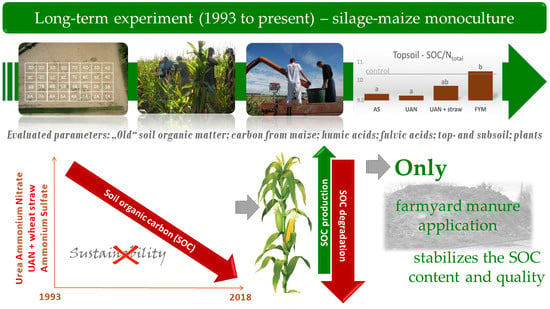
Soil Organic Matter Degradation in Long-Term Maize Cultivation and Insufficient Organic Fertilization
Abstract
:1. Introduction
2. Results
2.1. Yield Parameters
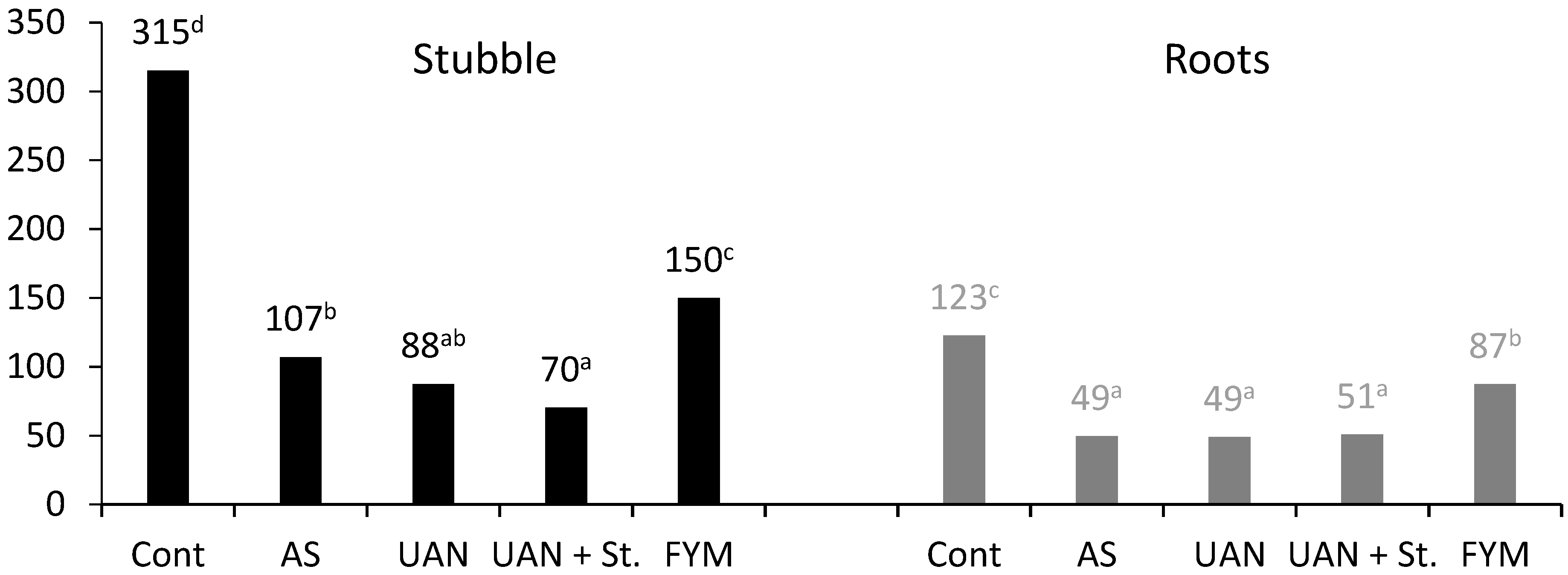
2.2. Carbon Produced by Maize
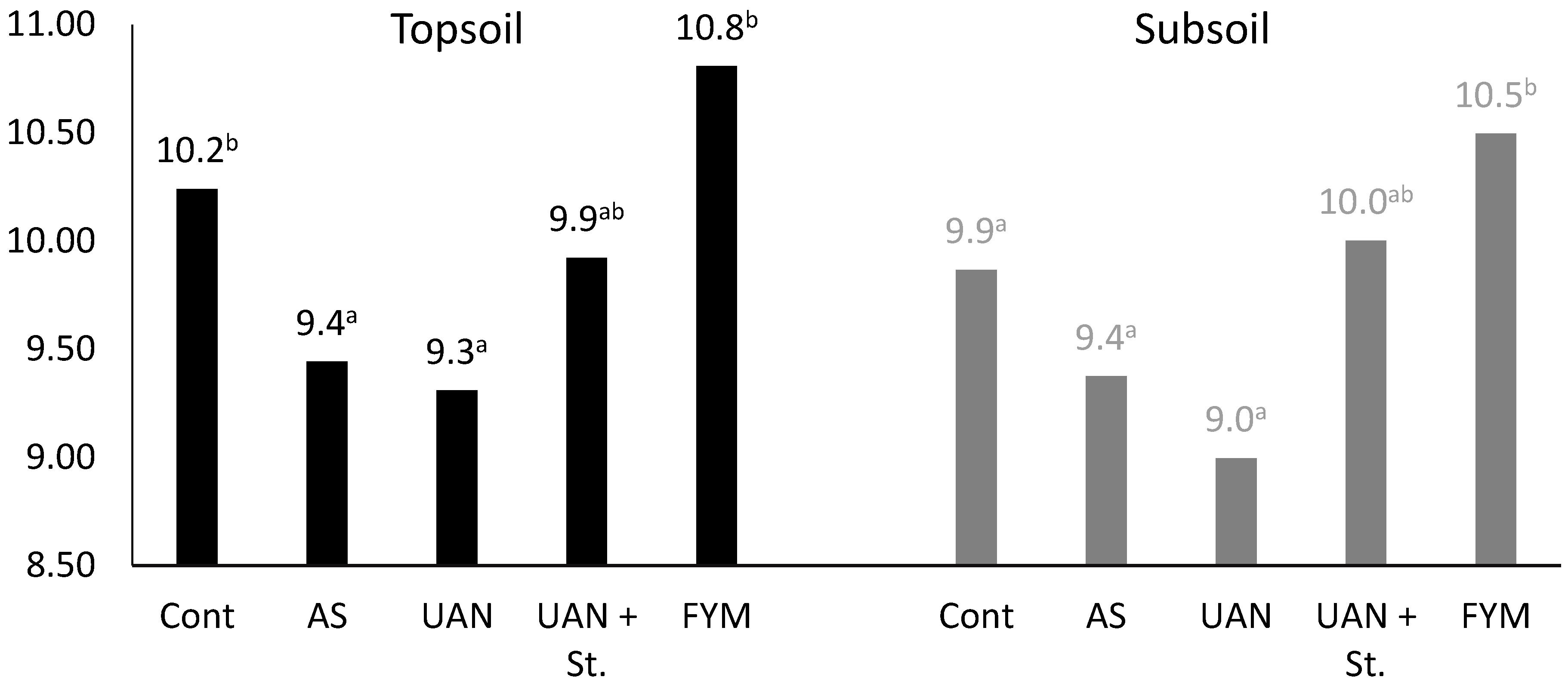
2.3. The Changes of Soil Organic Matter Carbon (CSOM) Contents
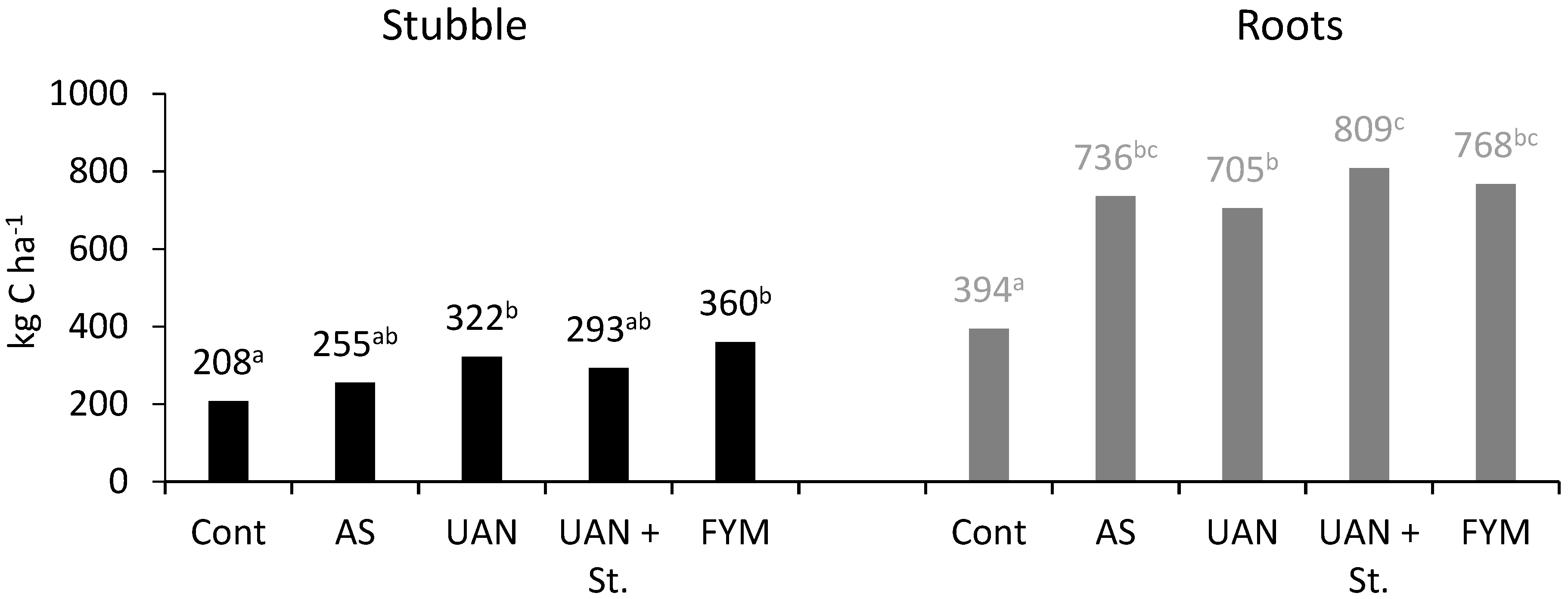
2.4. Carbon Balance
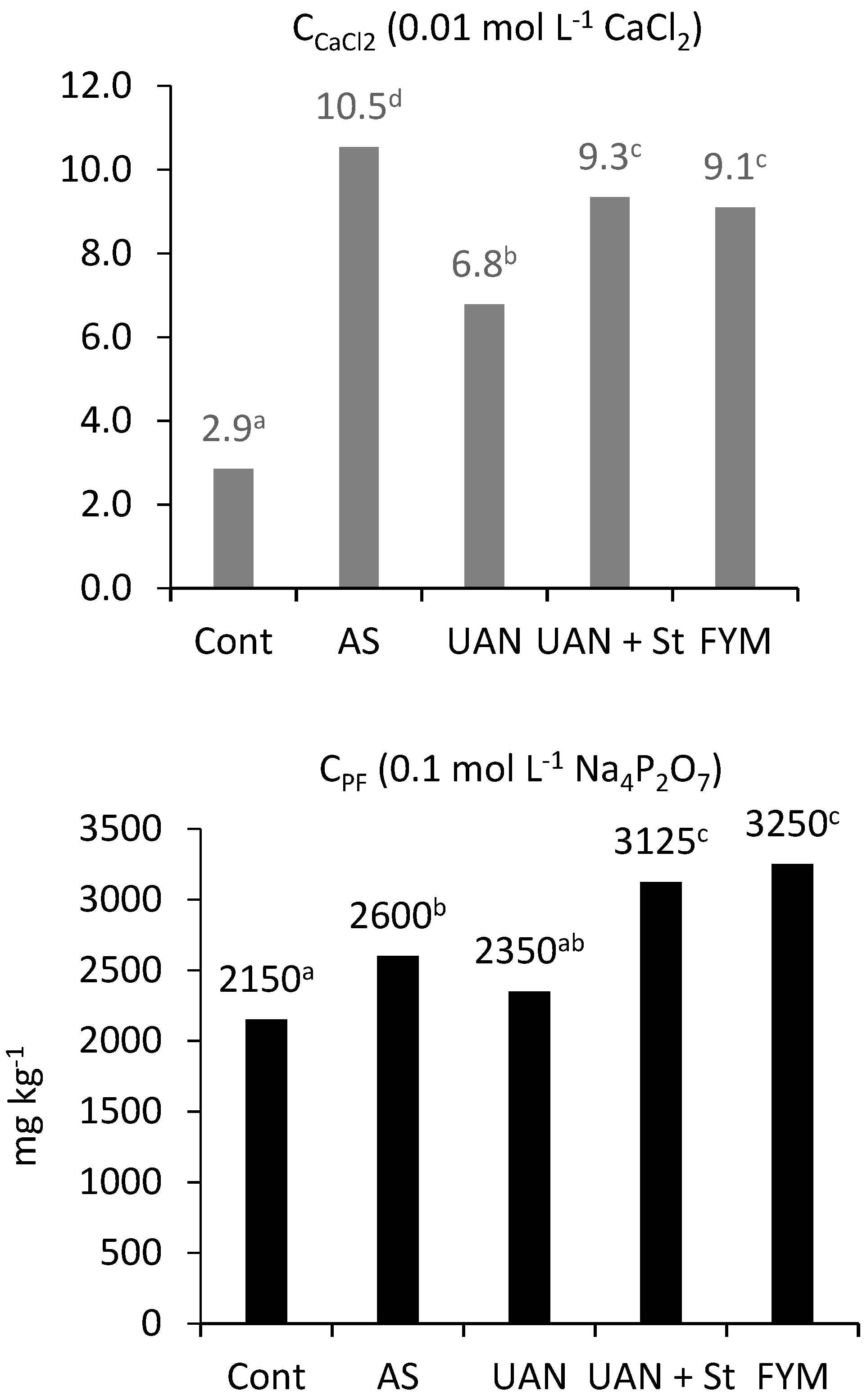
2.5. The Soil Organic Matter Quality Parameters
2.6. The Quality of Post-Harvest Residues
3. Discussion
4. Materials and Methods
4.1. Field Experiments
4.2. Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CSOM | Soil organic matter carbon compounds |
| C3 | “Old” soil organic matter |
| C4 | Carbon from maize |
| CHA | Carbon in humic acids |
| CFA | Carbon in fulvic acids |
| CHU | Carbon in humines |
| CHS | Carbon of humic substances |
| CCaCl2 | Carbon determined with 0.01 mol L−1 CaCl2 |
| CPF | Carbon determined with 0.1. mol L−1 Na4P2O7 |
| CDOC | Easily hydrolysable C fraction |
| Nt | total nitrogen content |
| DM | Dry matter |
| FYM | Farmyard manure |
| UAN | Urea ammonium nitrate |
| St | Straw |
| AS | Ammonium sulfate |
References
- Capriel, P. Trends in organic carbon and nitrogen contents in agricultural soils in Bavaria (South Germany) between 1986 and 2007. Eur. J. Soil Sci. 2013, 64, 445–454. [Google Scholar] [CrossRef]
- Loges, R.; Bunne, I.; Reinsch, T.; Malisch, C.; Kluß, C.; Herrmann, A.; Taube, F. Forage production in rotational systems generates similar yields compared to maize monocultures but improves soil carbon stocks. Eur. J. Agron. 2018, 97, 11–19. [Google Scholar] [CrossRef]
- Schnitzer, M. Organic matter, Principles and Processes. In Encyclopedia of Soils in the Environment, 3rd ed.; Hillel, D., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; pp. 85–93. [Google Scholar]
- Menšík, L.; Hlisnikovský, L.; Pospíšilová, L.; Kunzová, E. The effect of application of organic manures and mineral fertilizers on the state of soil organic matter and nutrients in the long-term field experiment. J. Soil Sediment. 2018, 18, 2813–2822. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Liang, B.C.; Drury, C.F.; MacKenzie, A.F.; McGill, W.B. Elucidation of the source and turnover of water soluble and microbial biomass carbon in agricultural soils. Soil Biol. Biochem. 2000, 32, 581–587. [Google Scholar] [CrossRef]
- Collins, H.P.; Blevins, R.L.; Gundy, L.G.; Christenson, D.R.; Dick, W.A.; Huggins, D.R.; Paul, E.A. Soil carbon dynamics in corn-based agroecosystems: Results from carbon-13 natural abundance. Soil Sci. Soc. Am. J. 1999, 63, 584–591. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, B.; John, B.; Ellerbrock, R.; Kaiser, M.; Flessa, H. Stabilization of maize in sandy soil in a long-term experiment. Eur. J. Soil Sci. 2003, 54, 117–126. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Ellert, B.H.; Drury, C.F.; Liang, B.C. Fertilization effects on soil organic matter turnover and corn residue C storage. Soil Sci. Soc. Am. J. 1996, 60, 472–476. [Google Scholar] [CrossRef]
- Bettina, J.; Ludwig, B.; Flessa, H. Carbon dynamics determined by natural 13C abundance in microcosm experiments with soils from long-term maize and rye monocultures. Soil Biol. Biochem. 2003, 35, 1193–1202. [Google Scholar] [CrossRef]
- Rasse, D.P.; Mulder, J.; Moni, C.; Chenu, C. Carbon Turnover Kinetics with Depth in a French Loamy soil. Soil Sci. Soc. Am. J. 2006, 70, 2097–2105. [Google Scholar] [CrossRef]
- Wanniarachchi, S.D.; Voroney, D.P.; Vyn, T.J.; Beyaert, R.P.; MacKenzie, A.F. Tillage effects on the dynamics of total and corn-residue derived soil organic matter in two Ontario soils. Can. J. Soil Sci. 1999, 79, 473–480. [Google Scholar] [CrossRef]
- Flessa, H.; Ludwig, B.; Heil, B.; Merbach, W. The origin of soil organic C, dissolved organic C and respiration in long-term maize experiment in Halle, Germany, determined by 13C natural abundance. J. Soil Sci. Plant. Nutr. 2000, 163, 157–163. [Google Scholar] [CrossRef]
- Campbell, C.A.; Lafond, G.P.; Biederbeck, V.O.; Wen, G.; Schoenau, J.; Hahn, D. Seasonal trends in soil biochemical attributes: Effects of crop management on a Black Chernozem. Can. J. Soil Sci. 1999, 79, 85–97. [Google Scholar] [CrossRef]
- Qin, R.; Stamp, P.; Richner, W. Impact of tillage on maize rooting in a Cambisol and Luvisol in Switzerland. Soil Till. Res. 2006, 85, 50–61. [Google Scholar] [CrossRef]
- Balesdent, J.; Balabane, M. Maize root-derived soil organic carbon estimated by natural 13C abundance. Soil Biol. Biochem. 1992, 24, 97–101. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Voroney, R.P.; Kachanoski, R.G. Turnover of carbon through the microbial biomass in soils with different texture. Soil Biol. Biochem. 1991, 23, 799–805. [Google Scholar] [CrossRef]
- Ajwa, H.; Rice, C.; Sotomayor, D. Carbon and nitrogen mineralization in tallgrass prairie and agricultural soil profiles. Soil. Sci. Soc. Am. J. 1998, 62, 942–951. [Google Scholar] [CrossRef]
- Eusterhues, K.; Rumpel, C.; Kleber, M.; Kogel-Knaber, I. Stabilisation of soil organic matter by interactions with minerals as revealed by mineral dissolution and oxidative degradation. Org. Geochem. 2003, 34, 1591–1600. [Google Scholar] [CrossRef]
- Anonymous. Biom, Czech Biomass Association. Available online: https://czbiom.cz/blog/2019/06/06/digestat/ (accessed on 16 March 2020).
- Anonymous. Cumulative Summaries about Soil Fund Based on Data from Cadastre of Czech Republic. Available online: https://www.cuzk.cz/Periodika-a-publikace/Statisticke-udaje/Souhrne-prehledy-pudniho-fondu/Rocenka_pudniho_fondu_2020.aspx (accessed on 16 March 2020).
- Vácha, R.; Čechmánková, J.; Duffková, R. Soil–Our Wealth [In Czech]; VÚMOP: Prague, Czech Republic, 2005; 226p. [Google Scholar]
- Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming and Creating Legends for Soil Maps; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; 193p, Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 1 September 2020).
- Hauzé, V.; Tran, G.; Edouard, N.; Lebas, F. Maize Silage. Feedipedia, a Programme by INRA, CIRAD, AFZ and FAO. 2017. Available online: https://www.feedipedia.org/node/13883 (accessed on 31 August 2020).
- Christensen, B.T. Wheat and barely straw decomposition under field conditions: Effect of soil type and plant cover on weight loss, nitrogen and potassium content. Soil Biol. Biochem. 1985, 17, 691–697. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Zhang, Y.; Hu, Y.; Wu, J.; Fu, X.; Le, Y. The variability and causes of organic carbon retention ability of different agricultural straw types returned to soil. Environ. Technol. 2017, 38, 538–548. [Google Scholar] [CrossRef]
- Guan, X.-K.; Wei, L.; Turner, C.N.; Ma, S.-C.; Yang, M.-D.; Wang, T.-C. Improved straw management practices promote in situ straw decomposition and nutrient release, and increase crop production. J. Clean Prod. 2020, 20, 119514. [Google Scholar] [CrossRef]
- Li, D.; Li, Z.; Zhao, B.; Zhang, J. Relationship between the chemical structure of straw and composition of main microbial groups during the decomposition of wheat and maize straws as affected by soil texture. Biol. Fert. Soils. 2020, 56, 11–24. [Google Scholar] [CrossRef]
- Schmidt, L.; Warnstorff, K.; Dörfel, H.; Leinweber, P.; Lange, H.; Merbach, W. The influence of fertilization and rotation on soil organic matter and plant yields in the long-term Eternal Rye trial in Halle (Saale), Germany. J. Plant. Nutr. Soil Sci. 2000, 163, 639–648. [Google Scholar] [CrossRef]
- Liang, B.C.; MacKenzie, A.F. Changes in soil organic carbon and nitrogen after six years of corn production. Soil Sci. 1992, 153, 307–313. [Google Scholar] [CrossRef]
- Balík, J.; Černý, J.; Kulhánek, M.; Sedlář, O. Soil carbon transformation in long-term field experiments with different fertilization treatments. Plant. Soil Environ. 2018, 64, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Ellerbrock, R.H.; Kaiser, M. Stability and composition of different soluble soil organic matter-evidence from δ13C and FTIR signatures. Geoderma 2005, 128, 28–37. [Google Scholar] [CrossRef]
- USDA NRCS. Soil Survey Field and Laboratory Methods Manual; National soil survey center: Lincoln, NE, USA, 2009; 436p.
- International Organization for Standardization. Soil Quality. Determination of Dry Bulk Density. Core Method: ISO 11272-2017; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Kononova, M. M Organičeskoje Veščestvo Počvy: Jego Priroda, Svojstva i Metody Izucenija; AN SSSR: Moscow, Russia, 1963. [Google Scholar]
- Buzek, F.; Cejkova, B.; Gersl, M.; Jackova, I.; Lnenickova, Z.; Gerslova, E. Carbon Isotope Study of soil Amendment with Maize Fermentation Digestate. IJPSS 2017, 14, 1–11. [Google Scholar] [CrossRef]
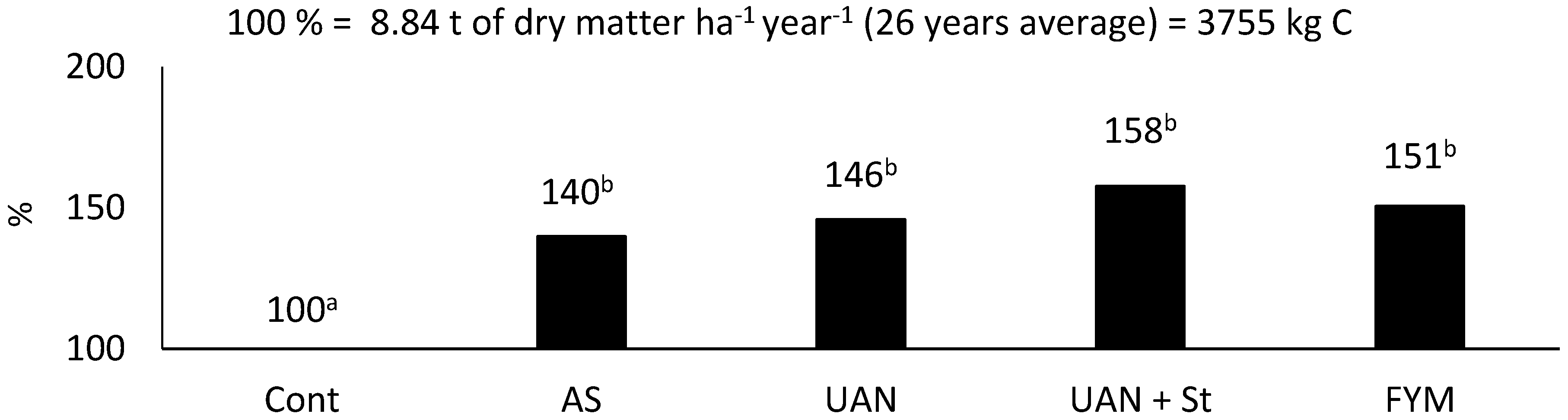
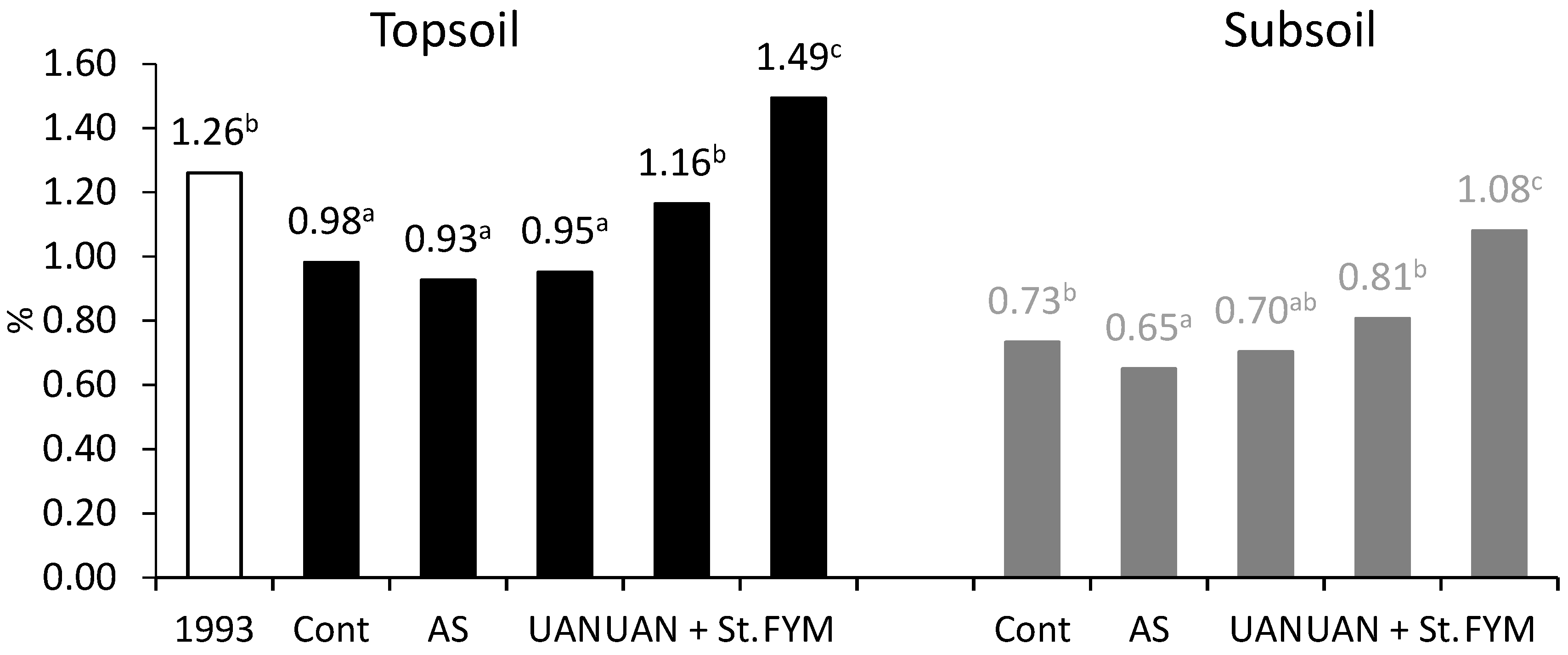
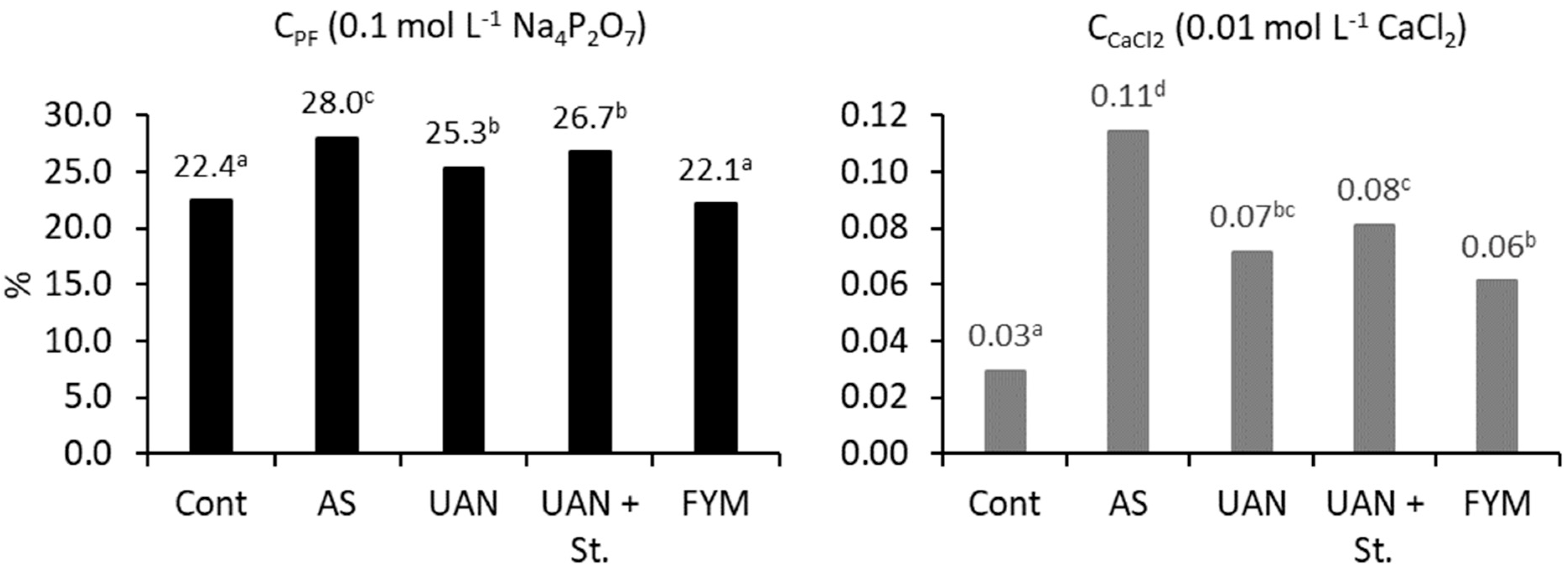

| Treatment | Above Ground Biomass | Roots + Exudates 3 | |||
|---|---|---|---|---|---|
| Transport by Harvest 1 Stubble 2 | ∑ | Roots 2 | Loges et al. [2] Rasse et al. [10] | ||
| Control | 3755 | 208 | 3963 | 394 | 548 |
| AS | 5210 | 255 | 5466 | 736 | 756 |
| UAN | 5421 | 322 | 5743 | 696 | 794 |
| UAN + St | 5854 | 293 | 6147 | 809 | 850 |
| FYM | 5615 | 360 | 5975 | 768 | 826 |
| Treatment | C in Topsoil 1 | C from Org. Fertilizer | C in Topsoil + C from Org. Fert. | C in Stubble 2 | C in Roots 2 | Total C | Loses/Harvest (%) 3 |
|---|---|---|---|---|---|---|---|
| Control | −485 | 0 | 485 | 208 | 394 | 1087 | 28.9 |
| AS | −571 | 0 | 571 | 255 | 736 | 1562 | 29.7 |
| UAN | −537 | 0 | 537 | 322 | 696 | 1555 | 28.4 |
| UAN + St | −173 | 2140 | 2313 | 293 | 809 | 3415 | 57.6 |
| FYM | 398 | 1603 | 1205 | 360 | 768 | 2333 | 41.1 |
| Treatment | Soil Depth (cm) | Origin of C | CSOM (g m−2) | CCaCl2 (g m−2) | CPF (g m−2) |
|---|---|---|---|---|---|
| Control | 0–30 | C3 + C4 | 4322 a | 1.2 a | 970 a |
| C3 | 3973 e | 0.99 d | 872 d | ||
| C4 | 349 g | 0.7 g | 98 g | ||
| 30–60 | C3 + C4 | 3408 o | |||
| C3 | 3221 r | ||||
| C4 | 187 s | ||||
| AS | 0–30 | C3 + C4 | 4101 a | 4.65 c | 1147 b |
| C3 | 3679 d | 3.75 f | 1005 f | ||
| C4 | 422 h | 0.9 h | 142 h | ||
| 30–60 | C3 + C4 | 3023 p | |||
| C3 | 2785 q | ||||
| C4 | 238 s | ||||
| UAN | 0–30 | C3 + C4 | 4190 a | 2.99 b | 1058 a,b |
| C3 | 3697 d | 2.46 e | 904 e,f | ||
| C4 | 493 h | 0.53 h | 154 h | ||
| 30–60 | C3 + C4 | 3269 o,p | |||
| C3 | 3073 q | ||||
| C4 | 196 s |
| GPS Coordinates | 50°4′22″ N; 14°10′19″ E |
|---|---|
| Altitude (m above sea level) | 410 |
| Mean annual temperature (°C) | 7.7 |
| Mean annual precipitation (mm) | 493 |
| Soil type [32] | Haplic Luvisol |
| Soil texture [ 32] | Loam |
| Clay (%) (<0.002 mm) | 5.4 |
| Silt (%) (0.002–0.05 mm) | 68.1 |
| Sand (%) (0.05–2 mm) | 26.5 |
| Bulk density (g cm−3) topsoil [33] | 1.47 |
| Bulk density (g cm−3) subsoil [33] | 1.55 |
| CSOM (%) | 1.26 |
| pH (CaCl2) | 6.5 |
| Cation exchange capacity (mmol(+) kg−1) | 118 |
| Treatment | kg N ha−1 year−1 | Organic Fertilizer (kg DM ha year−1) | C Content in DM (%) | C Supplied (kg ha−1year−1) | C Supplied during 26 Years (kg ha−1) | C/N in Org. Fert. |
|---|---|---|---|---|---|---|
| Control | — | — | — | — | — | — |
| AS | 120 | — | — | — | — | — |
| UAN | 120 | — | — | — | — | — |
| UAN + St | 120 + 33.5 1 | 5000 | 42.8 | 2140 | 55,640 | 79.3/1 |
| FYM | 120 | 5752 | 27.9 | 1603 | 41,678 | 13.4/1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balík, J.; Kulhánek, M.; Černý, J.; Sedlář, O.; Suran, P. Soil Organic Matter Degradation in Long-Term Maize Cultivation and Insufficient Organic Fertilization. Plants 2020, 9, 1217. https://doi.org/10.3390/plants9091217
Balík J, Kulhánek M, Černý J, Sedlář O, Suran P. Soil Organic Matter Degradation in Long-Term Maize Cultivation and Insufficient Organic Fertilization. Plants. 2020; 9(9):1217. https://doi.org/10.3390/plants9091217
Chicago/Turabian StyleBalík, Jiří, Martin Kulhánek, Jindřich Černý, Ondřej Sedlář, and Pavel Suran. 2020. "Soil Organic Matter Degradation in Long-Term Maize Cultivation and Insufficient Organic Fertilization" Plants 9, no. 9: 1217. https://doi.org/10.3390/plants9091217
APA StyleBalík, J., Kulhánek, M., Černý, J., Sedlář, O., & Suran, P. (2020). Soil Organic Matter Degradation in Long-Term Maize Cultivation and Insufficient Organic Fertilization. Plants, 9(9), 1217. https://doi.org/10.3390/plants9091217