Transcriptome-Wide Analysis of Nitrogen-Regulated Genes in Tea Plant (Camellia sinensis L. O. Kuntze) and Characterization of Amino Acid Transporter CsCAT9.1
Abstract
:1. Introduction
2. Results
2.1. Transcriptome Analysis Overview Based on PacBio RSII and NGS
2.2. Unigenes Annotation
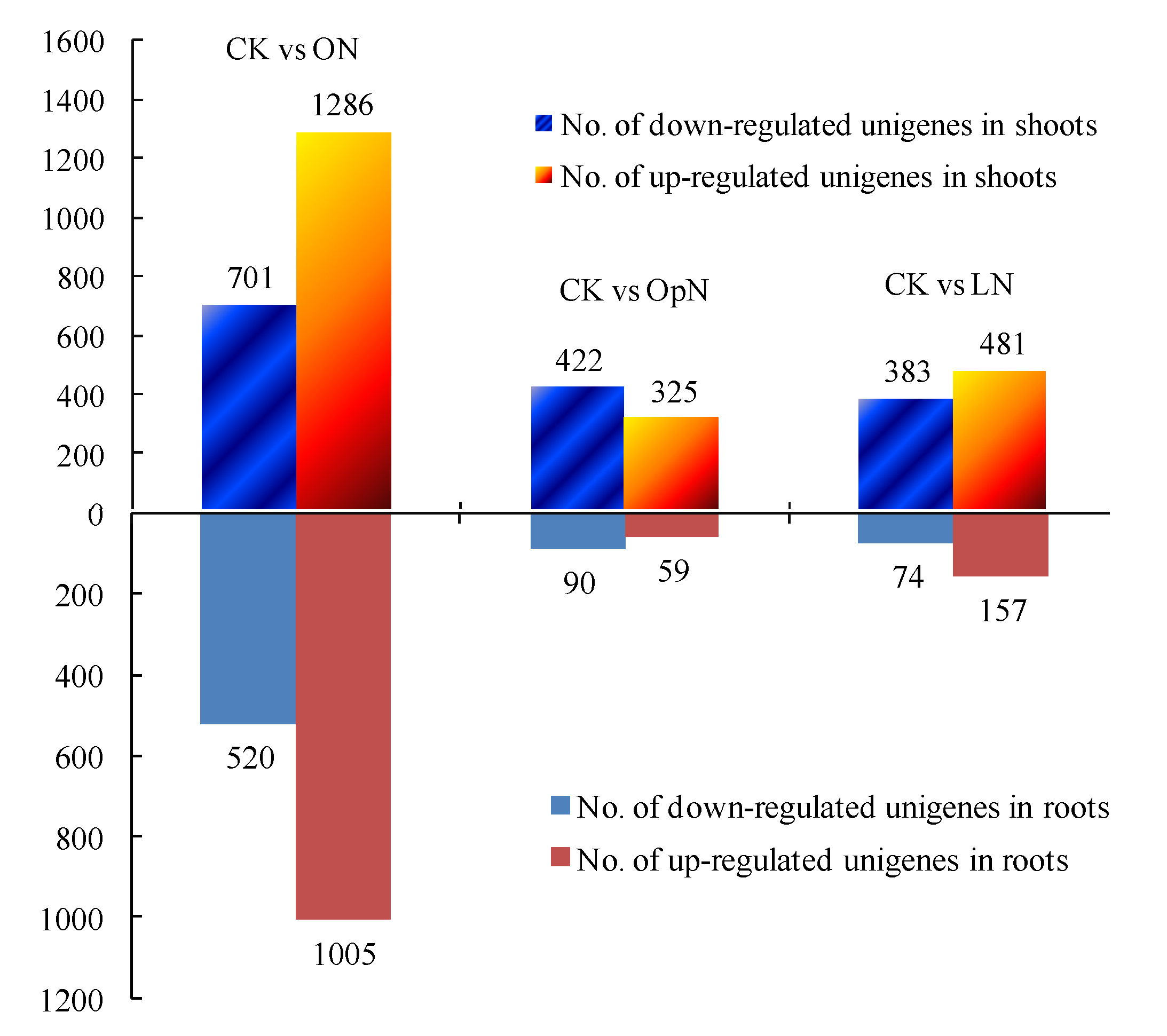
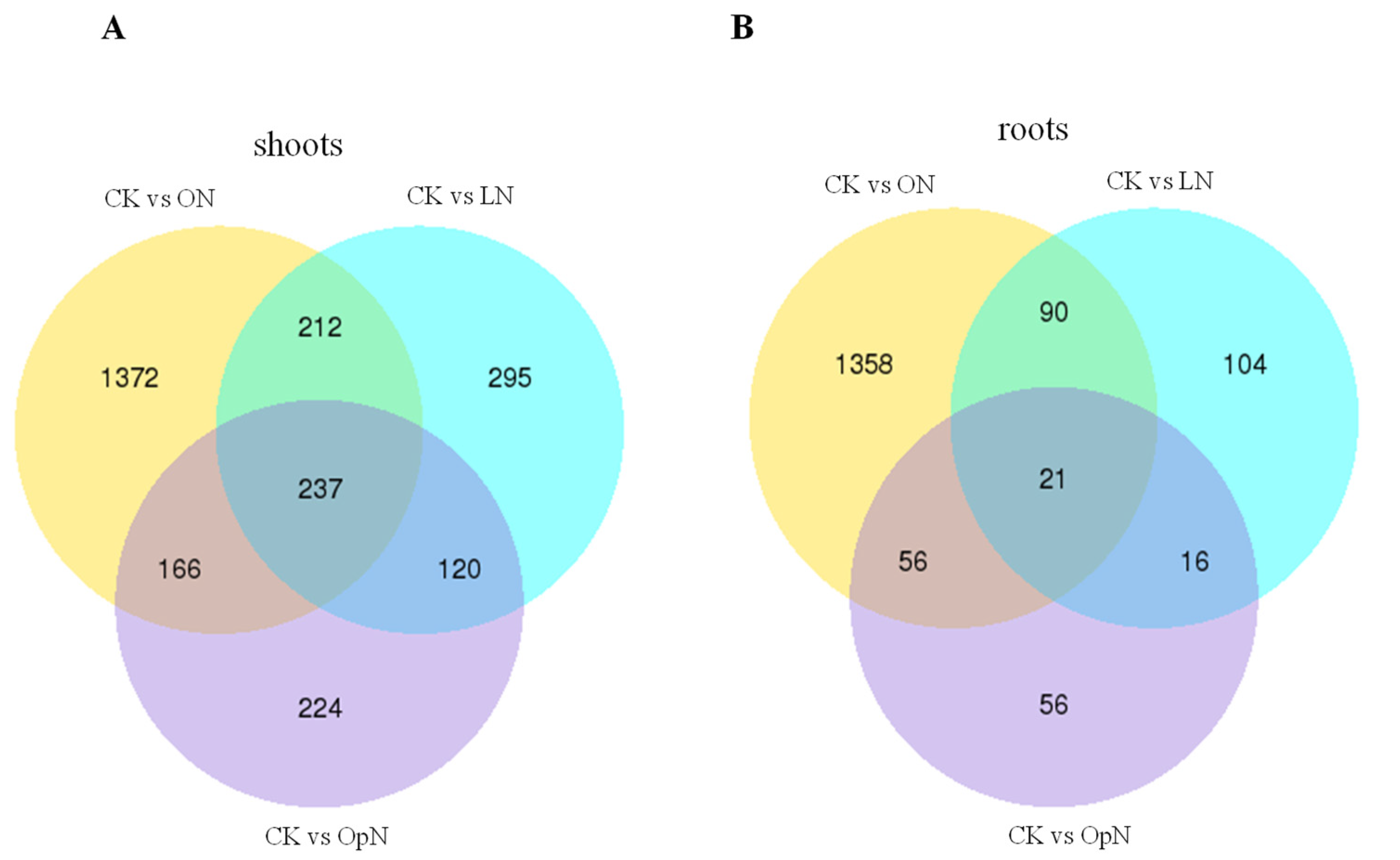
2.3. Global Analysis of Differentially Expressed Genes (DEGs)
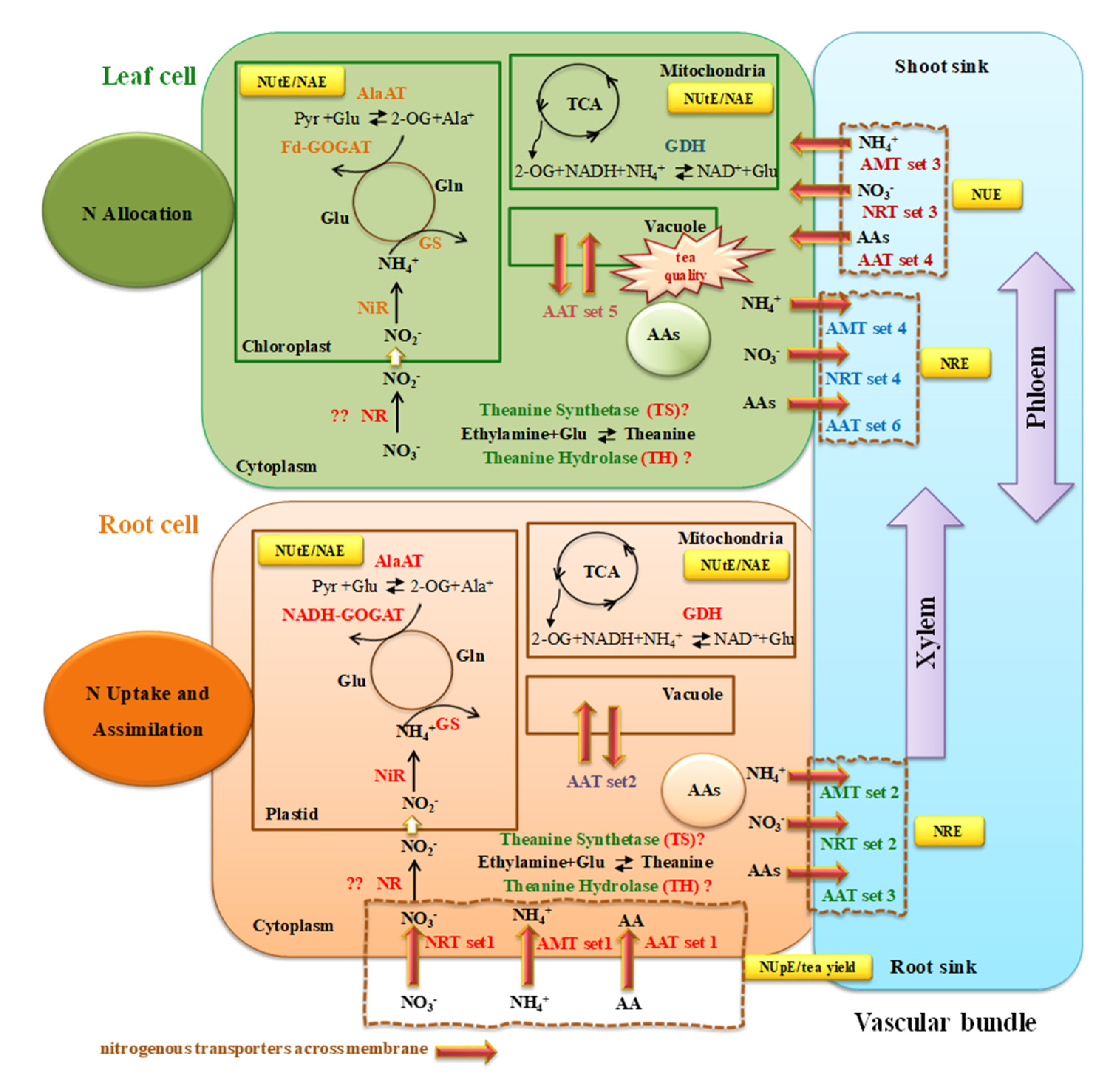
2.4. Transcript Analysis of Genes Involved in N Uptake and Transport
2.5. Transcript Analysis for Genes Involved in N Assimilation
2.6. RNA Sequencing Validation by qRT-PCR
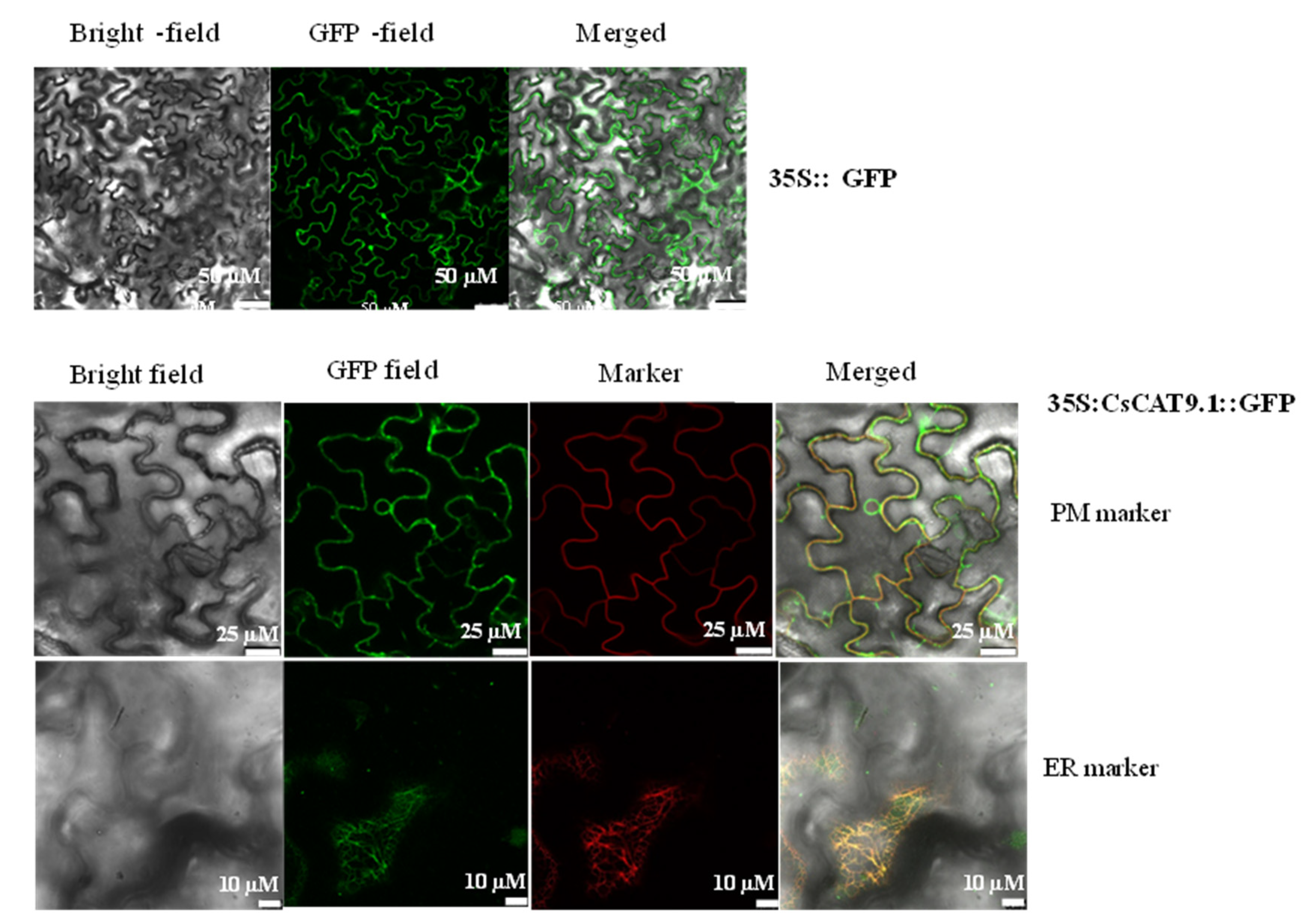
2.7. Subcellular Localization for a Putative Amino Acid Transporter of CsCAT9.1 with Differential Broad-Spectrum for Substrate Selectivity
3. Discussion
4. Materials and Methods
4.1. Tea Plant Materials and N Treatments
4.2. RNA Extraction, Next Generation Sequencing, SMRT Sequencing and Differential Expression Analysis
4.3. Verification of RNA-seq Data by qRT-PCR
4.4. Functional Annotation for Transcriptome Unigenes
4.5. CsCAT9.1 Cloning and Transformation in Arabidopsis
4.6. Subcellular Localization and Amino Acid Substrates Identification of CsCAT9.1
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.P.; Cui, Z.L.; Fan, M.S.; Vitousek, P.; Zhao, M.; Ma, W.Q.; Wang, Z.L.; Zhang, W.J.; Yan, X.Y.; Yang, J.C.; et al. Producing more grain with lower environmental costs. Nature 2014, 514, 486–491. [Google Scholar] [CrossRef]
- Stewart, W.M.; Dibb, D.W.; Johnston, A.E.; Smyth, T.J. The contribution of commercial fertilizer nutrients to food production. Agron. J. 2005, 97, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, A.D.M. Nitrogen use efficiency of crop plants, physiological constraints upon nitrogen absorption. Crit. Rev. Plant Sci. 2003, 22, 453–470. [Google Scholar] [CrossRef]
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Ni, K.; Shi, Y.Z.; Yi, X.Y.; Ji, L.F.; Ma, L.F.; Ruan, J.Y. Heavy nitrogen application increases soil nitrification through ammonia-oxidizing bacteria rather than archaea in acidic tea Camellia sinensis L., plantation soil. Sci. Total Environ. 2020, 717, 137248. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic nitrogen fertilizers deplete soil nitrogen, a global dilemma for sustainable cereal production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef] [Green Version]
- Hirel, B.; Chardon, F.; Durand, J. The contribution of molecular physiology to the improvement of nitrogen use efficiency in crops. J. Crop Sci. Biotech. 2007, 10, 123–132. [Google Scholar]
- Li, H.; Hu, B.; Chu, C.C. Nitrogen use efficiency in crops, lessons from Arabidopsis and rice. J. Exp. Bot. 2017, 68, 2477–2488. [Google Scholar] [CrossRef]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 82, 31–48. [Google Scholar] [CrossRef]
- Näsholm, T.; Persson, J. Plant acquisition of organic nitrogen in boreal forests. Physiol. Plant. 2001, 111, 419–426. [Google Scholar] [PubMed]
- Jones, D.L.; Kielland, K. Amino acid, peptide and protein mineralization dynamics in a taiga forest soil. Soil Biol. Biochem. 2012, 55, 60–69. [Google Scholar] [CrossRef]
- Gioseffi, E.; de Neergaard, A.; Schjoerring, J.K. Interactions between uptake of amino acids and inorganic nitrogen in wheat plants. Biogeosci. 2012, 9, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.Y.; Gerendás, J.; Härdter, R.; Sattelmacher, B. Effect of nitrogen form and root-zone pH on growth and nitrogen uptake of tea (Camellia sinensis) plants. Ann. Bot. 2007, 99, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Ruan, L.; Wei, K.; Wang, L.Y.; Cheng, H.; Zhang, F.; Wu, L.Y.; Bai, P.X.; Zhang, C.C. Characteristics of NH4+ and NO3− fluxes in tea (Camellia sinensis) roots measured by scanning ion-selective electrode technique. Sci. Rep. 2016, 6, 38370. [Google Scholar] [CrossRef]
- Tang, D.D.; Liu, M.Y.; Zhang, Q.F.; Ma, L.F.; Shi, Y.Z.; Ruan, J.Y. Preferential assimilation of NH4+ over NO3− in tea plant associated with genes involved in nitrogen transportation, utilization and catechins biosynthesis. Plant Sci. 2020, 291, 110369. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Q.Y.; Xia, E.H.; Gao, L.Z. Metabolomics and transcriptomics analyses reveal nitrogen influences on the accumulation of flavonoids and amino Acids in young shoots of tea plant Camellia sinensis L., associated with tea flavor. J. Agric. Food Chem. 2018, 66, 9828–9838. [Google Scholar] [CrossRef]
- Liu, M.Y.; Burgos, A.; Ma, L.F.; Zhang, Q.F.; Tang, D.D.; Ruan, J.Y. Lipidomics analysis unravels the effect of nitrogen fertilization on lipid metabolism in tea plant (Camellia sinensis L.). BMC Plant Biol. 2017, 17, 165. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.J.; Zhang, Y.; Han, W.X.; Tang, A.H.; Shen, J.L.; Cui, Z.L.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar]
- Han, M.; Okamoto, M.; Beatty, P.H.; Rothstein, S.J.; Good, A.G. The genetics of nitrogen use efficiency in crop plants. Annu. Rev. Genet. 2015, 49, 269–289. [Google Scholar] [CrossRef]
- Xu, G.H.; Fan, X.R.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Xiang, F.; Zhong, M.C.; Zhou, L.Y.; Liu, H.Y.; Li, S.J.; Wang, X.W. Transcriptome and metabolite analysis identifies nitrogen utilization genes in tea plant (Camellia sinensis). Sci. Rep. 2017, 7, 1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Wang, L.Y.; Bai, P.X.; Wei, K.; Zhang, Y.Z.; Ruan, L.; Wu, L.Y.; Cheng, H. Identification of regulatory networks and hub genes controlling nitrogen uptake in tea plants (Camellia sinensis (L.) O. Kuntze). J. Agric. Food Chem. 2020, 68, 2445–2456. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, H.; Wang, S.; Zhao, J.; Liu, C.; Gao, L.; Xia, E.H.; Lu, Y.; Tai, Y.L.; She, G.B.; et al. Draft genome sequence of Camellia sinensis var. sinensis provides insights into the evolution of the tea genome and tea quality. Proc. Natl. Acad. Sci. USA 2018, 115, E4151–E4158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.J.; Liang, Y.R. (Eds.) Flora of Chinese Clonal Tea Varieties; Shanghai Science and Technology Press: Shanghai, China, 2014. [Google Scholar]
- Ashihara, H. Occurrence, biosynthesis and metabolism of theanine γ-GlutamylL-ethylamide in plants, a comprehensive review. Nat. Prod. Commun. 2015, 10, 803–810. [Google Scholar]
- Ahmed, S.; Griffin, T.S.; Kraner, D.; Schaffner, M.K.; Sharma, D.; Hazel, M.; Leitch, A.R.; Orians, C.M.; Han, W.; Stepp, J.R.; et al. Environmental factors variably impact tea secondary metabolites in the context of climate change. Front. Plant Sci. 2019, 10, 939. [Google Scholar] [CrossRef] [Green Version]
- Han, W.Y.; Huang, J.G.; Li, X.; Li, Z.X.; Ahammed, G.J.; Yan, P.; Stepp, J.R. Altitudinal effects on the quality of green tea in east China, a climate change perspective. Eur. Food Res. Technol. 2017, 243, 323–330. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, B.J.; Chung, J.O.; Hwang, J.A.; Lee, S.J.; Lee, C.H.; Hong, Y.S. Geographical and climatic dependencies of green tea Camellia sinensis, metabolites, a 1H NMR-based metabolomics study. J. Agric. Food Chem. 2010, 58, 10582–10589. [Google Scholar] [CrossRef]
- Wang, L.Y.; Wei, K.; Jiang, Y.W.; Cheng, H.; Zhou, J.; He, W.; Zhang, C.C. Seasonal climate effects on flavanols and purine alkaloids of tea (Camellia sinensis L.). Eur. Food Res. Technol. 2011, 233, 1049–1055. [Google Scholar] [CrossRef]
- Fujiki, Y.; Teshima, H.; Kashiwao, S.; Kawano-Kawada, M.; Ohsumi, Y.; Kakinuma, Y.; Sekito, T. Functional identification of AtAVT3, a family of vacuolar amino acid transporters in Arabidopsis. FEBS Lett. 2017, 591, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Vuong, Q.V.; Bowyer, M.C.; Roach, P.D. L-Theanine, Properties synthesis and isolation from tea. J. Sci. Food Agric. 2011, 91, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.X.; Li, F.; Yang, T.Y.; Feng, L.; Zhang, S.P.; Li, F.D.; Li, W.H.; Xu, G.H.; Bao, S.L.; Wan, X.C.; et al. Theanine transporters identified in tea plants (Camellia sinensis L.). Plant J. 2020, 101, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yang, T.Y.; Zhang, Z.L.; Li, F.D.; Chen, Q.; Sun, J.; Shi, C.Y.; Deng, W.W.; Tao, M.M.; Tai, Y.L.; et al. Identification and characterization of cationic amino acid transporters CATs, in tea plant (Camellia sinensis). Plant Growth Regul. 2018, 84, 57–69. [Google Scholar] [CrossRef]
- Li, F.; Dong, C.; Yang, T.; Ma, J.; Zhang, S.; Wei, C.; Wan, X.; Zhang, Z. Seasonal theanine accumulation and related gene expression in the roots and leaf buds of tea plants (Camellia sinensis L.). Front. Plant Sci. 2019, 10, 1397. [Google Scholar] [CrossRef]
- Xia, E.H.; Li, F.D.; Tong, W.; Li, P.H.; Wu, Q.; Zhao, H.J.; Ge, R.H.; Li, R.P.; Li, Y.Y.; Zhang, Z.Z.; et al. Tea Plant Information Archive, a comprehensive genomics and bioinformatics platform for tea plant. Plant Biotech. J. 2019, 17, 1938–1953. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H.; Crozier, A. Caffeine: A well known but little mentioned compound in plant science. Trends Plant Sci. 2001, 6, 407–413. [Google Scholar] [CrossRef]
- Lu, M.; Han, J.; Zhu, B.; Jia, H.; Yang, T.; Wang, R.; Deng, W.W.; Zhang, Z.Z. Significantly increased amino acid accumulation in a novel albino branch of the tea plant (Camellia sinensis). Planta 2019, 249, 363–376. [Google Scholar] [CrossRef]
- Miller, A.J.; Fan, X.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate transport and signalling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef]
- Yuan, L.; Loqué, D.; Kojima, S.; Rauch, S.; Ishiyama, K.; Inoue, E.; Takahashi, H.; von Wirén, N. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell 2007, 19, 2636–2652. [Google Scholar] [CrossRef] [Green Version]
- Kiba, T.; Krapp, A. Plant nitrogen acquisition under low availability, Regulation of uptake and root architecture. Plant Cell Physiol. 2016, 57, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Vidmar, J.J.; Zhou, D.; Siddiqi, M.Y.; Schjoerring, J.K.; Touraine, B.; Glass, A.D.M. Regulation of high-affinity nitrate transporter genes and high-affinity nitrate influx by nitrogen pools in roots of barley. Plant Physiol. 2000, 123, 307–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Liu, Y.; Wang, L.Y.; Bai, P.X.; Ruan, L.; Zhang, C.C.; Wei, K.; Cheng, H. Molecular cloning and expression analysis of ammonium transporters in tea plants (Camellia sinensis. L.O. Kuntze.) under different nitrogen treatments. Gene 2018, 658, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Kong, H.L.; Li, Y.B.; Wang, L.Q.; Zhong, M.; Sun, L.; Gao, G.J.; Zhang, Q.L.; Luo, L.J.; Wang, G.W.; et al. OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat. Commun. 2014, 5, 4847. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Wu, B.; Wang, J.; Zhu, W.; Nie, H.P.; Qian, J.J.; Huang, W.T.; Fang, Z.M. Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 2018, 16, 1710–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, A.; Collier, R.; Trethewy, A.; Gould, G.; Sieker, R.; Tegeder, M. AAP1 regulates import of amino acids into developing Arabidopsis embryos. Plant J. 2009, 59, 540–555. [Google Scholar] [CrossRef]
- Santiago, J.P.; Tegeder, M. Connecting source with sink, the role of Arabidopsis AAP8 in phloem loading of amino acids. Plant Physiol. 2016, 171, 508–521. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.; Lee, J.; Kichey, T.; Gerentes, D.; Zivy, M.; Tatout, C.; Dubois, F.; Balliau, T.; Valot, B.; Davanture, M.; et al. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell 2006, 18, 3252–3274. [Google Scholar] [CrossRef] [Green Version]
- Brauer, E.K.; Rochon, A.; Bi, Y.M.; Bozzo, G.G.; Rothstein, S.J.; Shelp, B.J. Reappraisal of nitrogen use efficiency in rice overexpressing glutamine synthetase1. Physiol. Plant 2011, 141, 361–372. [Google Scholar] [CrossRef]
- Liu, Z.W.; Wu, Z.J.; Li, H.; Wang, Y.X.; Zhuang, J. L-Theanine content and related gene expression, novel insights into theanine biosynthesis and hydrolysis among different tea plant (Camellia sinensis L.) tissues and cultivars. Front. Plant Sci. 2017, 8, 498. [Google Scholar] [CrossRef] [Green Version]
- Shrawat, A.K.; Carroll, R.T.; Pauw, M.D.; Taylor, G.J.; Good, A.G. Genetic engineering of improved nitrogen use efficiency in rice by the tissue-specific expression of alanine aminotransferase. Plant Biotechnol. J. 2008, 6, 722–732. [Google Scholar] [CrossRef]
- Good, A.G.; Johnson, S.J.; Pauw, M.D.; Carroll, R.T.; Savidov, N.; Vidmar, J.; Lu, Z.J.; Taylor, G.; Stroeher, V. Engineering ni trogen use efficiency with alanine aminotransferase. Can. J. Bot. 2007, 85, 252–262. [Google Scholar]
- Kim, S.A.; Kwak, J.M.; Jae, S.; Wang, M.; Nam, H.G. Overexpression of the AtGluR2 gene encoding an Arabidopsis homolog of mammalian glutamate receptors impairs calcium utilization and sensitivity to ionic stress in transgenic plants. Plant Cell Physiol. 2001, 42, 74–84. [Google Scholar] [PubMed] [Green Version]
- Tegeder, M. Transporters for amino acids in plant cells, some functions and many unknowns. Curr. Opin. Plant Biol. 2012, 15, 315–321. [Google Scholar] [PubMed]
- Au, K.F.; Underwood, J.G.; Lee, L.; Wong, W.H. Improving PacBio long read accuracy by short read alignment. PLoS ONE 2012, 7, e46679. [Google Scholar]
- Love, M.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate, a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 1, 289–300. [Google Scholar]
- Quail, M.A.; Kozarewa, I.; Smith, F.; Scally, A.; Stephens, P.J.; Durbin, R.; Swerdlow, H.; Turner, D.J. A large genome center’s improvements to the Illumina sequencing system. Nat. Methods 2008, 5, 1005–1010. [Google Scholar]
- Gao, Y.Q.; Wu, W.H.; Wang, Y. The K+ channel KZM2 is involved in stomatal movement by modulating inward K+ currents in maize guard cells. Plant J. 2017, 92, 662–675. [Google Scholar]
- Zhao, H.; Huang, W.; Zhang, Y.G.; Zhang, Z.W.; Li, Y.; Tang, C.; Huang, J.; Ni, D.J. Natural variation of CsSTOP1 in tea plant (Camellia sinensis) related to aluminum tolerance. Plant Soil 2018, 431, 71–87. [Google Scholar]



| Databases | Number of Unigenes | Percentage |
|---|---|---|
| NR | 41,238 | 96.07% |
| SwissProt | 35,362 | 82.38% |
| KEGG | 18,264 | 42.55% |
| GO | 22,983 | 53.54% |
| KOG | 26,841 | 62.53% |
| Annotated in all databases | 10,615 | 24.73% |
| Annotated in at least one database | 41,325 | 96.28% |
| Total unigenes | 42,923 | 100% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, H.; Pilon-Smits, E.; Huang, W.; Wang, P.; Wang, M.; Guo, F.; Wang, Y.; Li, R.; Zhao, H.; et al. Transcriptome-Wide Analysis of Nitrogen-Regulated Genes in Tea Plant (Camellia sinensis L. O. Kuntze) and Characterization of Amino Acid Transporter CsCAT9.1. Plants 2020, 9, 1218. https://doi.org/10.3390/plants9091218
Zhang X, Liu H, Pilon-Smits E, Huang W, Wang P, Wang M, Guo F, Wang Y, Li R, Zhao H, et al. Transcriptome-Wide Analysis of Nitrogen-Regulated Genes in Tea Plant (Camellia sinensis L. O. Kuntze) and Characterization of Amino Acid Transporter CsCAT9.1. Plants. 2020; 9(9):1218. https://doi.org/10.3390/plants9091218
Chicago/Turabian StyleZhang, Xinwan, Hongling Liu, Elizabeth Pilon-Smits, Wei Huang, Pu Wang, Mingle Wang, Fei Guo, Yu Wang, Ruiyuan Li, Hua Zhao, and et al. 2020. "Transcriptome-Wide Analysis of Nitrogen-Regulated Genes in Tea Plant (Camellia sinensis L. O. Kuntze) and Characterization of Amino Acid Transporter CsCAT9.1" Plants 9, no. 9: 1218. https://doi.org/10.3390/plants9091218
APA StyleZhang, X., Liu, H., Pilon-Smits, E., Huang, W., Wang, P., Wang, M., Guo, F., Wang, Y., Li, R., Zhao, H., & Ni, D. (2020). Transcriptome-Wide Analysis of Nitrogen-Regulated Genes in Tea Plant (Camellia sinensis L. O. Kuntze) and Characterization of Amino Acid Transporter CsCAT9.1. Plants, 9(9), 1218. https://doi.org/10.3390/plants9091218





