Challenges for Ex Situ Conservation of Wild Bananas: Seeds Collected in Papua New Guinea Have Variable Levels of Desiccation Tolerance
Abstract
:1. Introduction
2. Results
2.1. Viability Evaluation of Seeds Stored in the Seed Bank
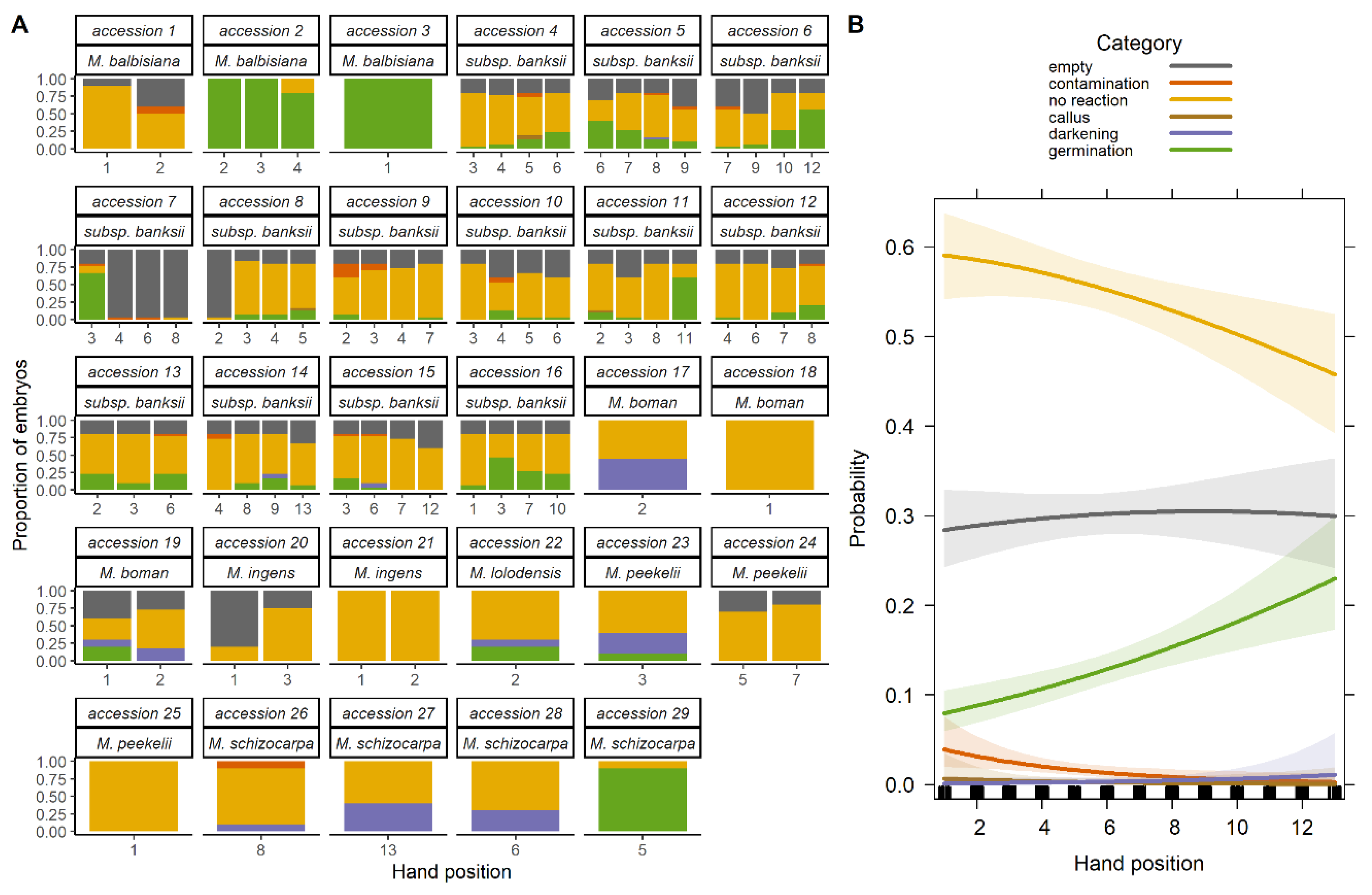
2.1.1. Overall Viability
2.1.2. Effect of Species
2.1.3. Effect of Position in the Infructescence
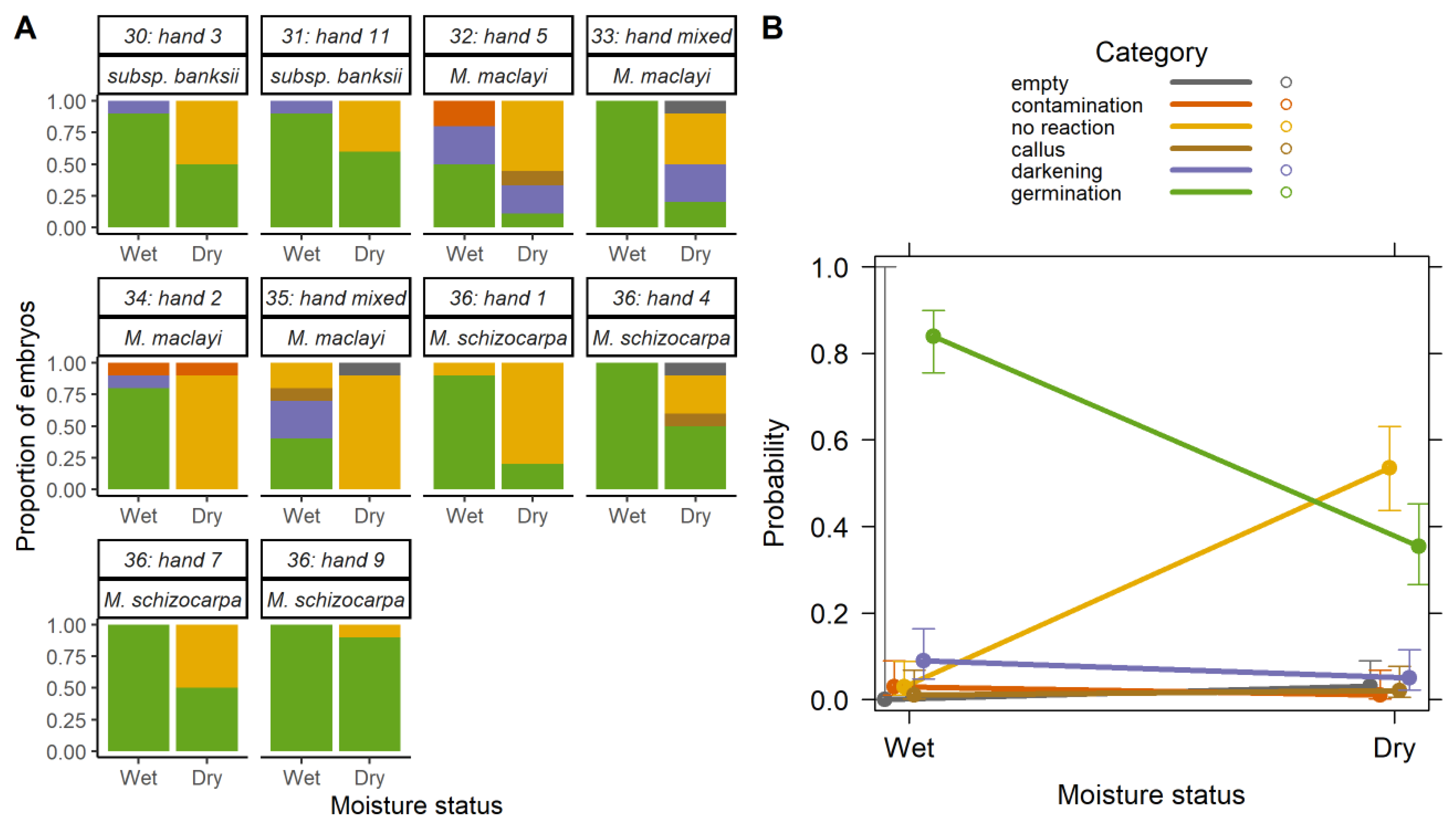
2.2. Effect of Desiccation
2.2.1. Overall Effect of Desiccation
2.2.2. Desiccation Tolerance and Species
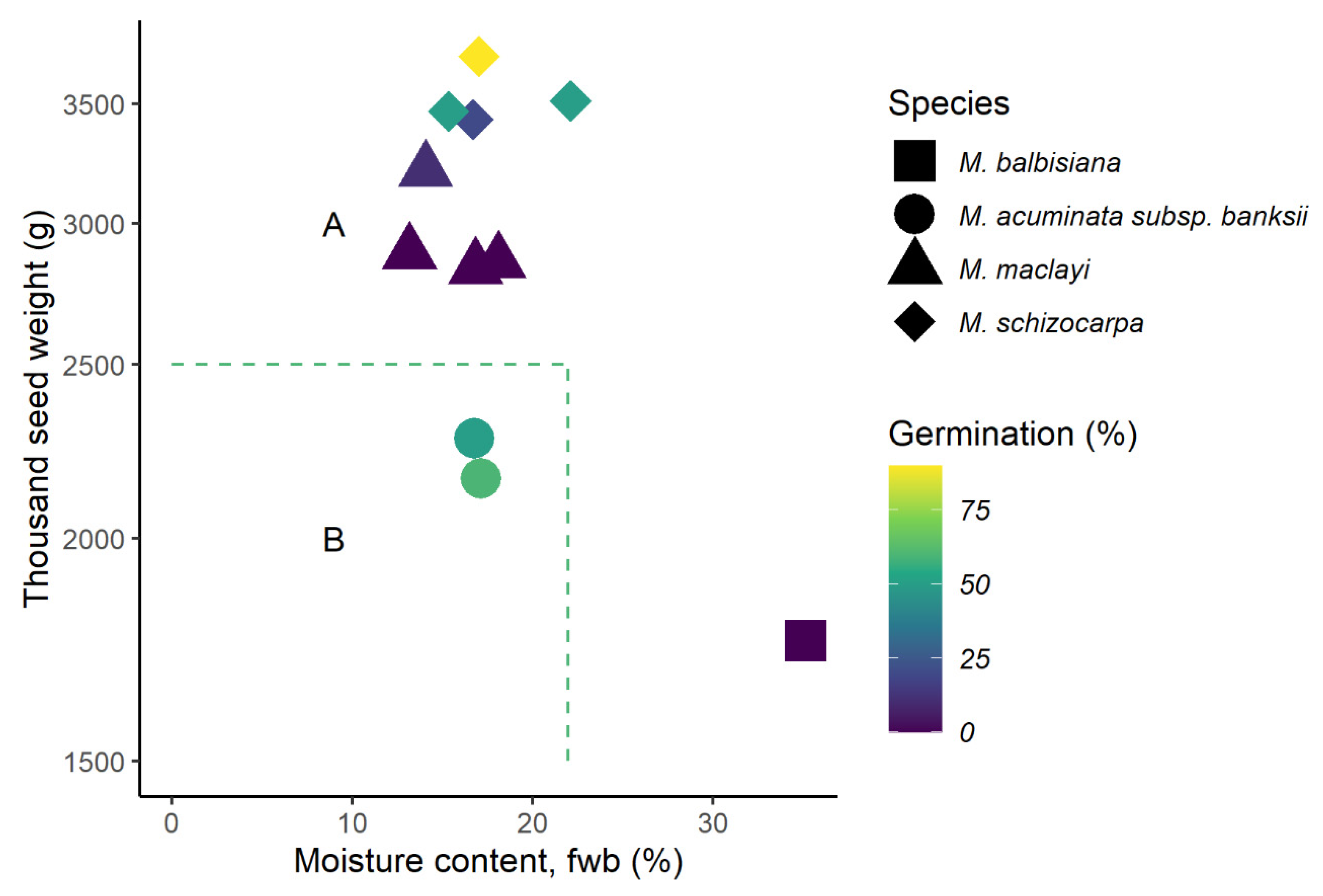
2.3. Prediction of Seed Storage Behaviour
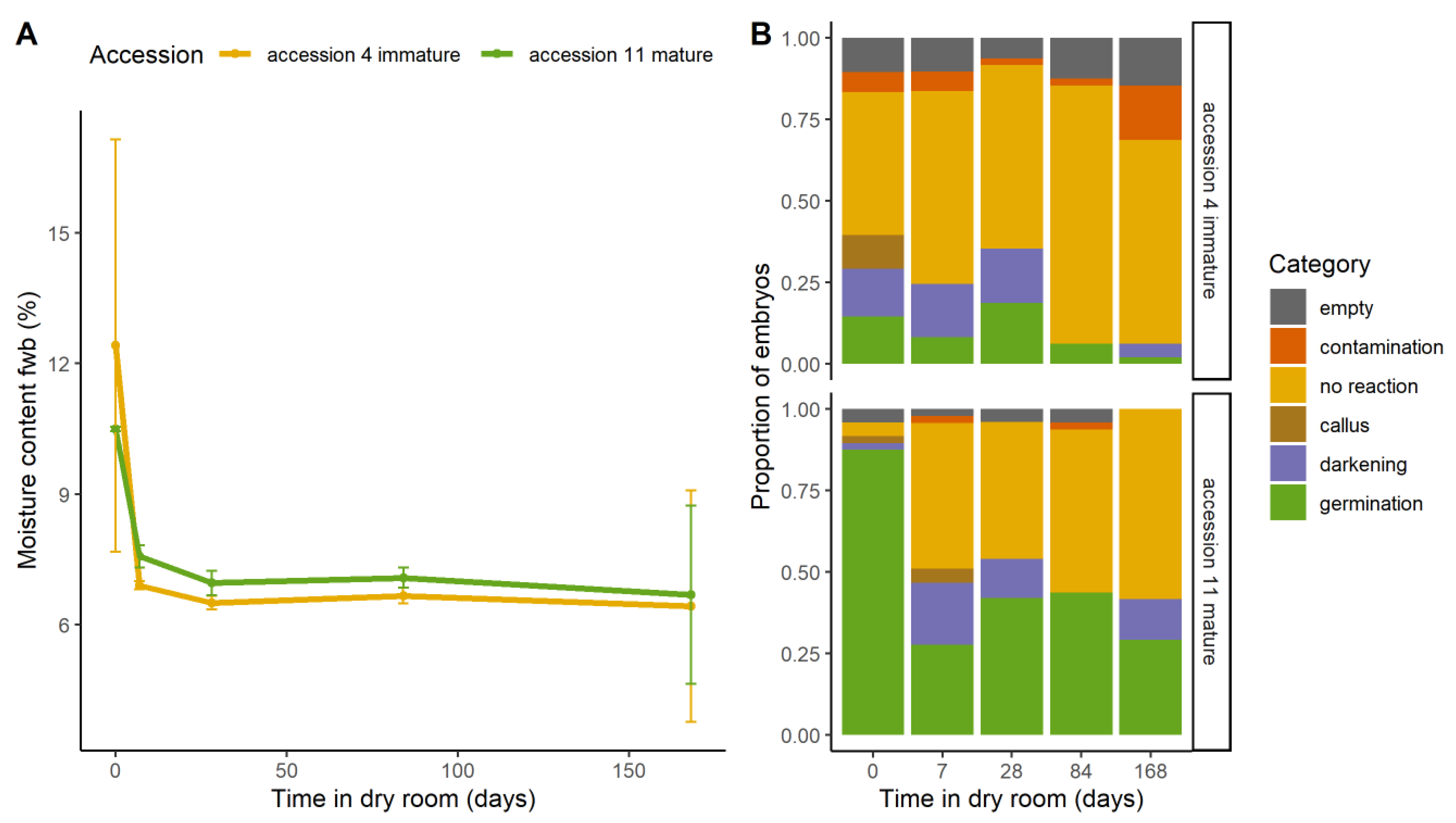
2.4. Dry storage and Maturity
2.4.1. Effect on Viability
2.4.2. Effect on Morphology
3. Discussion
3.1. Key Findings
3.2. Desiccation Sensitivity
3.3. Seed Storage Behaviour
3.4. Variation between Infructescences
3.4.1. Species and Climate
3.4.2. Seed Maturity
3.5. Variation within Infructescences
3.6. Limitations and Assumptions
3.6.1. Embryo Rescue
3.6.2. Conservation and Research Material
4. Materials and Methods
4.1. Study Region
4.2. Plant Material
4.2.1. Accessions
4.2.2. Seed Batches
4.3. Seed Collection, Field Evaluation and Transportation
4.4. Seed Processing
4.4.1. Extraction
4.4.2. Moisture Content Measurement
4.4.3. Storage
4.5. Viability Evaluation of Seeds Stored in the Seed Bank
4.6. Effect of Desiccation
4.7. Prediction of Seed Storage Behaviour
4.8. Survival during Dry Storage
4.8.1. Effect of Maturity
4.8.2. Effect on Morphology
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Redden, R.; Yadaz, S.S.; Maxted, N.; Dulloo, E.M.; Guarino, L.; Smith, P.E. Crop Wild Relatives and Climate Change; Redden, R., Yadaz, S.S., Maxted, N., Ehsan Dulloo, M., Guarino, L., Smith, P., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; p. 400. [Google Scholar]
- Guarino, L.; Lobell, D.B. A walk on the wild side. Nat. Clim. Chang. 2011, 1, 374–375. [Google Scholar] [CrossRef]
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and future use of wild relatives in crop breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Sampangi-Ramaiah, M.H.; Ravishankar, K.V.; Shivashankar, K.S.; Roy, T.K.; Rekha, A.; Hunashikatti, L.R. Developmental changes in the composition of leaf cuticular wax of banana influenced by wax biosynthesis gene expression: A case study in Musa acuminata and Musa balbisiana. Acta Physiol. Plant. 2019, 41, 9. [Google Scholar] [CrossRef]
- Vuylsteke, D.R.; Swennen, R.L.; Ortiz, R. Development and performance of black sigatoka resistant tetraploid hybrids of plantain (Musa spp. AAB group). Euphytica 1993, 65, 33–42. [Google Scholar] [CrossRef]
- Parac, E.P.; Lalusin, A.G.; Pangga, I.B.; Cruz, F.C.S. Characteristics of selected Hybrids of Abaca (Musa textilis Nee) with resistance to bunchy top. Philipp. Agric. Sci. 2020, 103, 1–12. [Google Scholar]
- Jarvis, A.; Lane, A.; Hijmans, R.J. The effect of climate change on crop wild relatives. Agric. Ecosyst. Environ. 2008, 126, 13–23. [Google Scholar] [CrossRef]
- Ford-Lloyd, B.V.; Schmidt, M.; Armstrong, S.J.; Barazani, O.; Engels, J.; Hadas, R.; Hammer, K.; Kell, S.P.; Kang, D.M.; Khoshbakht, K.; et al. Crop wild relatives: Undervalued, underutilized and under threat? Bioscience 2011, 61, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Schatz, G.E. Plants on the IUCN Red List: Setting priorities to inform conservation. Trends Plant Sci. 2009, 14, 638–642. [Google Scholar] [CrossRef]
- UN General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Secretariat of the Convention on Biological Diversity. Global Strategy for Plant Conservation; 2010. Available online: https://www.bgci.org/our-work/policy-and-advocacy/the-global-strategy-for-plant-conservation/ (accessed on 18 September 2020).
- Cohen, J.I.; Williams, J.T.; Plucknett, D.L.; Shands, H. Ex situ conservation of plant genetic resources: Global developments and environmental concerns. Science 1991, 253, 866–872. [Google Scholar] [CrossRef]
- FAO. Second Global Plan of Action for Plant Genetic Resources for Food and Agriculture; Commission on Genetic Resources for Food and Agriculture, Food and Agriculture Organization of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Maxted, N.; Dulloo, E.; Ford-Lloyd, B.V.; Iriondo, J.M.; Jarvis, A. Gap analysis: A tool for complementary genetic conservation assessment. Divers. Distrib. 2008, 14, 1018–1030. [Google Scholar] [CrossRef]
- Global Crop Diversity Trust. A global Rescue: Safeguarding the World’s Crop Wild Relatives; Global Crop Diversity Trust: Bonn, Germany, 2019. [Google Scholar]
- Pradheep, K.; Singh, M.; Sultan, S.M.; Singh, K.; Parimalan, R.; Ahlawat, S.P. Diversity in wild relatives of wheat: An expedition collection from cold-arid Indian Himalayas. Genet. Resour. Crop Evol. 2019, 66, 275–285. [Google Scholar] [CrossRef]
- Phillips, J.; Kyratzis, A.; Christoudoulou, C.; Kell, S.; Maxted, N. Development of a national crop wild relative conservation strategy for Cyprus. Genet. Resour. Crop Evol. 2014, 61, 817–827. [Google Scholar] [CrossRef]
- Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Khoury, C.K.; Muller, J.V.; Toll, J. Adapting agriculture to climate change: A global initiative to collect, conserve, and use crop wild relatives. Agroecol. Sustain. Food Syst. 2014, 38, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Holubec, V.; Hauptvogel, P.; Paprstein, F.; Podyma, W.; Sevcikoa, M.; Vymyslicky, T. Results of projects on collecting, mapping, monitoring, and conserving of plant genetic resources 1990–2008. Czech J. Genet. Plant Breed. 2010, 46, S2–S8. [Google Scholar] [CrossRef] [Green Version]
- Castañeda-Álvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16022. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online; Royal Botanic Gardens: Kew, UK, 2019. [Google Scholar]
- Häkkinen, M.; Väre, H. Typification and check-list of Musa L. names (Musaceae) with nomenclatural notes. Adansonia 2008, 30, 63–112. [Google Scholar]
- FAO. Banana Facts and Figures. Available online: http://www.fao.org/economic/est/est-commodities/bananas/bananafacts/en/#.XcWis-hKg2x (accessed on 8 November 2019).
- Daniells, J.; Jenny, C.; Karamura, D.; Tomekpe, K. Musalogue: Diversity in the Genus Musa; International Network for the Improvement of Banana and Plantain: Montpellier, France, 2001. [Google Scholar]
- Ruas, M.; Guignon, V.; Sempere, G.; Sardos, J.; Hueber, Y.; Duvergey, H.; Andrieu, A.; Chase, R.; Jenny, C.; Hazekamp, T.; et al. MGIS: Managing Banana (Musa spp.) genetic resources information and high-throughput genotyping data. Database 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Data Providers and the Crop Trust. Genesys Global Portal on Plant Genetic Resources. 2020. Available online: https://www.genesys-pgr.org/ (accessed on 17 September 2020).
- Cámara-Leret, R.; Frodin, D.G.; Adema, F.; Al, E. New Guinea has the world’s richest island flora. Nature 2020. [Google Scholar] [CrossRef]
- Perrier, X.; De Langhe, E.; Donohue, M.; Lentfer, C.; Vrydaghs, L.; Bakry, F.; Carreel, F.; Hippolyte, I.; Horry, J.P.; Jenny, C.; et al. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc. Natl. Acad. Sci. USA 2011, 108, 11311–11318. [Google Scholar] [CrossRef] [Green Version]
- Perrier, X.; Bakry, F.; Carreel, F.; Jenny, C.; Horry, J.P.; Lebot, V.; Hippolyte, I. Combining biological approaches to shed light on the evolution of edible bananas. Ethnobot. Res. Appl. 2009, 7, 199–216. [Google Scholar] [CrossRef] [Green Version]
- Christelová, P.; De Langhe, E.; Hřibová, E.; Čížková, J.; Sardos, J.; Hušáková, M.; Van den houwe, I.; Sutanto, A.; Kepler, A.K.; Swennen, R.; et al. Molecular and cytological characterization of the global Musa germplasm collection provides insights into the treasure of banana diversity. Biodivers. Conserv. 2017, 26, 801–824. [Google Scholar] [CrossRef]
- Sardos, J.; Perrier, X.; Dolezel, J.; Hribova, E.; Christelova, P.; Van den Houwe, I.; Kilian, A.; Roux, N. DArT whole genome profiling provides insights on the evolution and taxonomy of edible banana (Musa spp.). Ann. Bot. 2016, 118, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govaerts, R.; Häkkinen, M. World Checklist of Musaceae; Royal Botanic Gardens, Kew: Kew, UK, 2006. [Google Scholar]
- Arnaud, E.; Horry, J.P. Musalogue: A Catalogue of Musa Germplasm: Papua New Guinea Collecting Missions 1988–1989; International Network for the Improvement of Banana and Plantain: Montpellier, France, 1997; p. 127. [Google Scholar]
- Sachter-Smith, G.; Paufa, J.; Rauka, G.; Sardos, J.; Janssens, S. Bananas of the Autonomous Region of Bougainville: A Catalog of Banana Diversity Seen on the Islands of Bougainville and Buka, Papua New Guinea. 2017, p. 97. Available online: http://www.musalit.org/seeMore.php?id=17006 (accessed on 17 September 2020).
- Sardos, J.; Paofa, J.; Janssens, S.; Sachter-Smith, G.; Rauka, G.; Roux, N. Banana Collecting Mission in the Autonomous Region of Bougainville (AROB), Papua New Guinea. 2017. Available online: https://www.researchgate.net/publication/318259511 (accessed on 17 September 2020).
- Sardos, J.; Christelova, P.; Cizkova, J.; Paofa, J.; Sachter-Smith, G.L.; Janssens, S.B.; Rauka, G.; Ruas, M.; Daniells, J.W.; Dolezel, J.; et al. Collection of new diversity of wild and cultivated bananas (Musa spp.) in the Autonomous Region of Bougainville, Papua New Guinea. Genet. Resour. Crop Evol. 2018, 65, 2267–2286. [Google Scholar] [CrossRef] [Green Version]
- Eyland, D.; Breton, C.; Sardos, J.; Kallow, S.; Panis, B.; Swennen, R.; Paofa, J.; Tardieu, F.; Welcker, C.; Janssens, S.B.; et al. Filling the gaps in gene banks: Collecting, characterizing and phenotyping wild banana relatives of Papua new Guinea. Crop Sci. 2020. [Google Scholar] [CrossRef]
- Gargiulo, R.; Saubin, M.; Rizzuto, G.; West, B.; Fay, M.F.; Kallow, S.; Trivedi, C. Genetic diversity in British populations of Taxus baccata L.: Is the seedbank collection representative of the genetic variation in the wild? Biol. Conserv. 2019, 233, 289–297. [Google Scholar] [CrossRef]
- Hoban, S.; Kallow, S.; Trivedi, C. Implementing a new approach to effective conservation of genetic diversity, with ash (Fraxinus excelsior) in the UK as a case study. Biol. Conserv. 2018, 225, 10–21. [Google Scholar] [CrossRef]
- Li, D.Z.; Pritchard, H.W. The science and economics of ex situ plant conservation. Trends Plant Sci. 2009, 14, 614–621. [Google Scholar] [CrossRef]
- Whitehouse, K.J.; Hay, F.R.; Lusty, C. Why seed physiology is important for genebanking. Plants 2020, 9, 584. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the storage life of seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An intermediate category of seed storage behaviour. 1, Coffee. J. Exp. Bot. 1990, 41, 1167–1174. [Google Scholar] [CrossRef]
- Walters, C. Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Chin, H.F. Germination and Storage of Banana Seeds. In Proceedings of the Workshop on New Frontiers in Resistance Breeding for Nematode, Fusarium and Sigatoka, Kuala Lumpur, Malaysia, 2–5th October 1995. Frison, E., Horry, J.P., Waele, D.D., Eds.; International Network for the Improvement of Banana and Plantain: Montpellier, France, 1996; pp. 218–229. [Google Scholar]
- Simmonds, N.W. The germination of banana seeds. Trop. Agric. 1952, 29, 35–49. [Google Scholar]
- Stotzky, G.; Cox, E.A. Seed germination studies in Musa. II. Alternating temperature requirement for the germination of Musa balbisiana. Am. J. Bot. 1962, 49, 763–770. [Google Scholar] [CrossRef]
- Boyce, K.G. Report of the seed storage committee 1986–1989. Seed Sci. Technol. 1989, 17, 135–142. [Google Scholar]
- Chin, H.F.; Krishnapillay, B. Cryogenic storage of some horticultural species. In Proceedings of the IV International Symposium on Seed Research in Horticulture, Angers, France, 5–9 September 1988; pp. 107–112. [Google Scholar]
- Darjo, P.; Bakry, F. Conservation and germination of banana seeds. Fruits 1990, 45, 103–113. [Google Scholar]
- Nagano, S.; Mori, G.; Oda, M. Seed germinability in Musa velutina Wendl. & Drude is markedly lowered by 1 week in dry-storage. J. Hortic. Sci. Biotechnol. 2009, 84, 325–328. [Google Scholar]
- Fortescue, J.A.; Turner, D. Reproductive biology. In Banana Breeding: Progress and Challenges; Pillay, M., Tenkouano, A., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2011; pp. 145–179. [Google Scholar]
- Laliberté, B. Global Strategy for The Conservation and Use of Musa (Banana) Genetic Resources: A Consultative Document Prepared by The Global Musa Genetic Resources Network (MusaNet); Bioversity International: Rome, Italy, 2016; p. lxiv + 146. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. Handbook of Seed Technology for Genebanks; International Board for Plant Genetic Resources (IBPGR): Rome, Italy, 1985; Volume 2, p. 456. [Google Scholar]
- Batte, M.; Swennen, R.; Uwimana, B.; Akech, V.; Brown, A.; Tumuhimbise, R.; Hovmalm, H.P.; Geleta, M.; Ortiz, R. Crossbreeding East African Highland Bananas: Lessons learnt relevant to the botany of the crop after 21 years of genetic enhancement. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Hay, F.R.; Probert, R.J. Advances in seed conservation of wild plant species: A review of recent research. Conserv. Physiol. 2013, 1. [Google Scholar] [CrossRef]
- Rao, N.K.; Dulloo, M.E.; Engels, J.M.M. A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genet. Resour. Crop Evol. 2017, 64, 1061–1074. [Google Scholar] [CrossRef]
- Probert, R.; Adams, J.; Coneybeer, J.; Crawford, A.; Hay, F. Seed quality for conservation is critically affected by pre-storage factors. Aust. J. Bot. 2007, 55, 326–335. [Google Scholar] [CrossRef]
- Hay, F.; Probert, R. Collecting and handling seeds in the field. In Collecting Plant Genetic Diversity: Technical Guidelines–2011 Update; Guarino, L., Ramanatha Rao, V., Goldberg, E., Eds.; Bioiversity International: Rome, Italy, 2011. [Google Scholar]
- Pancholi, N.; Wetten, A.; Caligari, P.D.S. Germination of Musa velutina seeds: Comparison of in vivo and in vitro systems. In Vitro Cell. Dev. Biol. Plant 1995, 31, 127–130. [Google Scholar] [CrossRef]
- Afele, J.C.; De Langhe, E. Increasing in vitro germination of Musa balbisiana seed. Plant Cell Tissue Organ Cult. 1991, 27, 33–36. [Google Scholar] [CrossRef]
- Cox, E.A.; Stotzky, G.; Goos, R.D. In vitro culture of Musa balbisiana Colla embryos. Nature 1960, 185, 403–404. [Google Scholar] [CrossRef]
- IPGRI. Descriptors for Banana (Musa spp.); IPGRI: Rome, Italy, 1996. [Google Scholar]
- Hong, T.D.; Ellis, R.H. Ex situ biodiversity conservation by seed storage: Multiple-criteria keys to estimate seed storage behaviour. Seed Sci. Technol. 1996, 25, 157–161. [Google Scholar]
- Ellis, R.H.; Mai-Hong, T.; Hong, T.D.; Tan, T.T.; Xuan-Chuong, N.D.; Hung, L.Q.; Ngoc-Tam, B.; Le-Tam, V.T. Comparative analysis by protocol and key of seed storage behaviour of sixty Vietnamese tree species. Seed Sci. Technol. 2007, 35, 460–476. [Google Scholar] [CrossRef]
- Abdelnouresquivel, A.; Mora, A.; Villalobos, V. Cryopreservation of zygotic embryos of Musa acuminata (AA) and M. balbisiana (BB). Cryo-Lett. 1992, 13, 159–164. [Google Scholar]
- Berjak, P.; Pammenter, N.W. Progress in the understanding and manipulation of desiccation-sensitive (recalcitrant) seeds. Basic Appl. Asp. Seed Biol. 1997, 30, 689–703. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An intermediate category of seed storage behaviour. 2, effects of provenance, immaturity and imbibition on desiccation-tolerance in coffee. J. Exp. Bot. 1991, 42, 653–657. [Google Scholar] [CrossRef]
- Dussert, S.; Chabrillange, N.; Engelmann, F.; Anthony, F.; Louarn, J.; Hamon, S. Relationship between seed desiccation sensitivity, seed water content at maturity and climatic characteristics of native environments of nine Coffea L. species. Seed Sci. Res. 2000, 10, 293–300. [Google Scholar] [CrossRef]
- Walters, C. Understanding the mechanisms and kinetics of seed aging. Seed Sci. Res. 1998, 8, 223–244. [Google Scholar] [CrossRef]
- Berjak, P.; Pammenter, N.W. From Avicennia to Zizania: Seed recalcitrance in perspective. Ann. Bot. 2008, 101, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.H. Temporal patterns of seed quality development, decline, and timing of maximum quality during seed development and maturation. Seed Sci. Res. 2019, 29, 135–142. [Google Scholar] [CrossRef] [Green Version]
- De Langhe, E.; Perrier, X.; Donohue, M.; Denham, T. The original banana split: Multi-disciplinary implication of the generation of African and Pacific plantains in island southeast Asia. Ethnobot. Res. Appl. 2015, 14, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Argent, G.C.G. The wild bananas of Papua New Guinea. Notes Royal Bot. Gard. Edinb. 1976, 35, 77–114. [Google Scholar]
- Janssens, S.B.; Vandelook, F.; De Langhe, E.; Verstraete, B.; Smets, E.; Vandenhouwe, I.; Swennen, R. Evolutionary dynamics and biogeography of Musaceae reveal a correlation between the diversification of the banana family and the geological and climatic history of Southeast Asia. New Phytol. 2016, 210, 1453–1465. [Google Scholar] [CrossRef]
- Tweddle, J.C.; Dickie, J.B.; Baskin, C.C.; Baskin, J.M. Ecological aspects of seed desiccation sensitivity. J. Ecol. 2003, 91, 294–304. [Google Scholar] [CrossRef]
- Hay, F.R.; Probert, R.J. Seed maturity and the effects of different drying conditions on desiccation tolerance and seed longevity in foxglove (Digitalis purpurea L.). Ann. Bot. 1995, 76, 639–647. [Google Scholar] [CrossRef]
- Hong, T.D.; Ellis, R.H. A Protocol to Determine Seed Storage Behaviour; International Plant Genetic Resources Institute: Rome, Italy, 1996. [Google Scholar]
- Ellis, R.; Pieta Filho, C. The development of seed quality in spring and winter cultivars of barley and wheat. Seed Sci. Res. 1992, 2, 9–15. [Google Scholar] [CrossRef]
- Uma, S.; Lakshmi, S.; Saraswathi, M.S.; Akbar, A.; Mustaffa, M.M. Embryo rescue and plant regeneration in banana (Musa spp.). Plant Cell Tissue Organ Cult. 2010, 105, 105–111. [Google Scholar] [CrossRef]
- Marod, D.; Pinyo, P.; Duengkae, P.; Hiroshi, T. The role of wild banana (Musa acuminata Colla) on wildlife diversity in mixed deciduous forest, Kanchanaburi Province, Western Thailand. Kasetsart J. Nat. Sci. 2010, 44, 35–43. [Google Scholar]
- Meng, L.Z.; Gao, X.X.; Chen, J.; Martin, K. Spatial and temporal effects on seed dispersal and seed predation of Musa acuminata in southern Yunnan, China. Integr. Zool. 2012, 7, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Kulavmode, A.R.; Hegde, V.M.; Kambale, A.A. A note on seed dispersal of rock banana Ensete superbum by Asian palm civet Paradoxurus hermaphroditus at Sinhgad Fort, Maharashtra, India. J. Bombay Nat. Hist. Soc. 2015, 112, 25–26. [Google Scholar] [CrossRef]
- Kumar, K.P.S.; Bhowmik, D.; Duraivel, S.; Umadevi, M. Tradition and medicinal uses of banana. J. Pharmacogn. Phytochem. 2012, 1, 51–63. [Google Scholar]
- Whitehouse, K.J.; Hay, F.R.; Ellis, R.H. Increases in the longevity of desiccation-phase developing rice seeds: Response to high-temperature drying depends on harvest moisture content. Ann. Bot. 2015, 116, 247–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehouse, K.J.; Hay, F.R.; Ellis, R.H. High-temperature stress during drying improves subsequent rice (Oryza sativa L.) seed longevity. Seed Sci. Res. 2017, 27, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Whitehouse, K.J.; Hay, F.R.; Ellis, R.H. Improvement in rice seed storage longevity from high-temperature drying is a consistent positive function of harvest moisture content above a critical value. Seed Sci. Res. 2018, 28, 332–339. [Google Scholar] [CrossRef]
- Simmonds, N.W. Experiments on the germination of banana seeds. Trop. Agric. 1959, 36, 259–273. [Google Scholar]
- Michaels, H.J.; Benner, B.; Hartgerink, A.P.; Lee, T.D.; Rice, S.; Willson, M.F.; Bertin, R.I. Seed size variation: Magnitude, distribution, and ecological correlates. Evol. Ecol. 1988, 2, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Cavers, P.B.; Steel, M.G. Patterns of change in seed weight over time on individual plants. Am. Nat. 1984, 124, 324–335. [Google Scholar] [CrossRef]
- Boe, A.; Bortnem, R.; Johnson, P.J. Changes in weight and germinability of black medic seed over a growing season, with a new seed predator. In Proceedings of the 101st Annual Meeting of South-Dakota-Academy-of-Science, Univ Sioux Falls, Sioux Falls, SD, USA, 8–9 April 2016; pp. 105–117. [Google Scholar]
- Susko, D.J.; Lovett-Doust, L. Patterns of seed mass variation and their effects on seedling traits in Alliaria petiolata (Brassicaceae). Am. J. Bot. 2000, 87, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Vuylsteke, D. Factors influencing seed set in triploid Musa spp. and production of euploid hybrids. Ann. Bot. 1995, 75, 151–155. [Google Scholar] [CrossRef]
- Ssebuliba, R.; Makumbi, D.; Pillay, M. Patterns of seed set in East African Highland Banana (Musa spp.) hybrids. J. New Seeds 2009, 10, 160–170. [Google Scholar] [CrossRef]
- Asif, M.; Mak, C.; Othman, R. In vitro zygotic embryo culture of wild Musa acuminata ssp.malaccensis and factors affecting germination and seedling growth. Plant Cell Tissue Organ Cult. 2001, 67, 267–270. [Google Scholar] [CrossRef]
- FAO. International Treaty on Plant Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the worlds: A new map of life on Earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Clim. 2017, 25, 1965–1978. [Google Scholar] [CrossRef]
- Puteh, A.B.; Aris, E.M.; Sinniah, U.R.; Rahman, M.; Mohamad, R.B.; Abdullah, N.A.P. Seed anatomy, moisture content and scarification influence on imbibition in wild banana (Musa acuminata Colla) ecotypes. Afr. J. Biotechnol. 2011, 10, 14373–14379. [Google Scholar]
- Leist, N.; Kr ämer, S. Supplements 2011 to ISTA Working Sheets on Tetrazolium Testing; ISTA: Bassersdorf, Switzerland, 2011; Volume 1. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Vineesh, P.S.; Skaria, R.; Mukunthakumar, S.; Padmesh, P.; Decruse, S.W. Seed germination and cryostorage of Musa acuminata subsp. burmannica from Western ghats. S. Afr. J. Bot. 2015, 100, 158–163. [Google Scholar] [CrossRef]
- Ripley, B.; Venables, W. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Fox, J.; Hong, J. Effect displays in R for multinomial and proportional-odds logit models: Extensions to the effects package. J. Stat. Softw. 2009, 32, 1–24. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
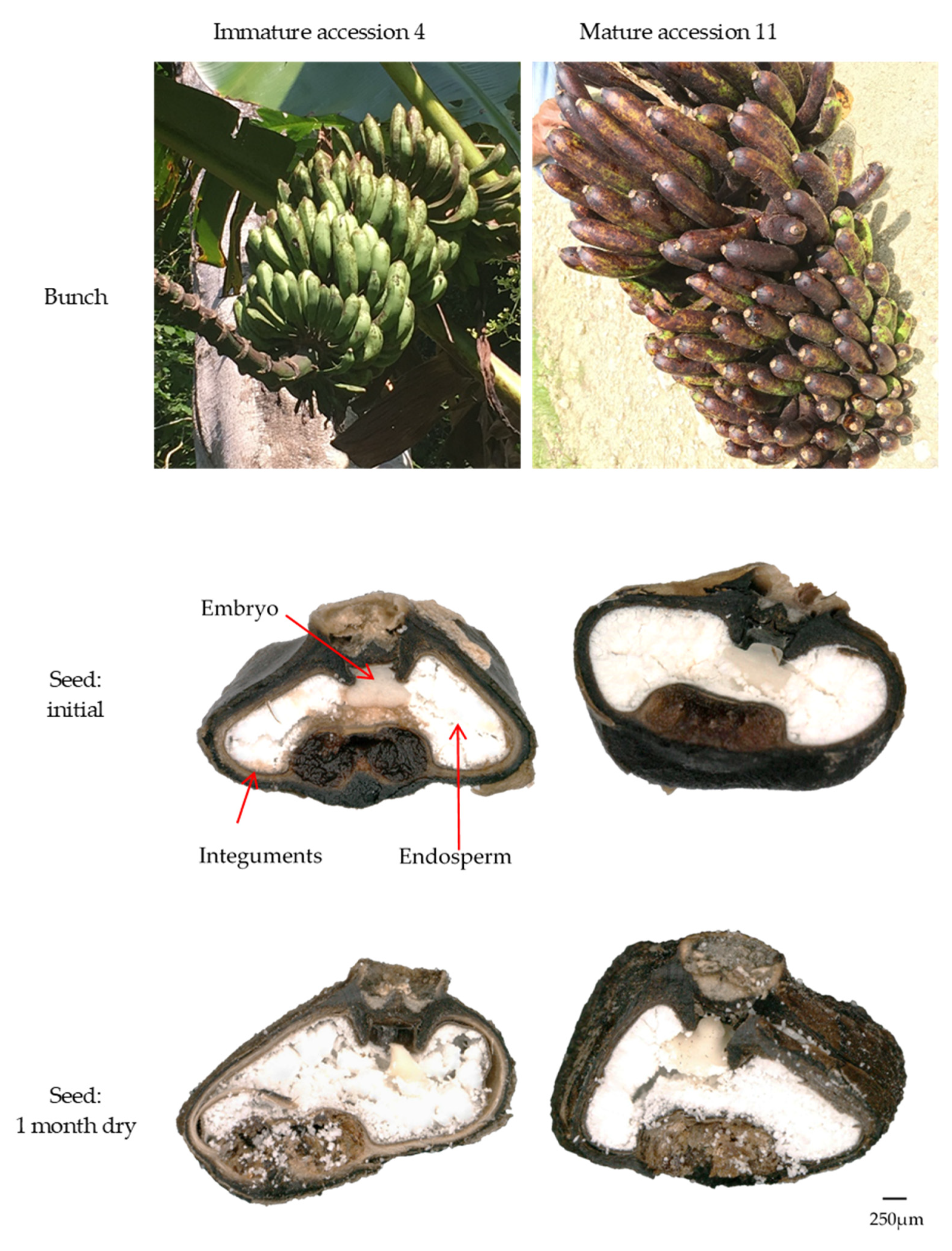
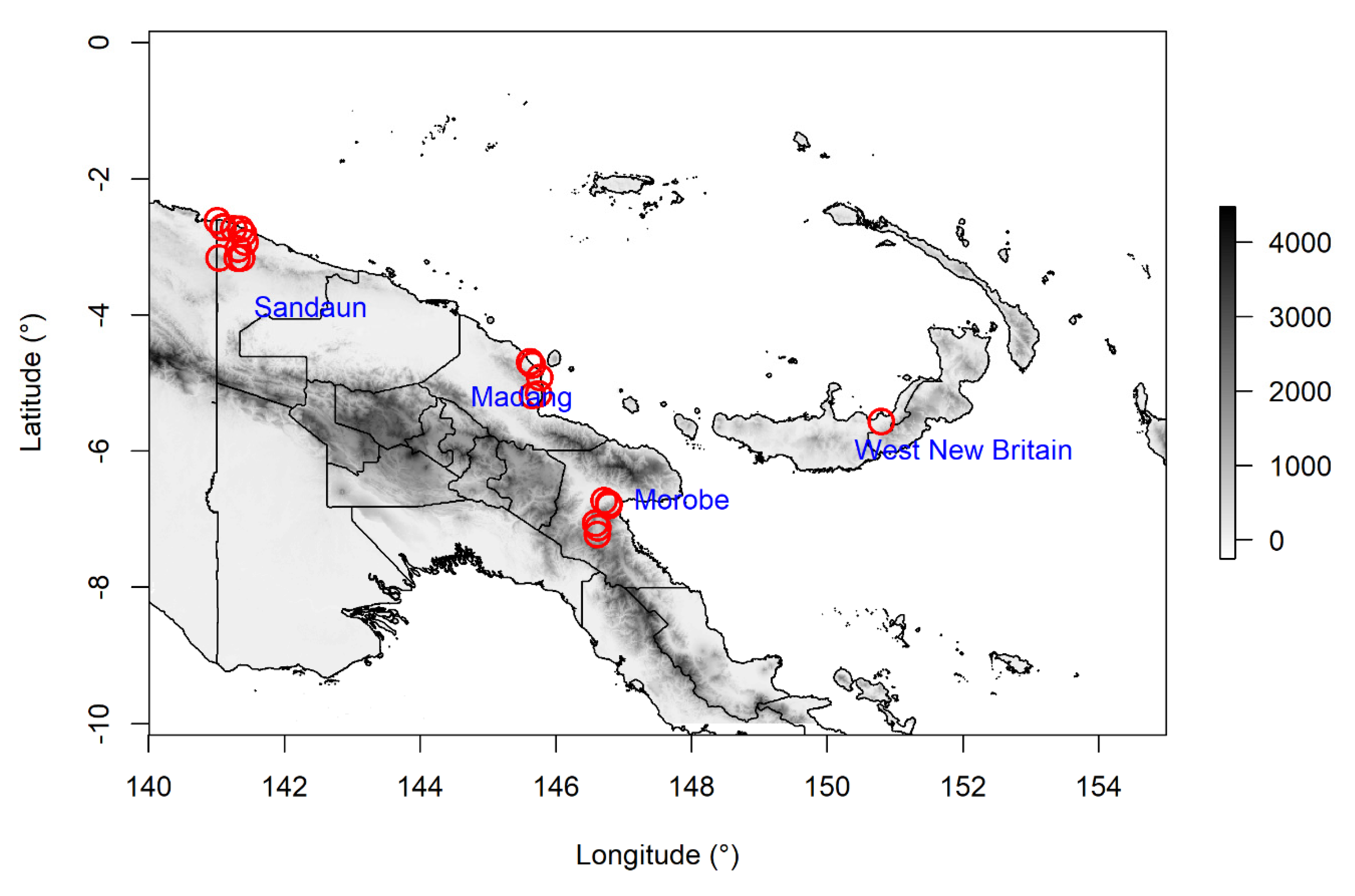
| Batch | Accession | Species | Province | Latitude | Longitude | Date Collected |
|---|---|---|---|---|---|---|
| 1 | 1 | M. balbisiana | Morobe | S 07°03′23″ | E 146°34′56″ | 14/05/2019 |
| 1 | 2 | M. balbisiana | Morobe | S 07°07′35″ | E 146°36′57″ | 14/05/2019 |
| 1 | 3 | M. balbisiana | Madang | S 04°41′49″ | E 145°36′49″ | 17/05/2019 |
| 1 | 4 | M. acuminata subsp.banksii | Morobe | S 06°43′34″ | E 146°42′40″ | 14/05/2019 |
| 1 | 5 | M. acuminata subsp.banksii | Morobe | S 06°43′34″ | E 146°42′40″ | 14/05/2019 |
| 1 | 6 | M. acuminata subsp.banksii | Morobe | S 07°13′42″ | E 146°36′32″ | 14/05/2019 |
| 1 | 7 | M. acuminata subsp.banksii | Morobe | S 07°13′42″ | E 146°36′32″ | 14/05/2019 |
| 1 | 8 | M. acuminata subsp.banksii | Morobe | S 06°47′14″ | E 146°47′00″ | 15/05/2019 |
| 1 | 9 | M. acuminata subsp.banksii | Morobe | S 06°46′04″ | E 146°46′46″ | 15/05/2019 |
| 1 | 10 | M. acuminata subsp.banksii | Madang | S 04°41′31″ | E 145°36′60″ | 17/05/2019 |
| 1 | 11 | M. acuminata subsp.banksii | Sandaun | S 02°43′58″ | E 141°15′20″ | 20/05/2019 |
| 1 | 12 | M. acuminata subsp.banksii | Sandaun | S 03°09′53″ | E 141°21′57″ | 21/05/2019 |
| 1 | 13 | M. acuminata subsp.banksii | Sandaun | S 03°09′51″ | E 141°18′18″ | 21/05/2019 |
| 1 | 14 | M. acuminata subsp.banksii | Sandaun | S 02°55′56″ | E 141°25′09″ | 21/05/2019 |
| 1 | 15 | M. acuminata subsp.banksii | Sandaun | S 02°42′17″ | E 141°05′36″ | 22/05/2019 |
| 1 | 16 | M. acuminata subsp.banksii | Sandaun | S 02°42′46″ | E 141°05′45″ | 22/05/2019 |
| 1 | 17 | M. boman | Sandaun | S 03°01′46″ | E 141°19′18″ | 21/05/2019 |
| 1 | 18 | M. boman | Sandaun | S 02°48′36″ | E 141°23′44″ | 21/05/2019 |
| 1 | 19 | M. boman | Sandaun | S 03°09′38″ | E 141°02′22″ | 21/05/2019 |
| 1 | 20 | M. ingens | Morobe | S 06°48′03″ | E 146°46′24″ | 15/05/2019 |
| 1 | 21 | M. ingens | Morobe | S 06°47′14″ | E 146°47′00″ | 15/05/2019 |
| 1 | 22 | M. lolodensis | Sandaun | S 03°01′46″ | E 141°19′18″ | 21/05/2019 |
| 1 | 23 | M. peekelii | Madang | S 05°09′44″ | E 145°44′51″ | 16/05/2019 |
| 1 | 24 | M. peekelii | Madang | S 04°55′07″ | E 145°45′51″ | 18/05/2019 |
| 1 | 25 | M. peekelii | Madang | S 04°55′07″ | E 145°45′51″ | 18/05/2019 |
| 1 | 26 | M. schizocarpa | Madang | S 04°44′17″ | E 145°38′60″ | 17/05/2019 |
| 1 | 27 | M. schizocarpa | Sandaun | S 02°44′19″ | E 141°21′11″ | 19/05/2019 |
| 1 | 28 | M. schizocarpa | Sandaun | S 02°43′58″ | E 141°15′20″ | 20/05/2019 |
| 1 | 29 | M. schizocarpa | Sandaun | S 02°37′02″ | E 141°00′52″ | 22/05/2019 |
| 2 | 30 | M. acuminata subsp.banksii | Madang | S 05°11′58″ | E 145°39′30″ | 16/10/2019 |
| 2 | 31 | M. acuminata subsp.banksii | Madang | S 05°11′58″ | E 145°39′30″ | 16/10/2019 |
| 2 | 32 | M. maclayi | West New Britain | S 05°33′54″ | E 150°47′29″ | 03/10/2019 |
| 2 | 33 | M. maclayi | West New Britain | S 05°33′54″ | E 150°47′29″ | 03/10/2019 |
| 2 | 34 | M. maclayi | West New Britain | S 05°33′54″ | E 150°47′29″ | 03/10/2019 |
| 2 | 35 | M. maclayi | West New Britain | S 05°33′54″ | E 150°47′29″ | 03/10/2019 |
| 2 | 36 | M. schizocarpa | Madang | S 05°11′58″ | E 145°39′30″ | 16/10/2019 |
| 2 | 37 | M. balbisiana | Madang | S 05°11′58″ | E 145°39′30″ | 15/10/2018 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallow, S.; Longin, K.; Sleziak, N.F.; Janssens, S.B.; Vandelook, F.; Dickie, J.; Swennen, R.; Paofa, J.; Carpentier, S.; Panis, B. Challenges for Ex Situ Conservation of Wild Bananas: Seeds Collected in Papua New Guinea Have Variable Levels of Desiccation Tolerance. Plants 2020, 9, 1243. https://doi.org/10.3390/plants9091243
Kallow S, Longin K, Sleziak NF, Janssens SB, Vandelook F, Dickie J, Swennen R, Paofa J, Carpentier S, Panis B. Challenges for Ex Situ Conservation of Wild Bananas: Seeds Collected in Papua New Guinea Have Variable Levels of Desiccation Tolerance. Plants. 2020; 9(9):1243. https://doi.org/10.3390/plants9091243
Chicago/Turabian StyleKallow, Simon, Kevin Longin, Natalia Fanega Sleziak, Steven B. Janssens, Filip Vandelook, John Dickie, Rony Swennen, Janet Paofa, Sebastien Carpentier, and Bart Panis. 2020. "Challenges for Ex Situ Conservation of Wild Bananas: Seeds Collected in Papua New Guinea Have Variable Levels of Desiccation Tolerance" Plants 9, no. 9: 1243. https://doi.org/10.3390/plants9091243
APA StyleKallow, S., Longin, K., Sleziak, N. F., Janssens, S. B., Vandelook, F., Dickie, J., Swennen, R., Paofa, J., Carpentier, S., & Panis, B. (2020). Challenges for Ex Situ Conservation of Wild Bananas: Seeds Collected in Papua New Guinea Have Variable Levels of Desiccation Tolerance. Plants, 9(9), 1243. https://doi.org/10.3390/plants9091243








