Variable Light Condition Improves Root Distribution Shallowness and P Uptake of Soybean in Maize/Soybean Relay Strip Intercropping System
Abstract
:1. Introduction
2. Results
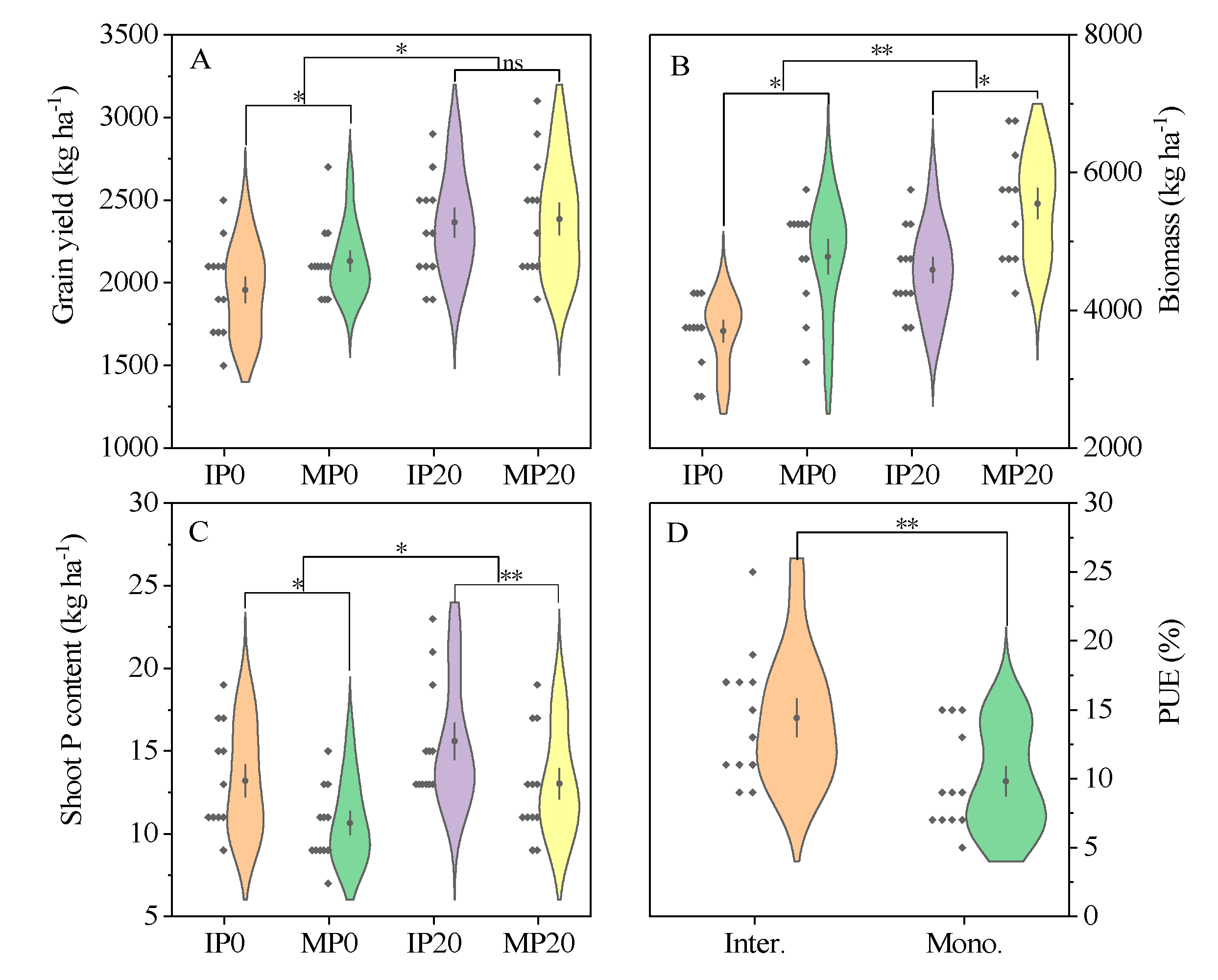
2.1. Yield and P Uptake Capability
2.2. Root Distribution and Morphological Traits
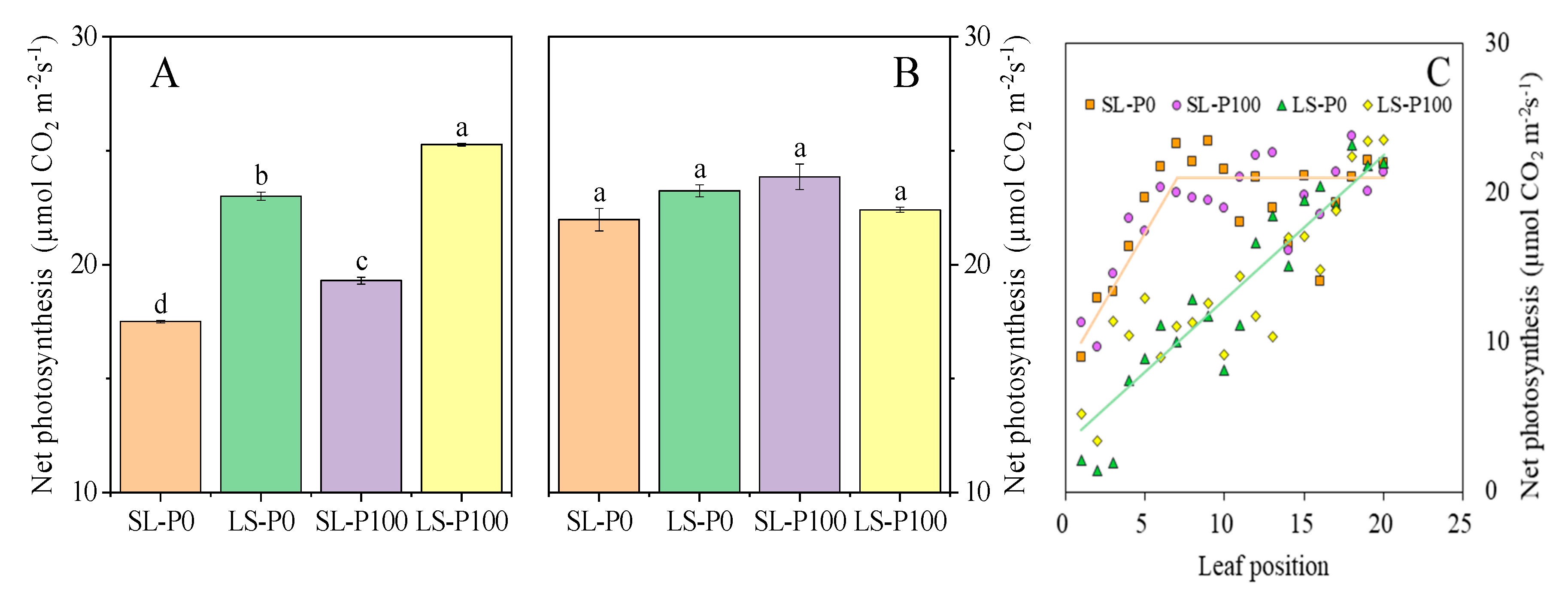
2.3. Photosynthetic Efficiency and Sucrose Accumulation
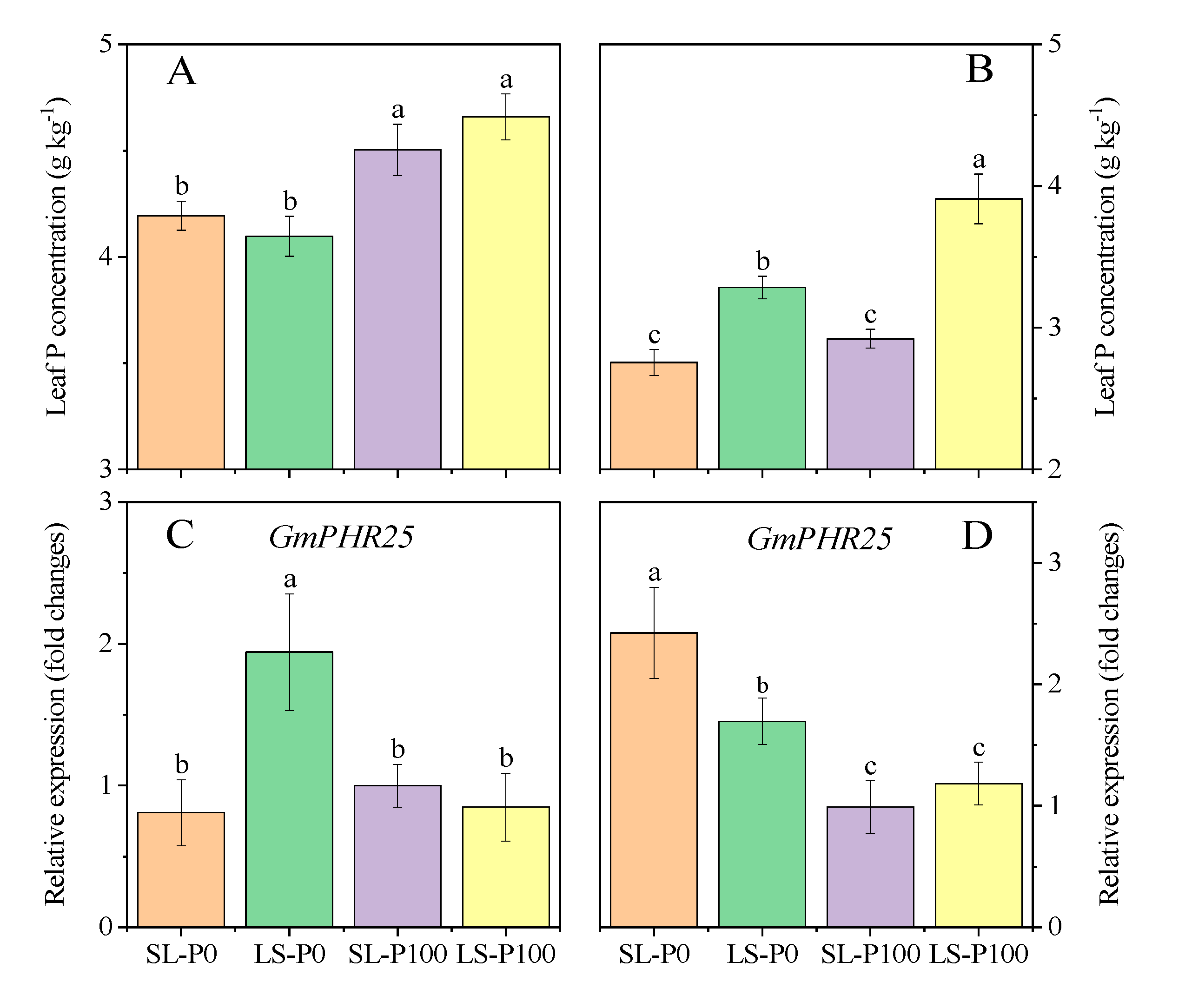
2.4. P Status in Leaf
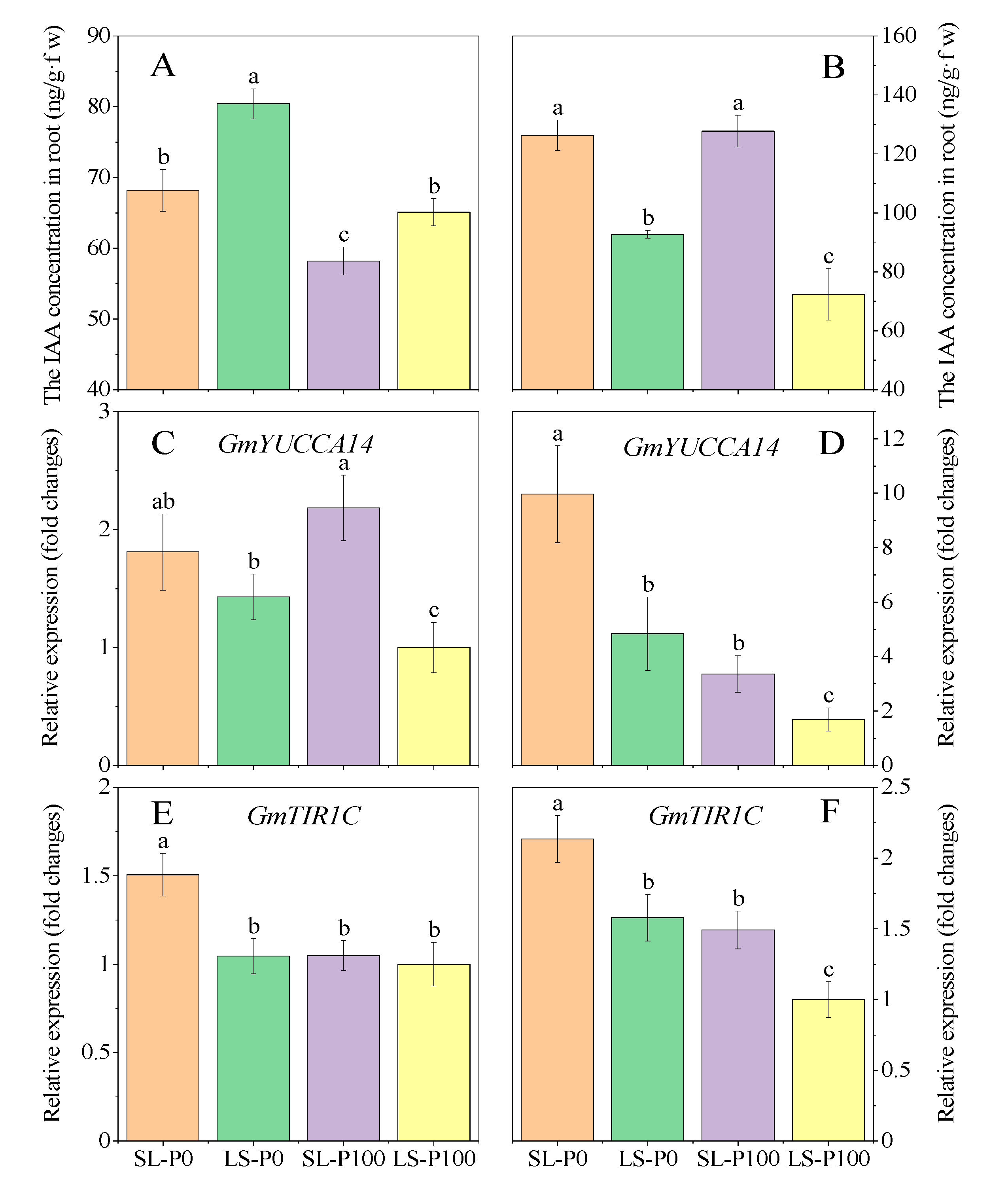
2.5. IAA Concentration and Expression of Auxin-Related Gene
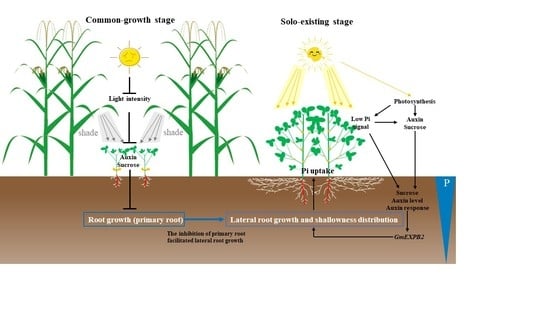
3. Discussion
3.1. Root Growth and Distribution in the Variable Light Condition of Inter-cropped soybeans
3.2. Auxin Concentration and Related Gene Expression Involves in Root Architecture
3.3. P Uptake Was Improved by the Relay Strip Inter-Cropping System
4. Materials and Methods
4.1. Field Experiment
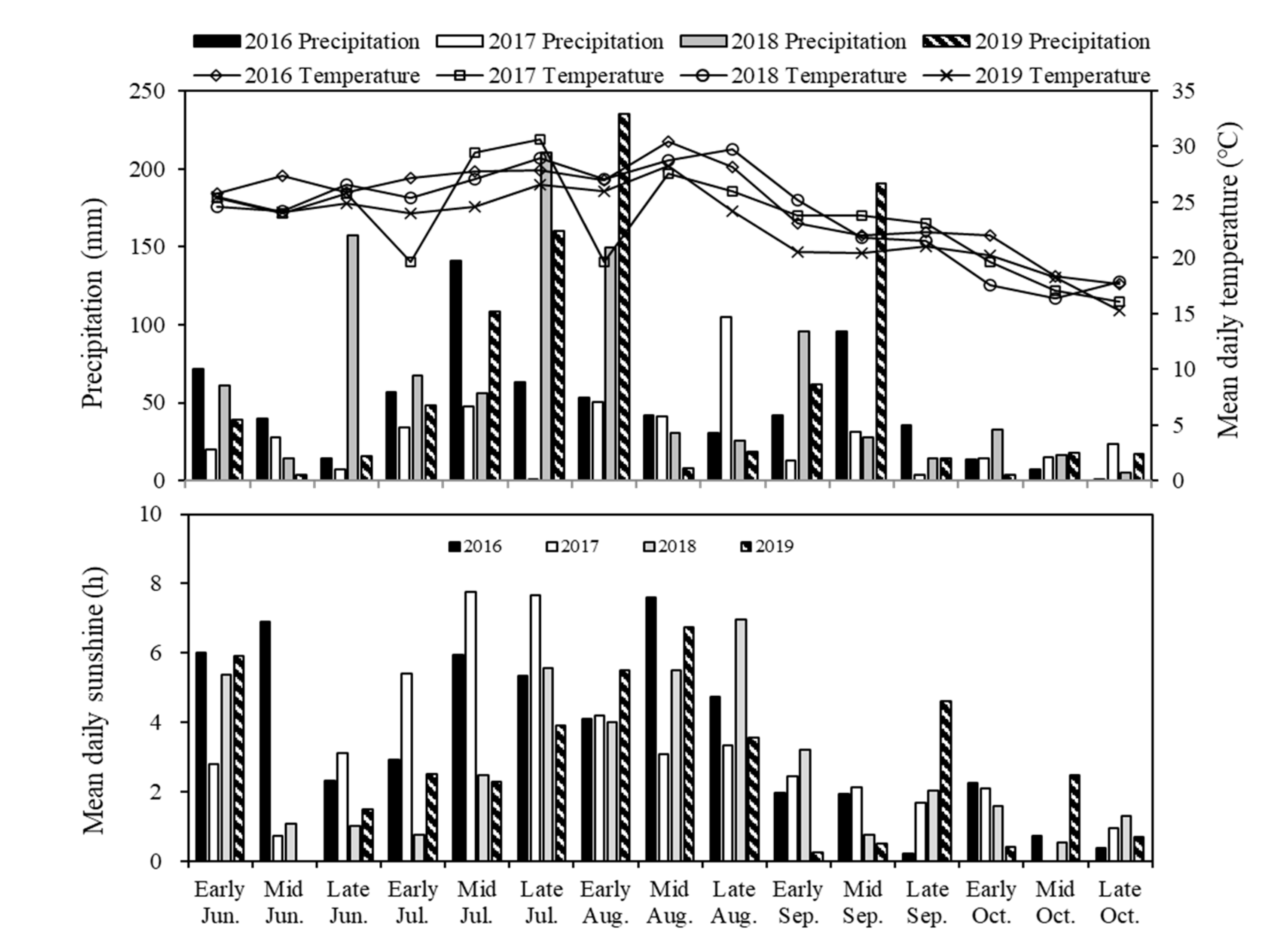
4.1.1. Site Description
4.1.2. Experiment Design and Crop Management
4.1.3. Plant Harvest and Measurements of Yield and P Concentration.
4.1.4. Determination of Root Distribution
4.2. Pot Experiment
4.2.1. Site Description
4.2.2. Experiment Design and Crop Management
4.2.3. Determination of P, Net Photosynthesis, Sucrose and Auxin Concentration.
4.2.4. Measurements of Root Morphology
4.2.5. Quantitative Real-Time PCR Analysis
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Ma, W.; Ji, Y.; Fan, M.; Oenema, O.; Zhang, F. Efficiency, economics, and environmental implications of phosphorus resource use and the fertilizer industry in China. Nutr. Cycl. Agroecosystems 2007, 80, 131–144. [Google Scholar] [CrossRef] [Green Version]
- Otani, T.; Ae, N. Phosphorus (P) uptake mechanisms of crops grown in soils with low P status. Soil Sci. Plant Nutr. 1996, 42, 155–163. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Vitousek, P. Chinese agriculture: An experiment for the world. Nature 2013, 497, 1476–4687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shen, J.; Zhang, J.; Zuo, Y.; Li, L.; Chen, X. Rhizosphere Processes and Management for Improving Nutrient Use Efficiency and Crop Productivity. Adv. Agron. 2010, 107, 1–32. [Google Scholar] [CrossRef]
- Du, J.; Han, T.; Gai, J.-Y.; Yong, T.; Sun, X.; Wang, X.-C.; Yang, F.; Liu, J.; Shu, K.; Liu, W.-G.; et al. Maize-soybean strip intercropping: Achieved a balance between high productivity and sustainability. J. Integr. Agric. 2018, 17, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Rahman, T.; Song, C.; Yang, F.; Su, B.; Cui, L.; Bu, W.; Yang, W.-Y. Relationships among light distribution, radiation use efficiency and land equivalent ratio in maize-soybean strip intercropping. Field Crop. Res. 2018, 224, 91–101. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Z.-G.; Zhao, J.-H.; Sun, J.-H.; Bao, X.-G.; Christie, P.; Zhang, F.; Li, L. Contribution of interspecific interactions and phosphorus application to sustainable and productive intercropping systems. Field Crop. Res. 2013, 154, 53–64. [Google Scholar] [CrossRef]
- Zhang, L.; Spiertz, H.; Zhang, S.; Li, B.; Van Der Werf, W. Nitrogen economy in relay intercropping systems of wheat and cotton. Plant Soil 2007, 303, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Brooker, R.W.; Bennett, A.E.; Cong, W.; Daniell, T.J.; George, T.S.; Hallett, P.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2014, 206, 107–117. [Google Scholar] [CrossRef]
- Li, L.; Tilman, D.; Lambers, H.; Zhang, F. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. P for Two, Sharing a Scarce Resource: Soil Phosphorus Acquisition in the Rhizosphere of Intercropped Species. Plant Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.F.; Luo, A.C.; Wei, X.H.; Yao, X.G. Genotypic variation of rice in phosphorus acquisition from iron phosphate: Contributions of root morphology and phosphorus uptake kinetics. Russ. J. Plant Physiol. 2007, 54, 230–236. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Tang, X.; Li, H.; Zhang, F.; Rengel, Z.; Whalley, W.R.; Davies, W.J.; Shen, J. Increased soil phosphorus availability induced by faba bean root exudation stimulates root growth and phosphorus uptake in neighbouring maize. New Phytol. 2015, 209, 823–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Postma, J.A.; York, L.M.; Lynch, J.P. Root foraging elicits niche complementarity-dependent yield advantage in the ancient ‘three sisters’ (maize/bean/squash) polyculture. Ann. Bot. 2014, 114, 1719–1733. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Bernard, L.; Brauman, A.; Daufresne, T.; Deleporte, P.; Desclaux, D.; Souche, G.; Placella, S.A.; Hinsinger, P. Increase in microbial biomass and phosphorus availability in the rhizosphere of intercropped cereal and legumes under field conditions. Soil Boil. Biochem. 2014, 75, 86–93. [Google Scholar] [CrossRef]
- Tang, X.; Placella, S.A.; Daydé, F.; Bernard, L.; Robin, A.; Journet, E.; Justes, É.; Hinsinger, P. Phosphorus availability and microbial community in the rhizosphere of intercropped cereal and legume along a P-fertilizer gradient. Plant Soil 2016, 407, 119–134. [Google Scholar] [CrossRef]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Li, H.; Zhang, C.; Zhang, F.; Van Der Werf, W. Yield gain, complementarity and competitive dominance in intercropping in China: A meta-analysis of drivers of yield gain using additive partitioning. Eur. J. Agron. 2020, 113, 125987. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, L.; Yang, H.; Gao, Y.; Liu, W.; Yang, W.-Y. Ameliorated light conditions increase the P uptake capability of soybean in a relay-strip intercropping system by altering root morphology and physiology in the areas with low solar radiation. Sci. Total. Environ. 2019, 688, 1069–1080. [Google Scholar] [CrossRef]
- Li, L.; Li, S.-M.; Sun, J.-H.; Zhou, L.-L.; Bao, X.-G.; Zhang, H.-G.; Zhang, F. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proc. Natl. Acad. Sci. USA 2007, 104, 11192–11196. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Tang, C.; Rengel, Z.; Zhang, F. Chickpea facilitates phosphorus uptake by intercropped wheat from an organic phosphorus source. Plant Soil 2003, 248, 297–303. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.; Li, X.; Christie, P.; Sun, J.; Yang, S.; Tang, C. Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutr. Cycl. Agroecosystems 2003, 65, 61–71. [Google Scholar] [CrossRef]
- Callaway, R.M. Positive Interactions and Interdependence in Plant Communities; Springer Science and Business Media LLC: Berlin, Germany, 2007. [Google Scholar]
- Yang, F.; Liao, D.; Wu, X.; Gao, R.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; Liu, J.; et al. Effect of aboveground and belowground interactions on the intercrop yields in maize-soybean relay intercropping systems. Field Crop. Res. 2017, 203, 16–23. [Google Scholar] [CrossRef]
- Bin Chen, X.; Yao, Q.; Gao, X.; Jiang, C.; Harberd, N.P.; Fu, X. Shoot-to-Root Mobile Transcription Factor HY5 Coordinates Plant Carbon and Nitrogen Acquisition. Curr. Boil. 2016, 26, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Dyachok, J.; Zhu, L.; Liao, F.; He, J.; Huq, E.; Blancaflor, E.B. SCAR Mediates Light-Induced Root Elongation in Arabidopsis through Photoreceptors and Proteasomes. Plant Cell 2011, 23, 3610–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correll, M.; Kiss, J.Z. The Roles of Phytochromes in Elongation and Gravitropism of Roots. Plant Cell Physiol. 2005, 46, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-J.; Park, Y.-J.; Ha, J.-H.; Baldwin, I.T.; Park, C.-M. Multiple Routes of Light Signaling during Root Photomorphogenesis. Trends Plant Sci. 2017, 22, 803–812. [Google Scholar] [CrossRef]
- Nagel, K.A.; Schurr, U.; Walter, A. Dynamics of root growth stimulation in Nicotiana tabacum in increasing light intensity. Plant Cell Environ. 2006, 29, 1936–1945. [Google Scholar] [CrossRef]
- Kircher, S.; Schopfer, P. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 11217–11221. [Google Scholar] [CrossRef] [Green Version]
- Müller, R.; Morant, M.; Jarmer, H.; Nilsson, L.; Nielsen, T.H. Genome-Wide Analysis of the Arabidopsis Leaf Transcriptome Reveals Interaction of Phosphate and Sugar Metabolism. Plant Physiol. 2006, 143, 156–171. [Google Scholar] [CrossRef] [Green Version]
- Saini, S.; Sharma, I.; Kaur, N.; Pati, P.K. Auxin: A master regulator in plant root development. Plant Cell Rep. 2013, 32, 741–757. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Sauer, M.; Napsucialy-Mendivil, S.; Ivanchenko, M.G.; Friml, J.; Shishkova, S.; Celenza, J.; Benková, E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc. Natl. Acad. Sci. USA 2008, 105, 8790–8794. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y. Auxin Biosynthesis and Its Role in Plant Development. Annu. Rev. Plant Boil. 2010, 61, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Van Gelderen, K.; Kang, C.; Pierik, R. Light Signaling, Root Development, and Plasticity. Plant Physiol. 2017, 176, 1049–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Jiang, Y.; Li, J.; Lin, F.; Holm, M.; Deng, X.W. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. In Proceedings of the Proceedings of the National Academy of Sciences. Proc. Natl. Acad. Sci. USA 2016, 113, 7655–7660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiltbrunner, A. Light-Activated Phytochrome A and B Interact with Members of the SPA Family to Promote Photomorphogenesis in Arabidopsis by Reorganizing the COP1/SPA Complex. Plant Cell 2015, 27, 189–201. [Google Scholar]
- Gangappa, S.N.; Botto, J.F. The Multifaceted Roles of HY5 in Plant Growth and Development. Mol. Plant 2016, 9, 1353–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassi, M.; Lu, Y.; Zhang, Y.; Wang, J.; Dhonukshe, P.; Blilou, I.; Dai, M.; Li, J.; Gong, X.; Jaillais, Y.; et al. COP1 mediates the coordination of root and shoot growth by light through modulation of PIN1- and PIN2-dependent auxin transport in Arabidopsis. Development 2012, 139, 3402–3412. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Raza, M.A.; Chen, Y.; Bin Khalid, M.H.; Meraj, T.A.; Ahsan, F.; Fan, Y.; Du, J.; Wu, X.; Song, C.; et al. Narrow-wide row planting pattern improves the light environment and seed yields of intercrop species in relay intercropping system. PLoS ONE 2019, 14, e0212885. [Google Scholar] [CrossRef]
- Coll, L.; Cerrudo, A.; Rizzalli, R.; Monzon, J.; Andrade, F.H. Capture and use of water and radiation in summer intercrops in the south-east Pampas of Argentina. Field Crop. Res. 2012, 134, 105–113. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, K.; Wang, K.; Huang, W.; Zhang, C.; Chen, Y. Effect of phosphate fertilizer on the improvement of yieldand nutrient use efficiency of soybean in wheat-soybean and wheat/maize/soybean systems. J. Plant Nutr. Fertil. 2015, 21, 336–345. [Google Scholar]
- Wu, Y.; Gong, W.; Yang, F.; Wang, X.; Yong, T.; Yang, W. Responses to shade and subsequent recovery of soya bean in maize-soya bean relay strip intercropping. Plant Prod. Sci. 2016, 19, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Osmont, K.S.; Sibout, R.; Hardtke, C.S. Hidden Branches: Developments in Root System Architecture. Annu. Rev. Plant Boil. 2007, 58, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin Control of Root Development. Cold Spring Harb. Perspect. Boil. 2010, 2, a001537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, H.; Wang, S.; Li, H. Genome-wide identification and expression analysis of the YUCCA gene family in soybean (Glycine max L.). Plant Growth Regul. 2016, 81, 265–275. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Zuo, Y.; Zhu, L.; Hastwell, A.H.; Chen, L.; Tian, Y.; Su, C.; Ferguson, B.J.; Li, X. GmYUC2a mediates auxin biosynthesis during root development and nodulation in soybean. J. Exp. Bot. 2019, 70, 3165–3176. [Google Scholar] [CrossRef]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef] [Green Version]
- López-Bucio, J.; Hernández-Abreu, E.; Sánchez-Calderón, L.; Nieto-Jacobo, M.F.; Simpson, J.; Herrera-Estrella, L. Phosphate Availability Alters Architecture and Causes Changes in Hormone Sensitivity in the Arabidopsis Root System. Plant Physiol. 2002, 129, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Hebert, Y.; Guingo, E.; Loudet, O. The Response of Root/Shoot Partitioning and Root Morphology to Light Reduction in Maize Genotypes. Crop. Sci. 2001, 41, 363–371. [Google Scholar] [CrossRef]
- Wissuwa, M.; Gamat, G.; Ismail, A.M. Is root growth under phosphorus deficiency affected by source or sink limitations? J. Exp. Bot. 2005, 56, 1943–1950. [Google Scholar] [CrossRef] [Green Version]
- Hammond, J.P.; White, P.J. Sucrose transport in the phloem: Integrating root responses to phosphorus starvation. J. Exp. Bot. 2007, 59, 93–109. [Google Scholar] [CrossRef]
- Cheng, L.; Tang, X.; Vance, C.P.; White, P.J.; Zhang, F.; Shen, J. Interactions between light intensity and phosphorus nutrition affect the phosphate-mining capacity of white lupin (Lupinus albus L.). J. Exp. Bot. 2014, 65, 2995–3003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Huang, S.; Gao, R.; Liu, W.; Yong, T.; Wang, X.; Wu, X.; Yang, W. Growth of soybean seedlings in relay strip intercropping systems in relation to light quantity and red:far-red ratio. Field Crop. Res. 2014, 155, 245–253. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, J.; Cheng, Y.; Raza, M.A.; Wu, X.; Wang, Z.; Liu, Q.; Wang, R.; Wang, X.; Yong, T.; et al. Effect of shading and light recovery on the growth, leaf structure, and photosynthetic performance of soybean in a maize-soybean relay-strip intercropping system. PLoS ONE 2018, 13, e0198159. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Jain, A.; Poling, M.D.; Karthikeyan, A.S.; Blakeslee, J.J.; Peer, W.A.; Titapiwatanakun, B.; Murphy, A.S.; Raghothama, K.G. Differential Effects of Sucrose and Auxin on Localized Phosphate Deficiency-Induced Modulation of Different Traits of Root System Architecture in Arabidopsis. Plant Physiol. 2007, 144, 232–247. [Google Scholar] [CrossRef] [Green Version]
- Nacry, P.; Canivenc, G.; Muller, B.; Azmi, A.; Van Onckelen, H.; Rossignol, M.; Doumas, P. A Role for Auxin Redistribution in the Responses of the Root System Architecture to Phosphate Starvation in Arabidopsis1. Plant Physiol. 2005, 138, 2061–2074. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Torres, C.-A.; López-Bucio, J.; Cruz-Ramírez, A.; Ibarra-Laclette, E.; Dharmasiri, S.; Estelle, M.; Herrera-Estrella, L. Phosphate Availability Alters Lateral Root Development in Arabidopsis by Modulating Auxin Sensitivity via a Mechanism Involving the TIR1 Auxin Receptor. Plant Cell 2008, 20, 3258–3272. [Google Scholar] [CrossRef] [Green Version]
- Bou-Torrent, J.; Galstyan, A.; Gallemi, M.; Cifuentes-Esquivel, N.; Molina-Contreras, M.J.; Salla-Martret, M.; Jikumaru, Y.; Yamaguchi, S.; Kamiya, Y.; Martinez-Garcia, J.F. Plant proximity perception dynamically modulates hormone levels and sensitivity in Arabidopsis. J. Exp. Bot. 2014, 65, 2937–2947. [Google Scholar] [CrossRef] [Green Version]
- De Wit, M.; Ljung, K.; Fankhauser, C. Contrasting growth responses in lamina and petiole during neighbor detection depend on differential auxin responsiveness rather than different auxin levels. New Phytol. 2015, 208, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Pucciariello, O.; Legris, M.; Rojas, C.C.R.; Iglesias, M.J.; Hernando, C.E.; Dezar, C.; Vázquez, M.; Yanovsky, M.J.; Finlayson, S.A.; Prat, S.; et al. Rewiring of auxin signaling under persistent shade. Proc. Natl. Acad. Sci. USA 2018, 115, 5612–5617. [Google Scholar] [CrossRef] [Green Version]
- Hersch, M.; Lorrain, S.; De Wit, M.; Trevisan, M.; Ljung, K.; Bergman, S.; Fankhauser, C. Light intensity modulates the regulatory network of the shade avoidance response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 6515–6520. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.L.; Russell, W.J. Root Development and Rooting Patterns of Soybean (Glycine max (L.) Merrill) Evaluated Under Field Conditions1. Agron. J. 1907, 63, 313–316. [Google Scholar] [CrossRef]
- Ticconi, C.A.; Delatorre, C.A.; Lahner, B.; Salt, D.E.; Abel, S. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J. 2004, 37, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Calderón, L.; López-Bucio, J.; Chacón-López, A.; Cruz-Ramirez, L.A.; Nieto-Jacobo, F.; Dubrovsky, J.G.; Herrera-Estrella, L.R.; Cruz-Ramirez, L.A. Phosphate Starvation Induces a Determinate Developmental Program in the Roots of Arabidopsis thaliana. Plant Cell Physiol. 2005, 46, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Svistoonoff, S.; Creff, A.; Reymond, M.; Sigoillot-Claude, C.; Ricaud, L.; Blanchet, A.; Nussaume, L.; Desnos, T. Root tip contact with low-phosphate media reprograms plant root architecture. Nat. Genet. 2007, 39, 792–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljung, K.; Nemhauser, J.L.; Perata, P. New mechanistic links between sugar and hormone signalling networks. Curr. Opin. Plant Boil. 2015, 25, 130–137. [Google Scholar] [CrossRef]
- Ruan, Y. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Boil. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Lynch, J.P.; Beebe, S.E. Adaptation of beans (Phaseolus vulganis L.) to low phosphorus availity. Hortience 1995, 30, 1165–1171. [Google Scholar]
- Strock, C.F.; De La Riva, L.M.; Lynch, J.P. Reduction in Root Secondary Growth as a Strategy for Phosphorus Acquisition. Plant Physiol. 2017, 176, 691–703. [Google Scholar] [CrossRef] [Green Version]
- Torrey, J.G. The induction of lateral roots by indoleacetic acid and root decapitation. Am. J. Bot. 1950, 37, 257–264. [Google Scholar] [CrossRef]
- Ge, Z.; Rubio, G.; Lynch, J.P. The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: Results from a geometric simulation model. Plant Soil 2000, 218, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Ochoa, I.; Nielsen, K.L.; Beck, D.; Lynch, J.P. Genetic variation for adventitious rooting in response to low phosphorus availability: Potential utility for phosphorusacquisition from stratified soils. Funct. Plant Boil. 2003, 30, 973–985. [Google Scholar] [CrossRef] [Green Version]
- Rangarajan, H.; Postma, J.A.; Lynch, J.P. Co-optimization of axial root phenotypes for nitrogen and phosphorus acquisition in common bean. Ann. Bot. 2018, 122, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.-J.; Lin, S.-I. Signaling Network in Sensing Phosphate Availability in Plants. Annu. Rev. Plant Boil. 2011, 62, 185–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Liao, H.; Lucas, W.J. Molecular mechanisms underlying phosphate sensing, signaling, and adaptation in plants. J. Integr. Plant Boil. 2014, 56, 192–220. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, L.; Sun, X.; Wang, X.; Chen, Y.; Rengel, Z.; Liu, W.; Yang, W. Light intensity influence maize adaptation to low P stress by altering root morphology. Plant Soil 2019, 447, 183–197. [Google Scholar] [CrossRef]
- Wang, H.; Inukai, Y.; Yamauchi, A. Root Development and Nutrient Uptake. Crit. Rev. Plant Sci. 2006, 25, 279–301. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of soil analysis. Am. Soc. Agron. 1982, 15, 99–100. [Google Scholar]
- Shi, H.; Wang, B.; Yang, P.; Li, Y.; Miao, F. Differences in Sugar Accumulation and Mobilization between Sequential and Non-Sequential Senescence Wheat Cultivars under Natural and Drought Conditions. PLoS ONE 2016, 11, e0166155. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]



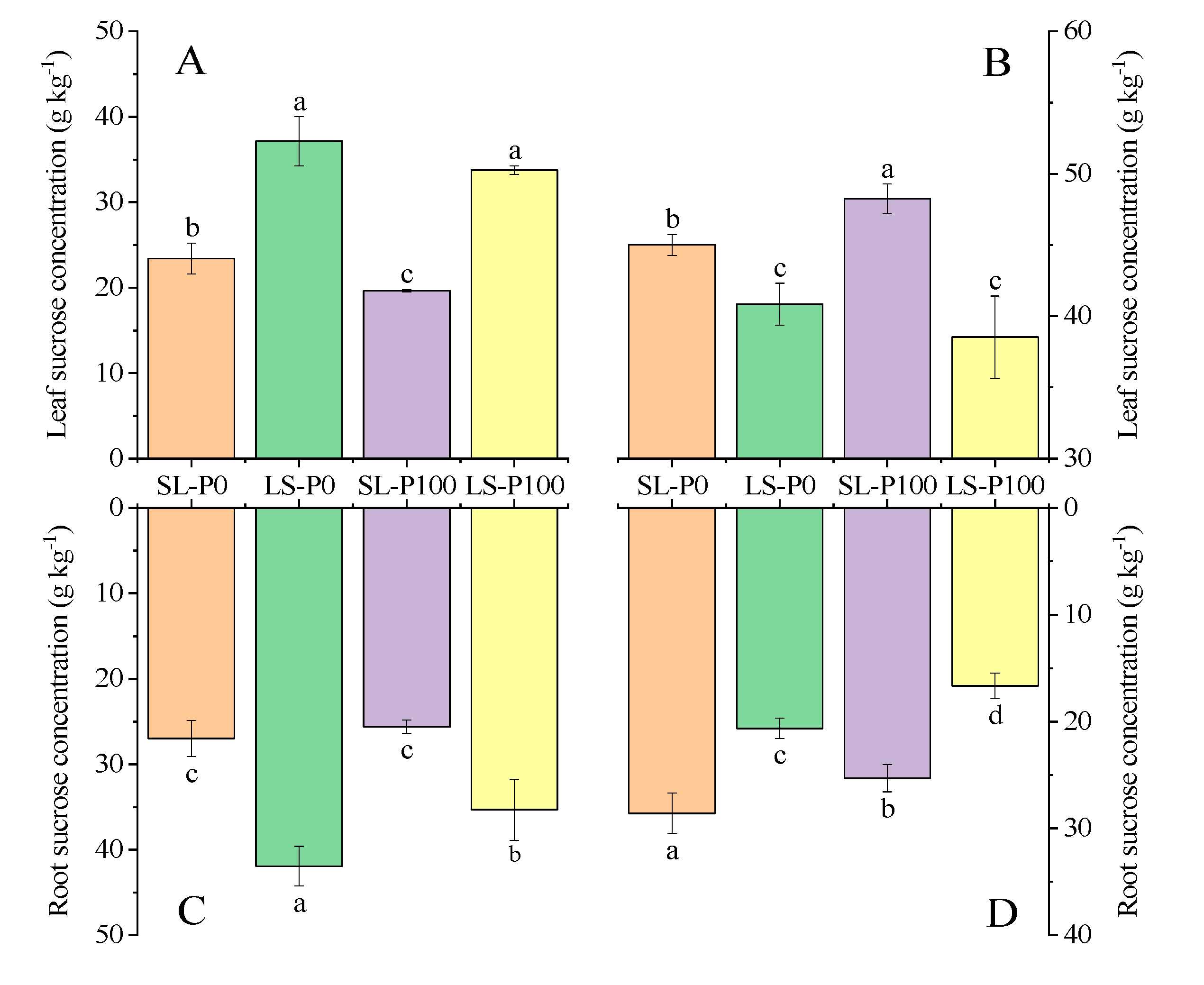
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhou, T.; Cheng, B.; Du, Y.; Qin, S.; Gao, Y.; Xu, M.; Lu, J.; Liu, T.; Li, S.; et al. Variable Light Condition Improves Root Distribution Shallowness and P Uptake of Soybean in Maize/Soybean Relay Strip Intercropping System. Plants 2020, 9, 1204. https://doi.org/10.3390/plants9091204
Wang L, Zhou T, Cheng B, Du Y, Qin S, Gao Y, Xu M, Lu J, Liu T, Li S, et al. Variable Light Condition Improves Root Distribution Shallowness and P Uptake of Soybean in Maize/Soybean Relay Strip Intercropping System. Plants. 2020; 9(9):1204. https://doi.org/10.3390/plants9091204
Chicago/Turabian StyleWang, Li, Tao Zhou, Bin Cheng, Yongli Du, Sisi Qin, Yang Gao, Mei Xu, Junji Lu, Ting Liu, Shuxian Li, and et al. 2020. "Variable Light Condition Improves Root Distribution Shallowness and P Uptake of Soybean in Maize/Soybean Relay Strip Intercropping System" Plants 9, no. 9: 1204. https://doi.org/10.3390/plants9091204
APA StyleWang, L., Zhou, T., Cheng, B., Du, Y., Qin, S., Gao, Y., Xu, M., Lu, J., Liu, T., Li, S., Liu, W., & Yang, W. (2020). Variable Light Condition Improves Root Distribution Shallowness and P Uptake of Soybean in Maize/Soybean Relay Strip Intercropping System. Plants, 9(9), 1204. https://doi.org/10.3390/plants9091204