Punica protopunica Balf., the Forgotten Sister of the Common Pomegranate (Punica granatum L.): Features and Medicinal Properties—A Review
Abstract
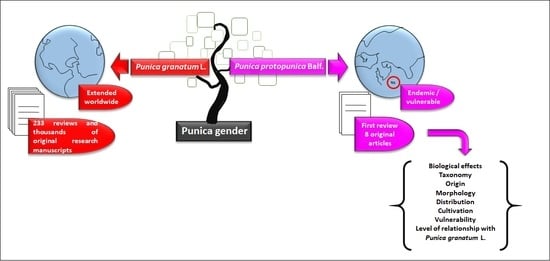
1. Introduction
2. Results
2.1. Taxonomic Positioning and Distribution
2.2. Morphology
2.3. Cultivation
2.4. Vulnerability and Conservation of the Species
2.5. Relationship between P. protopunica Balf., and P. granatum L.
2.6. Uses in Traditional Medicine
2.7. Bioactive Compounds of Pomegranate and Their Medicinal Properties
2.7.1. Phenolic Content and Antioxidant Activity
2.7.2. Antimicrobial, Antiviral and Antiprotozoal Activity
2.7.3. Anticancer Activity
2.7.4. Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Search Criteria
4.2. Data Extraction
5. Conclusions
6. Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conti, E.; Litt, A.; Sytsma, K.J. Circumscription of Myrtales and their relationships to other rosids: Evidence from rbcL sequence data. Am. J. Bot. 1996, 83, 221–233. [Google Scholar] [CrossRef]
- Dahlgren, R.; Thorne, R.F. The order Myrtales: Circumscription, variation, and relationships. Ann. Mo. Bot. Gard. 1984, 633–699. [Google Scholar] [CrossRef]
- Graham, S.A.; Graham, A. Ovary, fruit, and seed morphology of the Lythraceae. Int. J. Plant Sci. 2014, 175, 202–240. [Google Scholar] [CrossRef]
- Stevens, P.F. Angiosperm Phylogeny Website Missouri. Available online: http://www.mobot.org/mobot/research/apweb/welcome.html (accessed on 8 June 2020).
- The Plant List. Lythraceae—The Plant List. 2013. Available online: http://www.theplantlist.org/1.1/browse/A/Lythraceae/ (accessed on 20 August 2020).
- Zeynalova, A.M.; Novruzov, E.N. Origin, taxonomy and systematics of pomegranate. Proc. Inst. Bot. ANAS 2017, 37, 21–26. [Google Scholar]
- Shilkina, I.A. On the xylem anatomy of the genus Punica L. Bot. Zhurnal 1973, 58, 1628–1630. [Google Scholar]
- Graham, S.A.; Hall, J.; Sytsma, K.; Shi, S.-H. Phylogenetic analysis of the Lythraceae based on four gene regions and morphology. Int. J. Plant Sci. 2005, 166, 995–1017. [Google Scholar] [CrossRef]
- Conti, E.; Fischbach, A.; Sytsma, K.J. Tribal relationships in Onagraceae: Implications from rbcL sequence data. Ann. Mo. Bot. Gard. 1993, 672–685. [Google Scholar] [CrossRef]
- Conti, E.; Litt, A.; Wilson, P.G.; Graham, S.A.; Briggs, B.G.; Johnson, L.A.S.; Sytsma, K.J. Interfamilial relationships in Myrtales: Molecular phylogeny and patterns of morphological evolution. Syst. Bot. 1997, 629–647. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, S. Phylogenetics of Lythraceae sensu lato: A Preliminary Analysis Based on Chloroplast rbcL Gene, psaA-ycf3 Spacer, and Nuclear rDNA Internal Transcribed Spacer (ITS) Sequences. Int. J. Plant Sci. 2002, 163, 215–225. [Google Scholar] [CrossRef]
- Shi, S.; Huang, Y.; Tan, F.; He, X.; Boufford, D.E. Phylogenetic analysis of the Sonneratiaceae and its relationship to Lythraceae based on ITS sequences of nrDNA. J. Plant Res. 2000, 113, 253–258. [Google Scholar] [CrossRef]
- Narzary, D.; Ranade, S.A.; Divakar, P.K.; Rana, T.S. Molecular differentiation and phylogenetic relationship of the genus Punica (Punicaceae) with other taxa of the order Myrtales. Rheedea 2016, 26, 37–51. [Google Scholar]
- Stover, E.D.; Mercure, E.W. The pomegranate: A new look at the fruit of paradise. HortScience 2007, 42, 1088–1092. [Google Scholar] [CrossRef]
- Graham, S.A. Lythraceae. In Flowering Plants Eudicots; Springer: Berlin/Heidelberg, Germany, 2007; Volume 9, pp. 226–246. [Google Scholar] [CrossRef]
- International Plant Names Index (IPNI) Punica Protopunica|International Plant Names Index. Available online: https://www.ipni.org/n/554132-1 (accessed on 16 June 2020).
- Mars, M. Pomegranate plant material: Genetic resources and breeding, a review. Options Mediterr. Ser. A 2000, 42, 55–62. [Google Scholar]
- Morton, J.F.; Dowling, C.F. Fruits of Warm Climates; JF Morton: Miami, FL, USA, 1987; Volume 20534. [Google Scholar]
- Chandra, R.; Babu, K.D.; Jadhav, V.T.; Jaime, A.; Silva, T.D. Origin, history and domestication of pomegranate. Fruit Veg. Cereal Sci. Biotechnol. 2010, 2, 1–6. [Google Scholar]
- World of Flowering Plants Punica Protopunica (Socotran Pomegranate). Available online: https://worldoffloweringplants.com/punica-protopunica-socotran-pomegranate/ (accessed on 10 June 2020).
- Hedberg, I.; Hedberg, O. Conservation of vegetation in Africa south of the Sahara. In Proceedings of the A Symposium Held at the 6th Plenary Meeting of the” Association Pour L’étude Taxonomique de la Flore d’Afrique Tropicale”(AETFAT), Uppsala, Sweden, 12–16 September 1966. [Google Scholar]
- Royal Botanic Gardens Kew Punica Protopunica Balf. f|Plants of the World Online|Kew Science. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:554132-1 (accessed on 16 June 2020).
- Tardelli, M.; Baldini, R. Botanical report on the island of Socotra (Yemen). Port. Acta Biol. 2000, 19, 443–454. [Google Scholar]
- Abulhawa, T. Socotra: Enduring bonds between people and place. In World Heritage; UNESCO: Paris, France, 2015; pp. 16–21. [Google Scholar]
- Van-Damme, K. Socotra: Natural History of the Islands and Their People; Odyssey Books & Guides: Hong Kong, China, 2006. [Google Scholar]
- Kosenko, V.N. Palynomorphology of representatives of the family Punicaceae. Bot. Zhurnal 1985, 70, 39–41. [Google Scholar]
- Marriner, D. Socotra Pomegranate Tree (Punica Protopunica): The Vulnerable Predicament of the “Other Pomegranate.” DerdriuMarriner. 2020. Available online: https://wizzley.com/socotra-pomegranate-tree-punica-protopunica/ (accessed on 10 June 2020).
- Integrated Taxonomic Information System (ITIS) Standard Report Page: Punica Granatum. Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=27278#null (accessed on 20 June 2020).
- Miller, A. Punica Protopunica, Pomegranate Tree. The UICN Red List of Threatened Species. 2004. Available online: https://dx.doi.org/10.2305/IUCN.UK.2004.RLTS.T30404A9544416.en (accessed on 23 June 2020).
- Biodiversity Heritage Library. Proceedings of the Royal Society of Edinburgh; Neill and Company: Edinburgh, Scotland, 1882; Volume XI, Available online: https://www.biodiversitylibrary.org/page/48711006 (accessed on 23 June 2020).
- Balfour, I.B. Botany of Socotra. In Transactions of the Royal Society of Edinburgh; Grant, R., Ed.; Neill and Company: Edinburgh, Scotland, 1888; Volume 31, pp. 1–446. [Google Scholar]
- Al Shawish, F.; Hamed, F.; Al-Issa, I. Evaluation of some qualitative and chemical characteristics for the most important pomegranate (Punica granatum) accessions in Yemen. Damascus Univ. J. Agric. Sci. 2006, 22, 227–241. [Google Scholar]
- Youssef, M.; Alhammadi, A.S.; Ramírez-Prado, J.H.; Sánchez-Teyer, L.F.; Escobedo-GraciaMedrano, R.M. Remarks on genetic diversity and relationship of Punica protopunica and P. granatum assessed by molecular analyses. Genet. Resour. Crop Evol. 2018, 65, 577–590. [Google Scholar] [CrossRef]
- Miller, A.G.; Morris, M. Ethnoflora of the Soqotra Archipelago; Royal Botanic Garden Edinburgh: Edinburgh, Scotland, 2004; ISBN 1872291597. [Google Scholar]
- Van-Damme, K.; Banfield, L. Past and present human impacts on the biodiversity of Socotra Island (Yemen): Implications for future conservation. Zool. Middle East 2011, 54, 31–88. [Google Scholar] [CrossRef]
- UNESCO World Heritage Centre. Operational Guidelines for the Implementation of the World Heritage Convention; UNESCO: Paris, France, 1999; Available online: https://whc.unesco.org/en/guidelines/ (accessed on 26 June 2020).
- Pironnet, T.; Marsh, E. Proteger a las Personas a Través de la Naturaleza; World Wide Fund for Nature: Gland, Switzerland, 2016; ISBN 9782940529315. [Google Scholar]
- UNESCO World Heritage Centre. Socotra Archipelago (Yemen). Available online: https://whc.unesco.org/en/soc/3891 (accessed on 27 June 2020).
- Clearing House Mechanism of Biodiversity in Yemen Socotra Conservation and Development Programme. Available online: http://ye.chm-cbd.net/information-1/links/related-links/socotra-conservation-and-development-programme (accessed on 28 June 2020).
- Levin, G.M. Pomegranate, 1st ed.; East Libr. Drive Tempe Third Millenn.: St Petersburg, Russia, 2006; pp. 1–129. [Google Scholar]
- Muhammad, N.; Uddin, N.; Liu, M.; Ali, N. Ethno medicine, antioxidant potential and inter-specific variations of Punica L. species growing in Swat district, KP, Pakistan. Int. J. Bot. Stud. 2019, 4, 36–46. [Google Scholar]
- Mothana, R.A. Island Soqotra: A unique source of medicinal plants with anticancer, antimicrobial, antiviral and antiprotozoal potentials. UOFK 2015, 31, 45–49. [Google Scholar]
- Al-Dubai, A.S.; Al-Khulaidi, A.A. Medicinal and Aromatic Plants of Yemen (In Arabic) Sana’a. Obadi Center for Studies and Publishing: Sana’a, Yemen, 1996; pp. 125–130. [Google Scholar]
- Barzinji, A.K.R.; Nasher, A.K.; Mothana, R.A.; Al-Hamadi, M.M.S. In vitro Antimalarial activity of selected Yemeni plants used in traditional medicine. International Journal of Medicinal Plants. Photon 2014, 107, 526–535. [Google Scholar]
- Mothana, R.A.; Al-Musayeib, N.M.; Matheeussen, A.; Cos, P.; Maes, L. Assessment of the in vitro antiprotozoal and cytotoxic potential of 20 selected medicinal plants from the island of Soqotra. Molecules 2012, 17, 14349–14360. [Google Scholar] [CrossRef]
- Schopen, A. Traditionelle Heilmittel in Jemen; Steiner: Wiesbaden, Germany, 1983; ISBN 3515040676. [Google Scholar]
- Al-Huqail, A.A.; Elgaaly, G.A.; Ibrahim, M.M. Identification of bioactive phytochemical from two Punica species using GC–MS and estimation of antioxidant activity of seed extracts. Saudi J. Biol. Sci. 2018, 25, 1420–1428. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phyther. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Lindequist, U. Antimicrobial activity of some medicinal plants of the island Soqotra. J. Ethnopharmacol. 2005, 96, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Mothana, R.A.A.; Mentel, R.; Reiss, C.; Lindequist, U. Phytochemical screening and antiviral activity of some medicinal plants from the island Soqotra. Phyther. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 298–302. [Google Scholar] [CrossRef]
- Mothana, R.A.A.; Gruenert, R.; Lindequist, U.; Bednarski, P.J. Study of the anticancer potential of Yemeni plants used in folk medicine. Die Pharm. Int. J. Pharm. Sci. 2007, 62, 305–307. [Google Scholar]
- Vasconcelos, R.; Pujol-Buxó, E.; Llorente, G.A.; Saeed, A.; Carranza, S. Micro-hotspots for conservation: An umbrella tree species for the unique Socotran reptile fauna. Forests 2020, 11, 353. [Google Scholar] [CrossRef]
- Autin, J.; Bellahsen, N.; Leroy, S.; Husson, L.; Beslier, M.-O.; d’Acremont, E. The role of structural inheritance in oblique rifting: Insights from analogue models and application to the Gulf of Aden. Tectonophysics 2013, 607, 51–64. [Google Scholar] [CrossRef]
- Macey, J.R.; Kuehl, J.V.; Larson, A.; Robinson, M.D.; Ugurtas, I.H.; Ananjeva, N.B.; Rahman, H.; Javed, H.I.; Osman, R.M.; Doumma, A. Socotra Island the forgotten fragment of Gondwana: Unmasking chameleon lizard history with complete mitochondrial genomic data. Mol. Phylogenet. Evol. 2008, 49, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Balini, M. The Himalayan connection of the Middle Triassic brachiopod fauna from Socotra (Yemen). Bull. Geosci. 2018, 93, 2. [Google Scholar] [CrossRef]
- Kumar, P.A.; Sudhakaran, S.; Mohan, T.C.; Pamanna, D.; Kumar, P.R.; Shanthanna, P. Evaluation of colour enhance potential of three natural plant pigment sources (African tulip tree flower, red paprika, pomegranate peel) in goldfish (Carassius auratus). Int. J. Fish. Aquat. Stud. 2017, 5, 47–51. [Google Scholar]
- Fahan, A. The leaf. The flower. The seed. Plant Anat. Hakkibutz Hameuhad Publ. House Ltd. Jerus. 1976, 171–212. [Google Scholar]
- Verma, N.; Mohanty, A.; Lal, A. Pomegranate genetic resources and germplasm conservation: A review. Fruit Veg. Cereal Sci. Biotechnol. 2010, 4, 120–125. [Google Scholar]
- Frison, E.A.; Servinsky, J. Directory of European Institutions Holding Crop Genetic Resources Collections: Vol. 2. Index; European Cooperative Programme for Plant Genetic Resources: Rome, Italy, 1995; ISBN 9290432594. [Google Scholar]
- Heber, D.; Schulman, R.N.; Seeram, N.P. Pomegranates: Ancient Roots to Modern Medicine; CRC Press: Boca Raton, FL, USA, 2006; ISBN 1420009869. [Google Scholar] [CrossRef]
- Still, D.W. Chapter 13 Pomegranates: A Botanical Perspective. In Pomegranates: Ancient Roots to Modern Medicine; Seeram, N.P., Heber, D., Shulman, R.N., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 199–210. ISBN 1420009869. [Google Scholar]
- Da Silva, J.A.T.; Rana, T.S.; Narzary, D.; Verma, N.; Meshram, D.T.; Ranade, S.A. Pomegranate biology and biotechnology: A review. Sci. Hortic. 2013, 160, 85–107. [Google Scholar] [CrossRef]
- Mirjalili, S.A. Pomegranate: Biodiversity and genetic resources, a review. Rostaniha 2016, 17, 1–18. [Google Scholar]
- Tosco, R.B.; Navarro, M.L.R.; Scholz, S. Archipiélagos macaronésicos (VIII) y otras islas del mundo. Makaronesia Boletín Asoc. Amigos Mus. Cienc. Nat. Tenerife 2007, 9, 120–129. [Google Scholar]
- Oleski, A.; Lindequist, U.; Mothana, R.A.A.; Melzig, M.F. Screening of selected Arabian medicinal plant extracts for inhibitory activity against peptidases. Die Pharm. Int. J. Pharm. Sci. 2006, 61, 359–361. [Google Scholar]
- Amin, G.H. Popular medical plants of Iran. Farhang Publ. Tehran 1991, 61, 51–52. [Google Scholar]
- Lansky, E.; Shubert, S.; Neeman, I. Pharmacological and therapeutic properties of pomegranate. In Proceedings of the Symposium on Production, Processing and Marketing of Pomegranate in the Mediterranean Region: Advances in Research and Technology. Séminaires Méditerranéens (CIHEAM), Orihuela, Spain, 15–17 October 2000; pp. 231–235. [Google Scholar]
- Ma, K.I.; Du, M.; Liao, M.; Chen, S.; Yin, G.; Liu, Q.; Wei, Q.; Qin, G. Evaluation of wound healing effect of punica granatum L Peel extract on deep second-degree burns in rats. Trop. J. Pharm. Res. 2015, 14, 73–78. [Google Scholar] [CrossRef]
- Poyrazoğlu, E.; Gökmen, V.; Artιk, N. Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey. J. Food Compos. Anal. 2002, 15, 567–575. [Google Scholar] [CrossRef]
- Tanveer, A.; Farooq, U.; Akram, K.; Hayat, Z.; Shafi, A.; Nazar, H.; Ahmad, Z. Pomegranate extracts: A natural preventive measure against spoilage and pathogenic microorganisms. Food Rev. Int. 2015, 31, 29–51. [Google Scholar] [CrossRef]
- Chhetri, B.K.; Ali, N.A.A.; Setzer, W.N. A survey of chemical compositions and biological activities of Yemeni aromatic medicinal plants. Medicines 2015, 2, 67–92. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Rahemi, M. Seasonal changes of mineral nutrients and phenolics in pomegranate (Punica granatum L.) fruit. Sci. Hortic. 2007, 111, 120–127. [Google Scholar] [CrossRef]
- Ozgen, M.; Durgaç, C.; Serçe, S.; Kaya, C. Chemical and antioxidant properties of pomegranate cultivars grown in the Mediterranean region of Turkey. Food Chem. 2008, 111, 703–706. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Bhandari, P.R. Pomegranate (Punica granatum L). Ancient seeds for modern cure? Review of potential therapeutic applications. Int. J. Nutr. Pharmacol. Neurol. Dis. 2012, 2, 171. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Balli, D.; Cecchi, L.; Khatib, M.; Bellumori, M.; Cairone, F.; Carradori, S.; Zengin, G.; Cesa, S.; Innocenti, M.; Mulinacci, N. Characterization of Arils Juice and Peel Decoction of Fifteen Varieties of Punica granatum L.: A Focus on Anthocyanins, Ellagitannins and Polysaccharides. Antioxidants 2020, 9, 238–261. [Google Scholar] [CrossRef]
- Deng, Y.L.; Li, Y.L.; Zheng, T.T.; Hu, M.X.; Ye, T.H.; Xie, Y.; Yin, W.Y. The extract from Punica granatum (pomegranate) leaves promotes apoptosis and impairs metastasis in prostate cancer cells. J. Sichuan Univ. Med. Sci. Ed. 2018, 49, 8–12. [Google Scholar]
- Wang, Z.; Chen, Y. Antihypertensive Effect of Pomegranate Polyphenols in Spontaneously Hypertensive Rats. China Pharm. 2016, 19, 255–258. [Google Scholar]
- Neyrinck, A.M.; Van Hée, V.F.; Bindels, L.B.; De Backer, F.; Cani, P.D.; Delzenne, N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013, 109. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Jungbauer, A. Pomegranate: A fruit that ameliorates metabolic syndrome. Food Funct. 2013, 4, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Stowe, C.B. The effects of pomegranate juice consumption on blood pressure and cardiovascular health. Complement. Ther. Clin. Pract. 2011, 17, 113–115. [Google Scholar] [CrossRef]
- Betanzos-Cabrera, G.; Guerrero-Solano, J.A.; Martínez-Pérez, M.M.; Calderón-Ramos, Z.G.; Belefant-Miller, H.; Cancino-Diaz, J.C. Pomegranate juice increases levels of paraoxonase1 (PON1) expression and enzymatic activity in streptozotocin-induced diabetic mice fed with a high-fat diet. Food Res. Int. 2011, 44, 1381–1385. [Google Scholar] [CrossRef]
- Silva, N.C.C.; Fernandes Júnior, A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Mohan, M.; Waghulde, H.; Kasture, S. Effect of pomegranate juice on Angiotensin II-induced hypertension in diabetic wistar rats. Phyther. Res. 2010, 24, S196–S203. [Google Scholar] [CrossRef]
- Miguel, M.G.; Neves, M.A.; Antunes, M.D. Pomegranate (Punica granatum L.): A medicinal plant with myriad biological properties-A short review. J. Med. Plant Res. 2010, 4, 2836–2847. [Google Scholar]
- Hossin, F.L.A. Effect of pomegranate (Punica granatum) peels and it’s extract on obese hypercholesterolemic rats. Pak. J. Nutr. 2009, 8, 1251–1257. [Google Scholar] [CrossRef]
- Bagri, P.; Ali, M.; Aeri, V.; Bhowmik, M.; Sultana, S. Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem. Toxicol. 2009, 47, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Zhang, X.N.; Wang, W.; Xing, D.M.; Xie, W.D.; Su, H.; Du, L.J. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obes. 2007, 31, 1023–1029. [Google Scholar] [CrossRef]
- Rosenblat, M.; Hayek, T.; Aviram, M. Anti-oxidative effects of pomegranate juice (PJ) consumption by diabetic patients on serum and on macrophages. Atherosclerosis 2006, 187, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 2001, 158, 195–198. [Google Scholar] [CrossRef]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate Juice, Total Pomegranate Ellagitannins, and Punicalagin Suppress Inflammatory Cell Signaling in Colon Cancer Cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef]
- Hora, J.J.; Maydew, E.R.; Lansky, E.P.; Dwivedi, C. Chemopreventive Effects of Pomegranate Seed Oil on Skin Tumor Development in CD1 Mice. J. Med. Food 2003, 6, 157–161. [Google Scholar] [CrossRef]
- Khan, N.; Hadi, N.; Afaq, F.; Syed, D.N.; Kweon, M.-H.; Mukhtar, H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis 2007, 28, 163–173. [Google Scholar] [CrossRef]
- Kim, N.D.; Mehta, R.; Yu, W.; Neeman, I.; Livney, T.; Amichay, A.; Poirier, D.; Nicholls, P.; Kirby, A.; Jiang, W.; et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treat. 2002, 71, 203–217. [Google Scholar] [CrossRef]
- Malik, A.; Afaq, F.; Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Mukhtar, H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 14813–14818. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Lansky, E.P. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eur. J. Cancer Prev. 2004, 13. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Noratto, G.; Hingorani, L.; Talcott, S.T.; Mertens-Talcott, S.U. Protective Effects of Standardized Pomegranate (Punica granatum L.) Polyphenolic Extract in Ultraviolet-Irradiated Human Skin Fibroblasts. J. Agric. Food Chem. 2008, 56, 8434–8441. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Galli, G.V.; Bulgari, M.; Basilico, N.; Romeo, S.; Bhattacharya, D.; Taramelli, D.; Bosisio, E. Ellagitannins of the fruit rind of pomegranate (Punica granatum) antagonize in vitro the host inflammatory response mechanisms involved in the onset of malaria. Malar. J. 2010, 9, 208. [Google Scholar] [CrossRef]
- Cerdá, B.; Cerón, J.J.; Tomás-Barberán, F.A.; Espín, J.C. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J. Agric. Food Chem. 2003, 51, 3493–3501. [Google Scholar] [CrossRef]
- El Deeb, K.S.; Eid, H.H.; Ali, Z.Y.; Shams, M.M.; Elfiky, A.M. Bioassay-guided fractionation and identification of antidiabetic compounds from the rind of Punica Granatum Var. nana. Nat. Prod. Res. 2019, 1–4. [Google Scholar] [CrossRef]
- Gautam, R.K.; Sharma, S.; Sharma, K.; Gupta, G. Evaluation of Antiarthritic Activity of Butanol Fraction of Punica granatum Linn. Rind Extract against Freund’s Complete Adjuvant-Induced Arthritis in Rats. J. Environ. Pathol. Toxicol. Oncol. 2018, 37. [Google Scholar] [CrossRef]
- Kulkarni, A.P.; Mahal, H.S.; Kapoor, S.; Aradhya, S.M. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J. Agric. Food Chem. 2007, 55, 1491–1500. [Google Scholar] [CrossRef]
- Patel, C.; Dadhaniya, P.; Hingorani, L.; Soni, M.G. Safety assessment of pomegranate fruit extract: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2008, 46, 2728–2735. [Google Scholar] [CrossRef]
- Ouachrif, A.; Khalki, H.; Chaib, S.; Mountassir, M.; Aboufatima, R.; Farouk, L.; Benharraf, A.; Chait, A. Comparative study of the anti-inflammatory and antinociceptive effects of two varieties of Punica granatum. Pharm. Biol. 2012, 50, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Fallarero, A.; Peña, B.R.; Medina, M.E.; Gra, B.; Rivera, F.; Gutierrez, Y.; Vuorela, P.M. Studies on the toxicity of Punica granatum L.(Punicaceae) whole fruit extracts. J. Ethnopharmacol. 2003, 89, 295–300. [Google Scholar] [CrossRef] [PubMed]
| Classification | Denomination | Common Name |
|---|---|---|
| Order | Myrtales | - |
| Family | Lythraceae | Loosestrife |
| Subfamily | Punicoideae | - |
| Genera | Punica | Pomegranate |
| Species | Punica protopunica | Wild pomegranate, Socotra pomegranate |
| Punica protopunica Balf. | Morphological Characteristics |
|---|---|
| General habit | The tree can reach a height of 2.5 to 4.5 m. It is considered a small tree or shrub, but if it reaches more than 9.14 m it can be classified as a tree, that is, it can be considered as both main forms. There are trees that are wider than they are tall (for example, trees that grow on the slopes rocks of Socotra) and trees taller than they are wide (typical of trees growing on the Socotra limestone plateau.) Generally, the tree is equal in width to height, with an upright shrub appearance. |
| Bark, branches and trunk | The bark is reddish-brown when the tree is young, but changes to a grayish hue as it grows older. The branches have thorns. |
| Leaves | The leaves grow to a length of 3 cm, in pairs on the opposite sides of the stalk, they are perennial, their most common shape is elliptical or oblong, although there are also circular or oval and obovate leaves (a single branch can have leaves of all the shapes described). Its color is dark green, with a bright tone. |
| Flowers | The flowers have obovate or oval petals, although they are sometimes heart-shaped. Its color is light pink with glitter and its shape is “trumpet”. Flowering occurs from December until the summer of the following year. Their physiology allows them to produce fertilization and pollination. It is a self-crossing species. |
| Fruits | The shape of the fruits is almost identical to that of modified tangelos (Citrus x tangelo). They retain their floral chalice tube. The peel is hard and its color when ripe is light green to greenish yellow and may or may not contain pink reflections. Inside they present a spongy pericarp with membranes (endocarp) that separate the arils into compartments. In turn, each aril contains a membrane, pulp juice and a seed. |
| Seeds | The seeds are inside the arils and there are hundreds of them, they are relatively light. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero-Solano, J.A.; Jaramillo-Morales, O.A.; Jiménez-Cabrera, T.; Urrutia-Hernández, T.A.; Chehue-Romero, A.; Olvera-Hernández, E.G.; Bautista, M. Punica protopunica Balf., the Forgotten Sister of the Common Pomegranate (Punica granatum L.): Features and Medicinal Properties—A Review. Plants 2020, 9, 1214. https://doi.org/10.3390/plants9091214
Guerrero-Solano JA, Jaramillo-Morales OA, Jiménez-Cabrera T, Urrutia-Hernández TA, Chehue-Romero A, Olvera-Hernández EG, Bautista M. Punica protopunica Balf., the Forgotten Sister of the Common Pomegranate (Punica granatum L.): Features and Medicinal Properties—A Review. Plants. 2020; 9(9):1214. https://doi.org/10.3390/plants9091214
Chicago/Turabian StyleGuerrero-Solano, José Antonio, Osmar Antonio Jaramillo-Morales, Tania Jiménez-Cabrera, Thania Alejandra Urrutia-Hernández, Alejandro Chehue-Romero, Elena G. Olvera-Hernández, and Mirandeli Bautista. 2020. "Punica protopunica Balf., the Forgotten Sister of the Common Pomegranate (Punica granatum L.): Features and Medicinal Properties—A Review" Plants 9, no. 9: 1214. https://doi.org/10.3390/plants9091214
APA StyleGuerrero-Solano, J. A., Jaramillo-Morales, O. A., Jiménez-Cabrera, T., Urrutia-Hernández, T. A., Chehue-Romero, A., Olvera-Hernández, E. G., & Bautista, M. (2020). Punica protopunica Balf., the Forgotten Sister of the Common Pomegranate (Punica granatum L.): Features and Medicinal Properties—A Review. Plants, 9(9), 1214. https://doi.org/10.3390/plants9091214