X.; Liu, F.; Wang, C.; et al. YSZ-Based Acetone Sensor Using a Cd2SnO4 Sensing Electrode for Exhaled Breath Detection in Medical Diagnosis. Sens. Actuators B Chem. 2021, 345, 130321. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, A.; Chang, H.; Xia, B. Room-Temperature High-Performance Acetone Gas Sensor Based on Hydrothermal Synthesized SnO2-Reduced Graphene Oxide Hybrid Composite. RSC Adv. 2015, 5, 3016–3022. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y. Ultrasensitive and Low Detection Limit of Acetone Gas Sensor Based on ZnO/SnO2 Thick Films. RSC Adv. 2020, 10, 35958–35965. [Google Scholar] [CrossRef]
- Deng, Y. Semiconducting Metal Oxides for Gas Sensing; Springer: Singapore, 2019; ISBN 978-981-13-5852-4. [Google Scholar]
- Mazabuel-Collazos, A.; Rodríguez-Páez, J.E. Chemical Synthesis and Characterization of ZnO–TiO2 Semiconductor Nanocomposites: Tentative Mechanism of Particle Formation. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1739–1752. [Google Scholar] [CrossRef]
- Navale, S.T.; Yang, Z.B.; Liu, C.; Cao, P.J.; Patil, V.B.; Ramgir, N.S.; Mane, R.S.; Stadler, F.J. Enhanced Acetone Sensing Properties of Titanium Dioxide Nanoparticles with a Sub-Ppm Detection Limit. Sens. Actuators B Chem. 2018, 255, 1701–1710. [Google Scholar] [CrossRef]
- Cheng, X.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Ag Nanoparticles Modified TiO2 Spherical Heterostructures with Enhanced Gas-Sensing Performance. Sens. Actuators B Chem. 2011, 155, 716–721. [Google Scholar] [CrossRef]
- Bhowmik, B.; Manjuladevi, V.; Gupta, R.K.; Bhattacharyya, P. Highly Selective Low-Temperature Acetone Sensor Based on Hierarchical 3-D TiO2 Nanoflowers. IEEE Sens. J. 2016, 16, 3488–3495. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas Sensors Based on TiO2 Nanostructured Materials for the Detection of Hazardous Gases: A Review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Lou, Z.; Li, F.; Deng, J.; Wang, L.; Zhang, T. Branch-like Hierarchical Heterostructure (α-Fe2O3/TiO2): A Novel Sensing Material for Trimethylamine Gas Sensor. ACS Appl. Mater. Interfaces 2013, 5, 12310–12316. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Myung, J. ha Highly Selective Detection of Acetone by TiO2-SnO2 Heterostructures for Environmental Biomarkers of Diabetes. Sens. Actuators B Chem. 2021, 349, 130733. [Google Scholar] [CrossRef]
- Muñoz-Bolaños, J.D.; Rodríguez-Páez, J.E. WO3 Mono-Nanocrystals: Synthesis, Characterization and Evaluation of Their Electrical Behavior in Oxygen and Acetone Atmospheres. Mater. Sci. Eng. B 2021, 274, 115472. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, B.; Liu, W.; Zhou, X.; Liu, M.; Sun, X.; Lv, J.; Zhang, L.; Xu, W.; Bai, X.; et al. Highly Sensitive and Selective Acetone Sensor Based on Three-Dimensional Ordered WO3/Au Nanocomposite with Enhanced Performance. Sens. Actuators B Chem. 2020, 320, 128405. [Google Scholar] [CrossRef]
- Zahmouli, N.; Hjiri, M.; Leonardi, S.G.; El Mir, L.; Neri, G.; Iannazzo, D.; Espro, C.; Aida, M.S. High Performance Gd-Doped γ-Fe2O3 Based Acetone Sensor. Mater. Sci. Semicond. Process. 2020, 116, 105154. [Google Scholar] [CrossRef]
- Kohli, N.; Hastir, A.; Kumari, M.; Singh, R.C. Hydrothermally Synthesized Heterostructures of In2O3/MWCNT as Acetone Gas Sensor. Sens. Actuators A Phys. 2020, 314, 112240. [Google Scholar] [CrossRef]
- Harvey, S.P.; Mason, T.O.; Gassenbauer, Y.; Schafranek, R.; Klein, A. Surface versus Bulk Electronic/Defect Structures of Transparent Conducting Oxides: I. Indium Oxide and ITO. J. Phys. D Appl. Phys. 2006, 39, 3959–3968. [Google Scholar] [CrossRef]
- Wang, J.-C.; Shi, W.; Sun, X.-Q.; Wu, F.-Y.; Li, Y.; Hou, Y. Enhanced Photo-Assisted Acetone Gas Sensor and Efficient Photocatalytic Degradation Using Fe-Doped Hexagonal and Monoclinic WO3 Phase−Junction. Nanomaterials 2020, 10, 398. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Zhang, J.; Li, G.; Leng, D.; Gao, Y.; Gao, J.; Lu, H.; Li, X. Metal–Organic Framework-Derived Cu2O–CuO Octahedrons for Sensitive and Selective Detection of Ppb-Level NO2 at Room Temperature. Sens. Actuators B Chem. 2021, 328, 129045. [Google Scholar] [CrossRef]
- Choi, Y.M.; Cho, S.-Y.; Jang, D.; Koh, H.-J.; Choi, J.; Kim, C.-H.; Jung, H.-T. Ultrasensitive Detection of VOCs Using a High-Resolution CuO/Cu2O/Ag Nanopattern Sensor. Adv. Funct. Mater. 2019, 29, 1808319. [Google Scholar] [CrossRef]
- Van Duy, L.; Van Duy, N.; Hung, C.M.; Hoa, N.D.; Dich, N.Q. Urea Mediated Synthesis and Acetone-Sensing Properties of Ultrathin Porous ZnO Nanoplates. Mater. Today Commun. 2020, 25, 101445. [Google Scholar] [CrossRef]
- Ochoa-Muñoz, Y.H.; Mejía de Gutiérrez, R.; Rodríguez-Páez, J.E. Metal Oxide Gas Sensors to Study Acetone Detection Considering Their Potential in the Diagnosis of Diabetes: A Review. Molecules 2023, 28, 1150. [Google Scholar] [CrossRef]
- Cai, L.; Dong, X.; Wu, G.; Sun, J.; Chen, N.; Wei, H.; Zhu, S.; Tian, Q.; Wang, X.; Jing, Q.; et al. Ultrasensitive Acetone Gas Sensor Can Distinguish the Diabetic State of People and Its High-Performance Analysis by First-Principles Calculation. Sens. Actuators B Chem. 2022, 351, 130863. [Google Scholar] [CrossRef]
- Krishnan, S.T.; Devadhasan, J.P.; Kim, S. Recent Analytical Approaches to Detect Exhaled Breath Ammonia with Special Reference to Renal Patients. Anal. Bioanal. Chem. 2017, 409, 21–31. [Google Scholar] [CrossRef]
- Anderson, J.C.; Lamm, W.J.E.; Hlastala, M.P. Measuring Airway Exchange of Endogenous Acetone Using a Single-Exhalation Breathing Maneuver. J. Appl. Physiol. 2006, 100, 880–889. [Google Scholar] [CrossRef]
- Statheropoulos, M.; Agapiou, A.; Georgiadou, A. Analysis of Expired Air of Fasting Male Monks at Mount Athos. J. Chromatogr. B 2006, 832, 274–279. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, F.; Chen, Y.; Liu, J.; Sun, Y.; Luo, T.; Li, M.; Liu, J. A Novel Ammonia Sensor Based on High Density, Small Diameter Polypyrrole Nanowire Arrays. Sens. Actuators B Chem. 2009, 142, 204–209. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, M.; Zheng, K.; Ye, W.; Zhang, S.; Wang, B.; Long, X. High-Performance Room Temperature Ammonia Sensors Based on Pure Organic Molecules Featuring B–N Covalent Bond. Adv. Sci. 2024, 11, e2308483. [Google Scholar] [CrossRef]
- Chen, C.C.; Hsieh, J.C.; Chao, C.H.; Yang, W.S.; Cheng, H.T.; Chan, C.K.; Lu, C.J.; Meng, H.F.; Zan, H.W. Correlation between Breath Ammonia and Blood Urea Nitrogen Levels in Chronic Kidney Disease and Dialysis Patients. J. Breath Res. 2020, 14, 036002. [Google Scholar] [CrossRef]
- Jayasree, T.; Bobby, M.; Muttan, S. Sensors for Detecting Ammonia from the Exhaled Breath of Renal Disorder Patients. Sensor Lett. 2015, 13, 1003–1008. [Google Scholar] [CrossRef]
- Jayasree, T.; Bobby, M.; Muttan, S. Sensor Data Classification for Renal Dysfunction Patients Using Support Vector Machine. J. Med. Biol. Eng. 2015, 35, 759–764. [Google Scholar] [CrossRef]
- Kumar, G.; Mishra, S.; Jain, A. Development of Breath Ammonia Analysis System for Disease Diagnosis. Asian J. Biochem. Pharm. Res. 2013, 3, 36–43. [Google Scholar]
- Jayasree, T.; Muttan, S. Study of Gas Sensors for the Detection of Volatile Organic Compounds in Breath. Appl. Mech. Mater. 2014, 573, 785–790. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath Analysis by Nanostructured Metal Oxides as Chemo-Resistive Gas Sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Senesac, L.; Thundat, T.G. Nanosensors for Trace Explosive Detection. Mater. Today 2008, 11, 28–36. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, D.; Li, N.; Zhang, L.; Yang, J. Diabetes Identification and Classification by Means of a Breath Analysis System; Springer Nature: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Fens, N.; van der Schee, M.P.; Brinkman, P.; Sterk, P.J. Exhaled Breath Analysis by Electronic Nose in Airways Disease. Established Issues and Key Questions. Clin. Exp. Allergy 2013, 43, 705–715. [Google Scholar] [CrossRef]
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of Volatile Lung Cancer Markers by Gas Chromatography-Mass Spectrometry: Comparison with Discrimination by Canines. Anal. Bioanal. Chem. 2012, 404, 141–146. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Astolfi, M.; Rispoli, G.; Anania, G.; Artioli, E.; Nevoso, V.; Zonta, G.; Malagù, C. Tin, Titanium, Tantalum, Vanadium and Niobium Oxide-Based Sensors to Detect Colorectal Cancer Exhalations in Blood Samples. Molecules 2021, 26, 466. [Google Scholar] [CrossRef] [PubMed]
- Poļaka, I.; Mežmale, L.; Anarkulova, L.; Kononova, E.; Vilkoite, I.; Veliks, V.; Ļeščinska, A.M.; Stonāns, I.; Pčolkins, A.; Tolmanis, I.; et al. The Detection of Colorectal Cancer through Machine Learning-Based Breath Sensor Analysis. Diagnostics 2023, 13, 3355. [Google Scholar] [CrossRef] [PubMed]
- Bax, C.; Robbiani, S.; Zannin, E.; Capelli, L.; Ratti, C.; Bonetti, S.; Novelli, L.; Raimondi, F.; Di Marco, F.; Dellacà, R.L. An Experimental Apparatus for E-Nose Breath Analysis in Respiratory Failure Patients. Diagnostics 2022, 12, 776. [Google Scholar] [CrossRef]
- Wanniarachchi, P.C.; Kumarasinghe, K.G.U.; Jayathilake, C. Recent Advancements in Chemosensors for the Detection of Food Spoilage. Food Chem. 2024, 436, 137733. [Google Scholar] [CrossRef]
- Park, J.-C.; Yuk, Y.; Lee, S. Recent Trends in Gas Sensors for Food Spoilage Monitoring. J. Sens. Sci. Technol. 2024, 33, 412–418. [Google Scholar] [CrossRef]
- Li, H.-Y.; Huang, L.; Wang, X.-X.; Lee, C.-S.; Yoon, J.-W.; Zhou, J.; Guo, X.; Lee, J.-H. Molybdenum Trioxide Nanopaper as a Dual Gas Sensor for Detecting Trimethylamine and Hydrogen Sulfide. RSC Adv. 2017, 7, 3680–3685. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, W.; Wei, J.; Lan, K.; Yan, S.; Chen, R.; Qin, G. Ultrasensitive Flexible Olfactory Receptor-Derived Peptide Sensor for Trimethylamine Detection by the Bending Connection Method. ACS Sens. 2022, 7, 3513–3520. [Google Scholar] [CrossRef]
- Genzardi, D.; Núñez Carmona, E.; Poeta, E.; Gai, F.; Caruso, I.; Fiorilla, E.; Schiavone, A.; Sberveglieri, V. Unraveling the Chicken Meat Volatilome with Nanostructured Sensors: Impact of Live and Dehydrated Insect Larvae Feeding. Sensors 2024, 24, 4921. [Google Scholar] [CrossRef]
- Chung, J.H.; Cho, Y.-H.; Hwang, J.; Lee, S.H.; Lee, S.; Park, S.-H.; Sohn, S.; Cho, D.; Lee, K.; Shim, Y.-S. Heterostructures of SnO2-Decorated Cr2O3 Nanorods for Highly Sensitive H2S Detection. J. Sens. Sci. Technol. 2024, 33, 40–47. [Google Scholar] [CrossRef]
- Shu, J.; Qiu, Z.; Lv, S.; Zhang, K.; Tang, D. Cu2+-Doped SnO2 Nanograin/Polypyrrole Nanospheres with Synergic Enhanced Properties for Ultrasensitive Room-Temperature H2S Gas Sensing. Anal. Chem. 2017, 89, 11135–11142. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Qiu, L.; Wang, T.; Yu, H.; Yang, W.; Dong, X.; Yang, Y. P–N Heterojunction Formation: Metal Sulfide@Metal Oxide Chemiresistor for ppb H2S Detection from Exhaled Breath and Food Spoilage at Flexible Room Temperature. ACS Sens. 2024, 9, 3433–3443. [Google Scholar] [CrossRef]
- Sholehah, A.; Karmala, K.; Huda, N.; Utari, L.; Septiani, N.L.W.; Yuliarto, B. Structural Effect of ZnO-Ag Chemoresistive Sensor on Flexible Substrate for Ethylene Gas Detection. Sens. Actuators A Phys. 2021, 331, 112934. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.M.; Oh, M.-H.; Huh, Y.S.; Jang, H.W. Food Quality Assessment Using Chemoresistive Gas Sensors: Achievements and Future Perspectives. Sustain. Food Technol. 2024, 2, 266–280. [Google Scholar] [CrossRef]
- Dang, T.T.L.; Tran, V.D.N.; Chu, T.X.; Chu, M.H.; Nguyen, V.D.; Nguyen, D.H. Eco-Friendly Facile Synthesis of Co3O4–Pt Nanorods for Ethylene Detection towards Fruit Quality Monitoring. Sens. Actuators A Phys. 2023, 362, 114607. [Google Scholar] [CrossRef]
- Jaisutti, R.; Khemphet, S.; Pudwat, S.; Petchsang, N.; Kim, Y.-H.; Osotchan, T.; Zhu, Z. UV-Induced Room-Temperature Ethylene Sensors Based on Ag-Decorated ZnO Nanoflowers for Fruit Ripeness Monitoring. ACS Appl. Nano Mater. 2024, 7, 16575–16584. [Google Scholar] [CrossRef]
- Poeta, E.; De Chiara, M.L.V.; Cefola, M.; Caruso, I.; Genzardi, D.; Núñez-Carmona, E.; Pace, B.; Palumbo, M.; Sberveglieri, V. Quality Monitoring of Table Grapes Stored in Controlled Atmosphere Using an S3 + MOS Nanosensor Device. Postharvest Biol. Technol. 2025, 227, 113587. [Google Scholar] [CrossRef]
- Canhoto, O.; Magan, N. Electronic Nose Technology for the Detection of Microbial and Chemical Contamination of Potable Water. Sens. Actuators B Chem. 2005, 106, 3–6. [Google Scholar] [CrossRef]
- Ventura-Aguilar, R.I.; Lucas-Bautista, J.A.; Arévalo-Galarza, M.d.L.; Bosquez-Molina, E. Volatile Organic Compounds as a Diagnostic Tool for Detecting Microbial Contamination in Fresh Agricultural Products: Mechanism of Action and Analytical Techniques. Processes 2024, 12, 1555. [Google Scholar] [CrossRef]
- Berna, A.Z.; Lammertyn, J.; Saevels, S.; Di Natale, C.; Nicolaï, B.M. Electronic Nose Systems to Study Shelf Life and Cultivar Effect on Tomato Aroma Profile. Sensors 2004, 97, 324–333. [Google Scholar] [CrossRef]
- Carmona, E.N.; Sberveglieri, V.; Pulvirenti, A. Detection of Microorganisms in Water and Different Food Matrix by Electronic Nose. In Proceedings of the 2013 Seventh International Conference on Sensing Technology (ICST), Wellington, New Zealand, 3–5 December 2013; pp. 699–703. [Google Scholar] [CrossRef]
- Falasconi, M.; Gobbi, E.; Pardo, M.; Della Torre, M.; Bresciani, A.; Sberveglieri, G. Detection of Toxigenic Strains of Fusarium verticillioides in Corn by Electronic Olfactory System. Sens. Actuators B Chem. 2005, 108, 250–257. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Z.; Wang, Y.; Long, M.; Wu, W.; Kuca, K. Fumonisin B1: Mechanisms of Toxicity and Biological Detoxification Progress in Animals. Food Chem. Toxicol. 2021, 149, 111977. [Google Scholar] [CrossRef]
- Greco, G.; Núñez-Carmona, E.; Genzardi, D.; Bianchini, L.; Piccoli, P.; Zottele, I.; Tamanini, A.; Motolose, C.; Scalmato, A.; Sberveglieri, G.; et al. Tailored Gas Sensors as Rapid Technology to Support the Jams Production. Chemosensors 2023, 11, 403. [Google Scholar] [CrossRef]
- Olsen, M.; Lindqvist, R.; Bakeeva, A.; Su-lin, L.L.; Sulyok, M. Distribution of Mycotoxins Produced by Penicillium spp. Inoculated in Apple Jam and Crème Fraiche during Chilled Storage. Int. J. Food Microbiol. 2019, 292, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Mahata, B.; Acharyya, S.; Banerji, P.; Guha, P.K. Assessment of Fish Adulteration Using SnO2 Nanopetal-Based Gas Sensor and Machine Learning. Food Chem. 2024, 438, 138039. [Google Scholar] [CrossRef]
- Almario, A.A.; Calabokis, O.P.; Barrera, E.A. Smart E-Tongue Based on Polypyrrole Sensor Array as Tool for Rapid Analysis of Coffees from Different Varieties. Foods 2024, 13, 3586. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Pietro, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Sberveglieri, V. Validation of Parmigiano Reggiano Cheese Aroma Authenticity, Categorized through the Use of an Array of Semiconductors Nanowire Device (S3). Materials 2016, 9, 81. [Google Scholar] [CrossRef]
- Martins, V.d.C.; de Oliveira Godoy, R.L.; Senna Gouvêa, A.C.M.; Pessanha de Araujo Santiago, M.C.; Borguini, R.G.; de Oliveira Braga, E.C.; Pacheco, S.; de Mattos do Nascimento, L.S. Fraud Investigation in Commercial Coffee by Chromatography. Food Qual. Saf. 2018, 2, 121–133. [Google Scholar] [CrossRef]
- Oliveira, R.C.S.; Oliveira, L.S.; Franca, A.S.; Augusti, R. Evaluation of the Potential of SPME-GC-MS and Chemometrics to Detect Adulteration of Ground Roasted Coffee with Roasted Barley. J. Food Compos. Anal. 2009, 22, 257–261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).