Phosphate Deprivation Can Impair Mechano-Stimulated Cytosolic Free Calcium Elevation in Arabidopsis Roots
Abstract
1. Introduction
2. Results
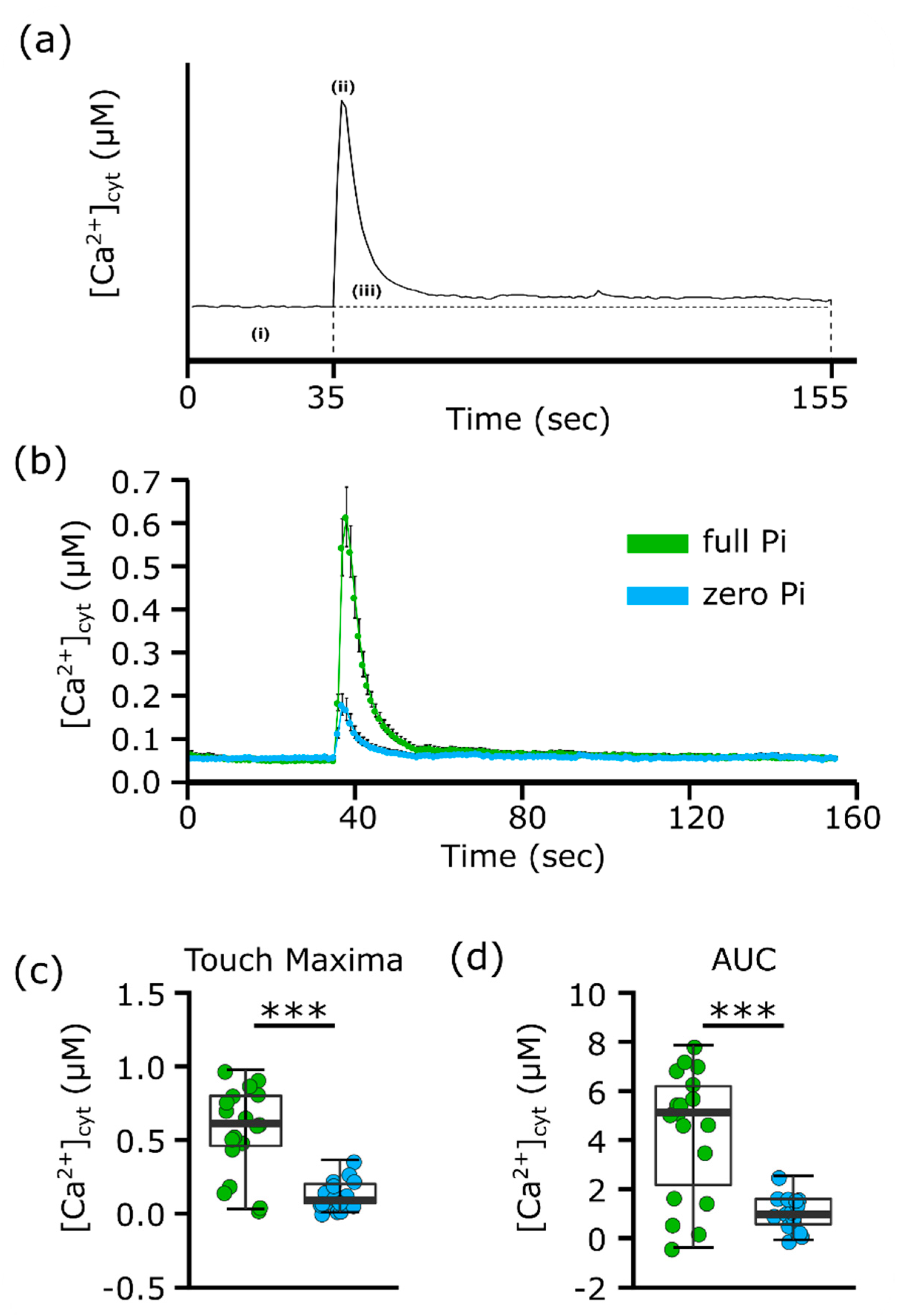
2.1. Phosphate Starvation Contributes to Impaired [Ca2+]cyt Elevation in Response to Mechanical Stimulation
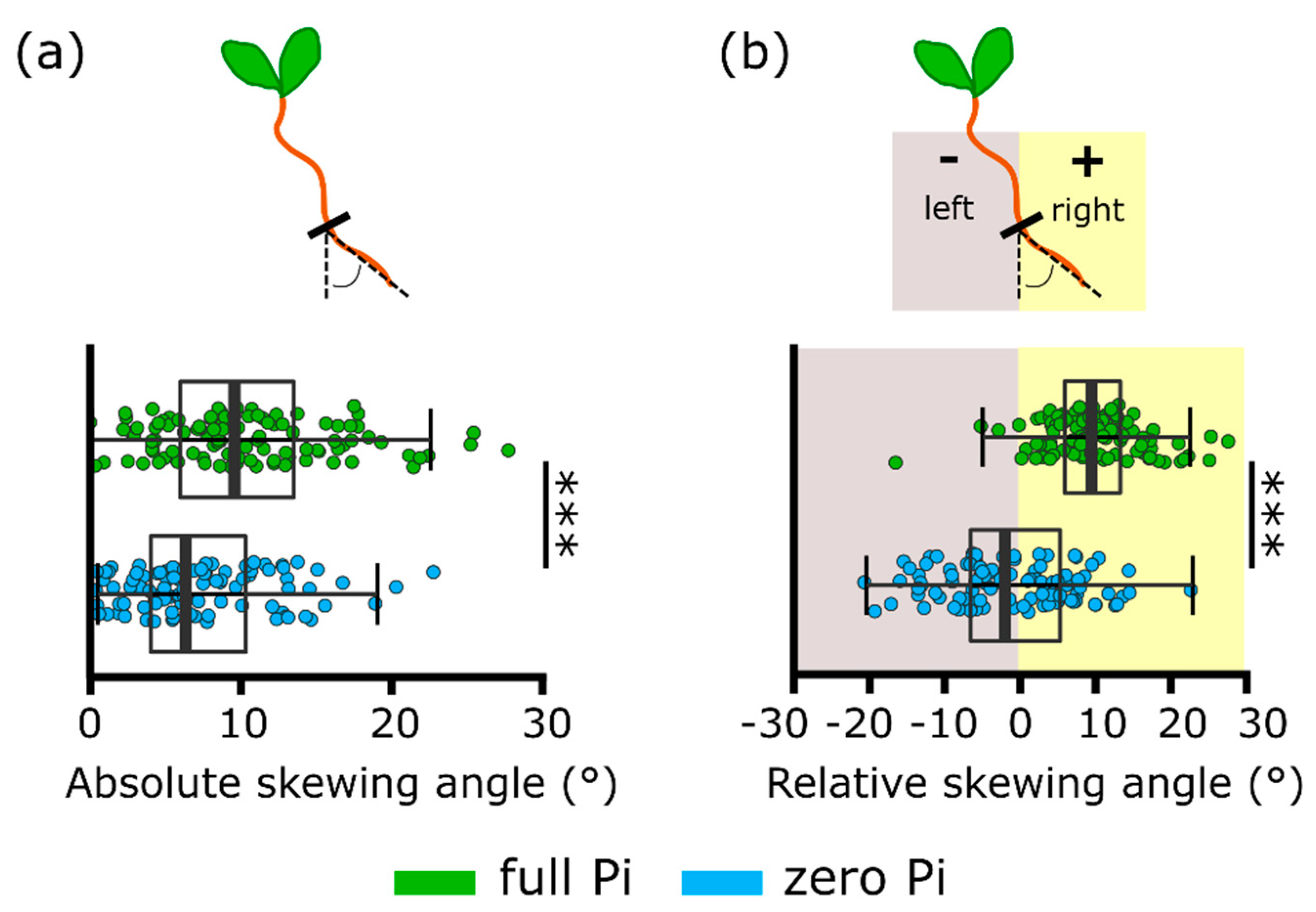
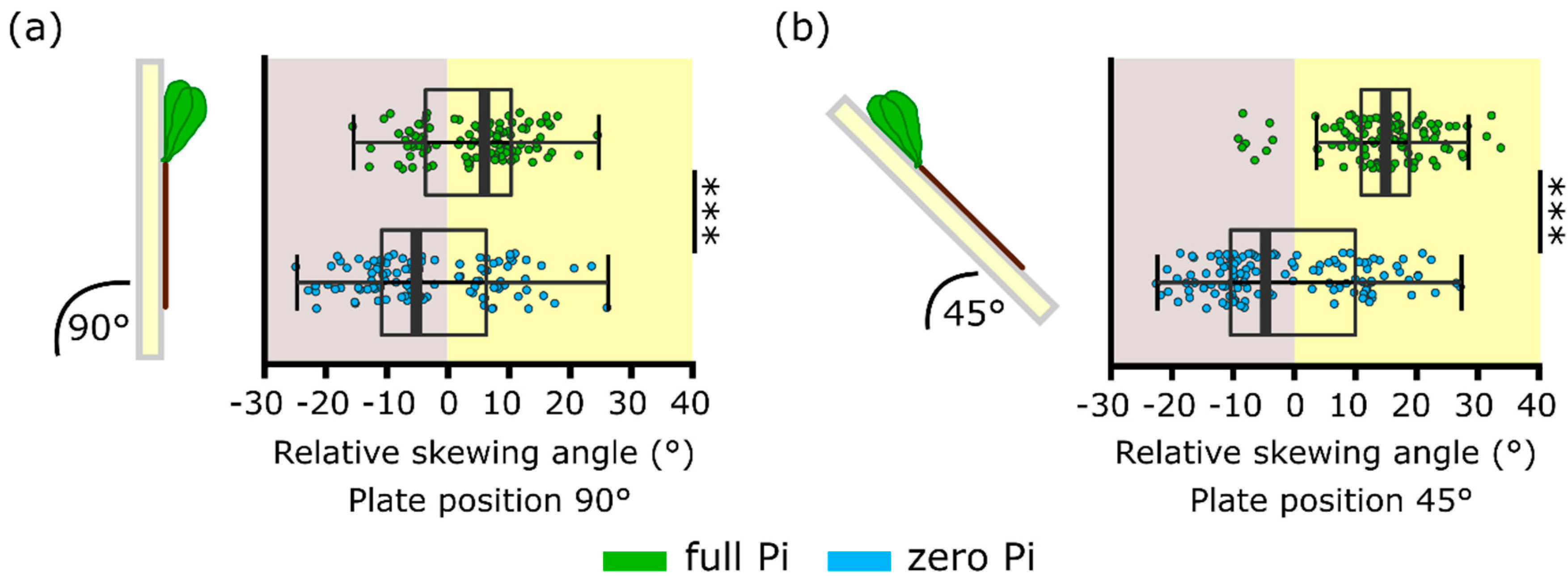
2.2. Phosphate Starvation Results in Impaired Root Skewing
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Aequorin Luminometry
4.3. Root Skewing
4.4. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakagawa, Y.; Katagiri, T.; Shinozaki, K.; Qi, Z.; Tatsumi, H.; Furuichi, T.; Kishigami, A.; Sokabe, M.; Kojima, I.; Sato, S.; et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 2007, 104, 3639–3644. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.L.; Monshausen, G.B.; Krol, A.; Gilroy, S. Mechanical stimuli modulate lateral root organogenesis. Plant Physiol. 2009, 151, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-W.; Miller, N.D.; Dai, C.; Spalding, E.P.; Monshausen, G.B. The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr. Biol. 2014, 24, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Kolb, E.; Legué, V.; Bogeat-Triboulot, M.B. Physical root-soil interactions. Phys. Biol. 2017, 14, 065004. [Google Scholar] [CrossRef]
- Okamoto, T.; Takatani, S.; Noutoshi, Y.; Motose, H.; Takahashi, T. Omeprazole enhances mechanical stress-induced root growth reduction in Arabidopsis thaliana. Plant Cell Physiol. 2018, 59, 1581–1591. [Google Scholar] [CrossRef]
- Knight, M.R.; Campbell, A.K.; Smith, S.M.; Trewavas, A.J. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 1991, 352, 524–526. [Google Scholar] [CrossRef]
- Knight, M.R.; Smith, S.M.; Trewavas, A.J. Wind-induced plant motion immediately increases cytosolic calcium. Proc. Natl. Acad. Sci. USA 1992, 89, 4967–4971. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Renhu, N.; Naito, M.; Nakamura, A.; Shiba, H.; Yamamoto, T.; Suzaki, T.; Iida, H.; Miura, K. Ca2+-permeable mechanosensitive channels MCA1 and MCA2 mediate cold-induced cytosolic Ca2+ increase and cold tolerance in Arabidopsis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Hou, C.; Tian, W.; Kleist, T.; He, K.; Garcia, V.; Bai, F.; Hao, Y.; Luan, S.; Li, L. DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 2014, 24, 632–635. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Basu, D.; Haswell, E.S. The mechanosensitive ion channel MSL10 potentiates responses to cello swelling in Arabidopsis seedlings. BioRxiv 2020. [Google Scholar] [CrossRef]
- Tran, D.; Galletti, R.; Neumann, E.D.; Dubois, A.; Sharif-Naeni, R.; Geitmann, A.; Frachisse, J.-M.; Hamant, O.; Ingram, G.C. A mechanosensitive Ca2+ channel activity is dependent on the developmental regulator DEK1. Nat. Commun. 2017, 8, 1009. [Google Scholar] [CrossRef] [PubMed]
- Guerringue, Y.; Thomine, S.; Frachisse, J.-M. Sensing and transducing forces in plants with MSL10 and DEK1 mechanosensors. FEBS Lett. 2018, 592, 1968–1979. [Google Scholar] [CrossRef]
- Ackermann, F.; Stanislas, T. The plasma membrane—An integrating compartment for mechano-signaling. Plants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Bacete, L.; Hamann, T. The role of mechanoperception in plant cell wall integrity maintenance. Plants 2020, 9, 574. [Google Scholar] [CrossRef]
- Legué, V.; Blancaflor, E.; Wymer, C.; Perbal, G.; Fantin, D.; Gilroy, S. Cytoplasmic free Ca2+ in Arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997, 114, 789–800. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Bibikova, T.N.; Weisenseel, M.H.; Gilroy, S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 2009, 21, 2341–2356. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, B.C.; Gilroy, S.; Chehab, E.W.; Braam, J. CML24 is involved in root mechanoresponses and cortical microtubule orientation in Arabidopsis. J. Plant Growth Regul. 2011, 30, 467–479. [Google Scholar] [CrossRef]
- Matthus, E.; Wilkins, K.A.; Swarbreck, S.M.; Doddrell, N.H.; Doccula, F.G.; Costa, A.; Davies, J.M. Phosphate starvation alters abiotic-stress-induced cytosolic free calcium increase in roots. Plant Physiol. 2019, 179, 1754–1767. [Google Scholar] [CrossRef]
- Matthus, E.; Wilkins, K.A.; Davies, J.M. Iron availability modulates the Arabidopsis thaliana root calcium signature evoked by exogenous ATP. Plant Signal. Behav. 2019. [Google Scholar] [CrossRef]
- Rutherford, R.; Masson, P. Arabidopsis thaliana sku mutant seedlings show exaggerated surface-dependent alteration in root growth vector. Plant Physiol. 1996, 111, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Califar, B.; Sng, N.J.; Zupanska, A.; Paul, A.-L.; Ferl, R.J. Root skewing-associated genes impact the spaceflight response of Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Emonet, A.; Tendon, V.D.; Marhavy, P.; Wu, D.; Lahaye, T.; Geldner, N. Co-incidence of damage and microbial patterns controls localized immune responses in roots. Cell 2020, 180, 440–453.e8. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Bassham, D.C. Root growth movements: Waving and skewing. Plant Sci. 2014, 221, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Balzergue, C.; Dartevelle, T.; Godon, C.; Laugier, E.; Meisrimler, C.-N.; Teulon, J.-M.; Creff, A.; Bissler, M.; Brouchoud, C.; Hagège, A.; et al. Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation. Nat. Commun. 2017. [Google Scholar] [CrossRef]
- Schultz, E.R.; Zupanska, A.K.; Sng, N.J.; Paul, A.-L.; Ferl, R.J. Skewing in Arabidopsis roots involves disparate environmental signaling pathways. BMC Plant Biol. 2017, 17, 31. [Google Scholar] [CrossRef]
- Nakamura, Y. Phosphate starvation and membrane lipid remodeling in seed plants. Prog. Lipid Res. 2013, 52, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Otsuki, H.; Narisawa, T.; Kobayashi, M.; Sawai, S.; Kamide, Y.; Kusano, M.; Aoki, T.; Hirai, M.Y.; Saito, K. A new class of plant lipid is essential for protection against phosphorus depletion. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Toev, T.; Heisters, M.; Teller, J.; Moore, K.L.; Hause, G.; Dinesh, D.C.; Bürstenbinder, K.; Abel, S. Iron-dependent callose deposition adjusts root meristem maintenance to phosphate availability. Dev. Cell 2015, 33, 216–230. [Google Scholar] [CrossRef]
- Hoehenwarter, W.; Mönchgesang, S.; Neumann, S.; Majovsky, P.; Abel, S.; Müller, J. Comparative expression profiling reveals a role of the root apoplast in local phosphate response. BMC Plant Biol. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Foroozani, M.; Zahraeifard, S.; Oh, D.H.; Wang, G.N.; Dassanayake, M.; Smith, A.P. Low-phosphate chromatin dynamics predict a cell wall remodeling network in rice shoots. Plant Physiol. 2020, 182, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.; Li, W.; Schmidt, W. Complementary proteome and transcriptome profiling in phosphate-deficient Arabidopsis roots reveals multiple levels of gene regulation. Mol. Cell. Proteom. 2012, 11, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Zhou, X.; Dong, L.; Guo, J.; Chen, Y.; Zhang, Y.; Wu, L.; Xu, M. iTRAQ-based analysis of the Arabidopsis proteome reveals insights into the potential mechanisms of anthocyanin accumulation regulation in response to phosphate deficiency. J. Proteom. 2018. [Google Scholar] [CrossRef]
- Lin, W.-D.; Liao, Y.-Y.; Yang, T.J.W.; Pan, C.-Y.; Buckhout, T.J.; Schmidt, W. Coexpression-based clustering of Arabidopsis root genes predicts functional modules in early phosphate deficiency signaling. Plant Physiol. 2011, 155, 1383–1402. [Google Scholar] [CrossRef]
- Leitão, N.; Dangeville, P.; Carter, R.; Charpentier, M. Nuclear calcium signatures are associated with root development. Nat. Commun. 2019, 10, 4865. [Google Scholar] [CrossRef]
- Muchhal, U.S.; Liu, C.M.; Raghothama, K.G. Ca2+-ATPase is expressed differentially in phosphate-starved roots of tomato. Physiol. Plant 1997, 101, 540–544. [Google Scholar] [CrossRef]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N.; et al. A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef]
- Kellermeier, F.; Armengaud, P.; Seditas, T.J.; Danku, J.; Salt, D.E.; Amtmann, A. Analysis of the root system architecture of Arabidopsis provides a quantitative readout of crosstalk between nutritional signals. Plant Cell 2014, 26, 1480–1496. [Google Scholar] [CrossRef]
- Williamson, L.C.; Ribrioux, S.P.C.P.; Fitter, A.H.; Leyser, H.M.O. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, M.T.; Ray, S.; Saengwilai, P.; Luthe, D.; Lynch, J.P.; Brown, K.M. Buffered delivery of phosphate to Arabidopsis alters response to low phosphate. J. Exp. Bot. 2018. [Google Scholar] [CrossRef]
- Nacry, P.; Canivenc, G.; Muller, B.; Azmi, A.; van Onckelen, H.; Rossignol, M.; Doumas, P. A role for auxin redistribution in the responses of root system architecture to phosphate starvation in Arabidopsis. Plant Physiol. 2005, 138, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.; Borchers, A. Handedness in plant cell exapnsion: A mutant perspective on helical growth. New Phytol. 2020, 225, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Swarbreck, S.M.; Guerringue, Y.; Matthus, E.; Jamieson, F.J.C.; Davies, J.M. Impairment in karrikin but not strigolactone sensing enhances root skewing in Arabidopsis thaliana. Plant J. 2019, 98, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Marti, M.C.; Stancombe, M.A.; Webb, A.A.R. Cell- and stimulus type-specific intracellular free Ca2+ signals in Arabidopsis. Plant Physiol. 2013, 163, 625–634. [Google Scholar] [CrossRef]
- Valentine, T.A.; Hallett, P.D.; Binnie, K.; Young, M.W.; Squire, G.R.; Hawes, C.; Bengough, A.G. Soil strength and macropore volume limit root elongation rates in many agricultural soils. Ann. Bot. 2012, 110, 259–270. [Google Scholar] [CrossRef]
- Chehab, E.W.; Yao, C.; Henderson, Z.; Kim, S.; Braam, J. Arabidopsis touch-induced morphogenesis is jasmonate-mediated and protects against stress. Curr. Biol. 2012, 22, 701–706. [Google Scholar] [CrossRef]
- Lange, M.J.P.; Lange, T. Touch-induced changes in Arabidopsis morphology dependent on gibberellin breakdown. Nat. Plants 2015. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthus, E.; Doddrell, N.H.; Guillaume, G.; Mohammad-Sidik, A.B.; Wilkins, K.A.; Swarbreck, S.M.; Davies, J.M. Phosphate Deprivation Can Impair Mechano-Stimulated Cytosolic Free Calcium Elevation in Arabidopsis Roots. Plants 2020, 9, 1205. https://doi.org/10.3390/plants9091205
Matthus E, Doddrell NH, Guillaume G, Mohammad-Sidik AB, Wilkins KA, Swarbreck SM, Davies JM. Phosphate Deprivation Can Impair Mechano-Stimulated Cytosolic Free Calcium Elevation in Arabidopsis Roots. Plants. 2020; 9(9):1205. https://doi.org/10.3390/plants9091205
Chicago/Turabian StyleMatthus, Elsa, Nicholas H. Doddrell, Gaëtan Guillaume, Amirah B. Mohammad-Sidik, Katie A. Wilkins, Stéphanie M. Swarbreck, and Julia M. Davies. 2020. "Phosphate Deprivation Can Impair Mechano-Stimulated Cytosolic Free Calcium Elevation in Arabidopsis Roots" Plants 9, no. 9: 1205. https://doi.org/10.3390/plants9091205
APA StyleMatthus, E., Doddrell, N. H., Guillaume, G., Mohammad-Sidik, A. B., Wilkins, K. A., Swarbreck, S. M., & Davies, J. M. (2020). Phosphate Deprivation Can Impair Mechano-Stimulated Cytosolic Free Calcium Elevation in Arabidopsis Roots. Plants, 9(9), 1205. https://doi.org/10.3390/plants9091205





