Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice
Abstract
1. Introduction
2. Results
2.1. Plant Growth, Leaf Greenness, and Effective Quantum Yield of Photosystem II
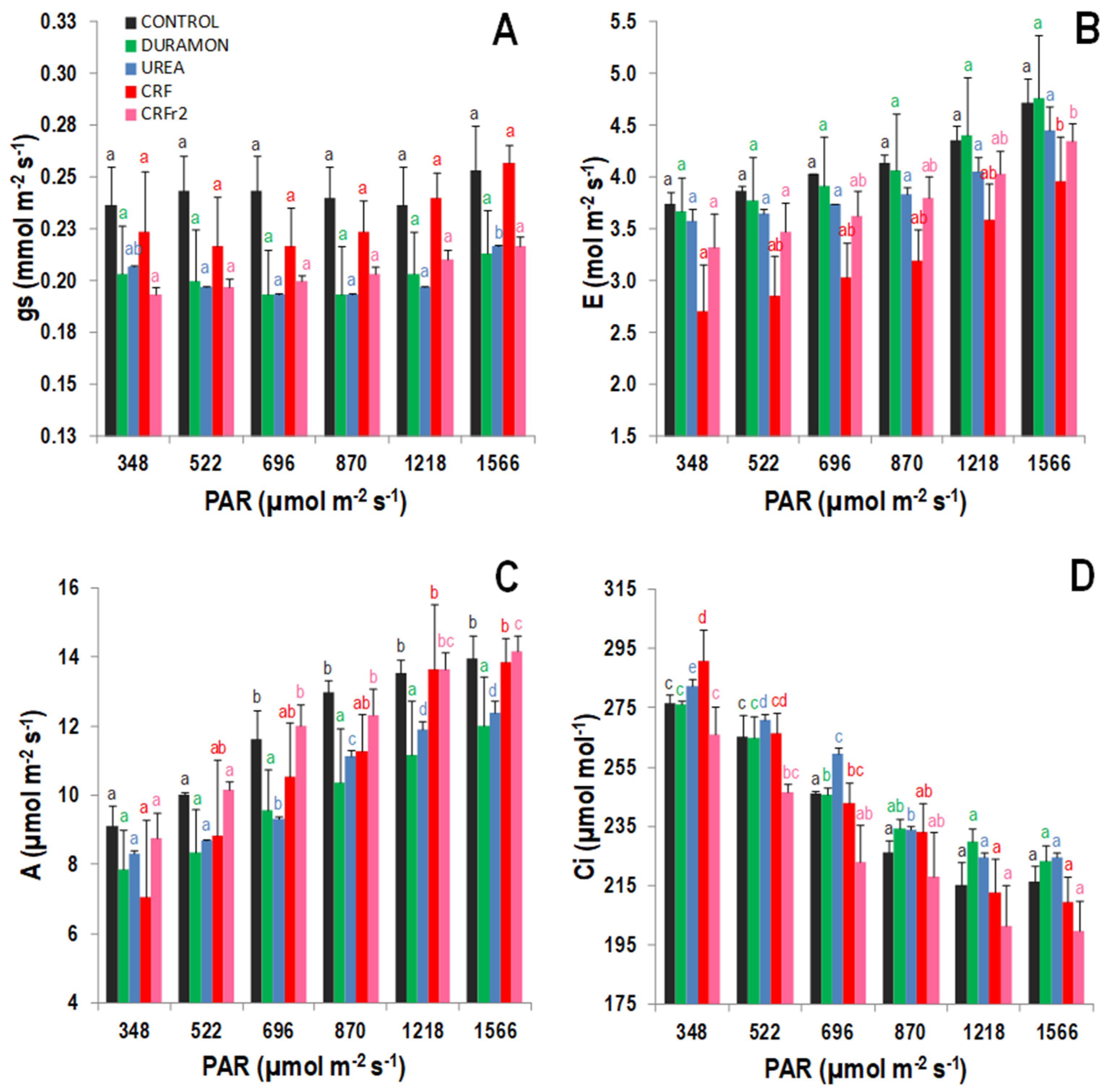
2.2. Gas Exchange Analysis
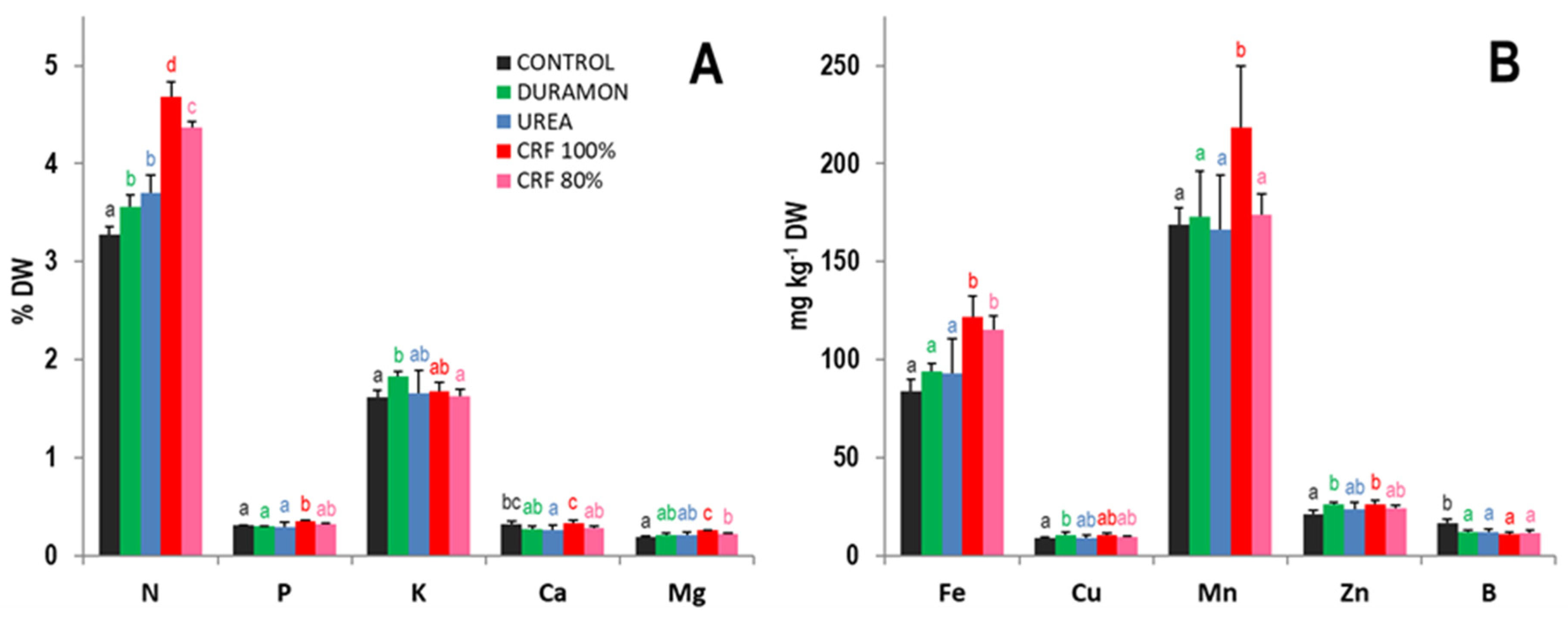
2.3. Foliar Nutrient Content
2.4. Hormone Activity
2.5. Growth, Yield, and Cereal Grain Composition
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Applied Fertilizers and Treatments
4.3. Soil Fertility Characterization
4.4. Growth Parameters
4.5. Leaf Gas Exchange and Photosynthetic Parameters
4.6. Foliar Nutrient Analysis
4.7. Hormone Activity
4.8. Yield and Cereal Grain Composition
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 August 2020).
- Mi, W.H.; Yang, X.; Wu, L.H.; Ma, Q.X.; Liu, Y.L.; Zhang, X. Evaluation of Nitrogen Fertilizer and Cultivation Methods for Agronomic Performance of Rice. Agron. J. 2016, 108, 1907–1916. [Google Scholar] [CrossRef]
- Miao, Y.X.; Stewart, B.A.; Zhang, F.S. Long-term experiments for sustainable nutrient management in China. A review. Agron. Sustain. Dev. 2011, 31, 397–414. [Google Scholar] [CrossRef]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2005; Volume 87, pp. 85–156. [Google Scholar]
- Raun, W.R.; Johnson, G.V. Improving nitrogen use efficiency for cereal production. Agron. J. 1999, 91, 357–363. [Google Scholar] [CrossRef]
- Naher, U.A.; Abu Saleque, M.; Panhwar, Q.A.; Radziah, O.; Jusop, S. Techniques of efficient fertilizer management for wetland rice—A review. Aust. J. Crop Sci. 2011, 5, 1661–1669. [Google Scholar]
- Pan, J.; Liu, Y.; Zhong, X.; Lampayan, R.M.; Singleton, G.R.; Huang, N.; Liang, K.; Peng, B.; Tian, K. Grain yield, water productivity and nitrogen use efficiency of rice under different water management and fertilizer-N inputs in South China. Agric. Water Manag. 2017, 184, 191–200. [Google Scholar] [CrossRef]
- Santos, A.B.; Fageria, N.K.; Prabhu, A.S. Rice ratooning management practices for higher yields. Commun. Soil Sci. Plan. 2003, 34, 881–918. [Google Scholar] [CrossRef]
- Fageria, N.K.; Slaton, N.A.; Baligar, V.C. Nutrient management for improving lowland rice productivity and sustainability. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2003; Volume 80, pp. 63–152. [Google Scholar]
- McAllister, C.H.; Beatty, P.H.; Good, A.G. Engineering nitrogen use efficient crop plants: The current status. Plant Biotechnol. J. 2012, 10, 1011–1025. [Google Scholar] [CrossRef]
- Cassman, K.G.; Peng, S.; Olk, D.C.; Ladha, J.K.; Reichardt, W.; Dobermann, A.; Singh, U. Opportunities for increased nitrogen-use efficiency from improved resource management in irrigated rice systems. Field Crops Res. 1998, 56, 7–39. [Google Scholar] [CrossRef]
- Dalal, R.C.; Wang, W.J.; Robertson, G.P.; Parton, W.J. Nitrous oxide emission from Australian agricultural lands and mitigation options: A review. Aust. J. Soil Res. 2003, 41, 165–195. [Google Scholar] [CrossRef]
- Dubey, A.; Mailapalli, D.R. Development of control release urea fertilizer model for water and nitrogen movement in flooded rice. Paddy Water Environ. 2018, 16, 1–13. [Google Scholar] [CrossRef]
- Crews, T.E.; Peoples, M.B. Can the synchrony of nitrogen supply and crop demand be improved in legume and fertilizer-based agroecosystems? A review. Nutr. Cycl. Agroecosyst. 2005, 72, 101–120. [Google Scholar] [CrossRef]
- Freney, J.R.; Trevitt, A.C.F.; Dedatta, S.K.; Obcemea, W.N.; Real, J.G. The interdependence of ammonia volatilization and denitrification as nitrogen loss processes in flooded rice fields in the Philippines. Biol. Fert. Soils 1990, 9, 31–36. [Google Scholar] [CrossRef]
- Eichner, M.J. Nitrous-oxide emissions from fertilized soils—Summary of available data. J. Environ. Qual. 1990, 19, 272–280. [Google Scholar] [CrossRef]
- Roshanravan, B.; Soltani, S.M.; Mahdavi, F.; Rashid, S.A.; Yusop, M.K. Preparation of encapsulated urea-kaolinite controlled release fertiliser and their effect on rice productivity. Chem. Speciation Bioavailab. 2014, 26, 249–256. [Google Scholar] [CrossRef]
- Shaviv, A.; Mikkelsen, R.L. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation—A review. Fertil. Res. 1993, 35, 1–12. [Google Scholar] [CrossRef]
- Chien, S.H.; Prochnow, L.I.; Cantarella, H. Recent developments of fertilizer production and use to improve nutrient efficiency and minimize environmental impacts. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2009; Volume 102, pp. 267–322. [Google Scholar]
- Harrison, R.; Webb, J. A review of the effect of N fertilizer type on gaseous emissions. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2001; Volume 73, pp. 65–108. [Google Scholar]
- Ando, H.; Kakuda, K.; Nakayama, M.; Yokoto, K. Yield of no-tillage direct-seeded lowland rice as influenced by different sources and application methods of fertilizer nitrogen. Soil Sci. Plant Nutr. 2000, 46, 105–115. [Google Scholar] [CrossRef][Green Version]
- Linquist, B.A.; Liu, L.J.; van Kessel, C.; van Groenigen, K.J. Enhanced efficiency nitrogen fertilizers for rice systems: Meta-analysis of yield and nitrogen uptake. Field Crops Res. 2013, 154, 246–254. [Google Scholar] [CrossRef]
- Azeem, B.; KuShaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on materials & methods to produce controlled release coated urea fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef]
- Wakimoto, K. Utilization advantages of controlled release nitrogen fertilizer on paddy rice cultivation. JARQ Jpn. Agric. Res. Q. 2004, 38, 15–20. [Google Scholar] [CrossRef]
- Carson, L.C.; Ozores-Hampton, M. Factors Affecting Nutrient Availability, Placement, Rate, and Application Timing of Controlled-release Fertilizers for Florida Vegetable Production Using Seepage Irrigation. Horttechnology 2013, 23, 553–562. [Google Scholar] [CrossRef]
- Medina, L.C.; Sartain, J.B.; Obreza, T.A.; Hall, W.L.; Thiex, N.J. Evaluation of a Soil Incubation Method to Characterize Nitrogen Release Patterns of Slow- and Controlled-Release Fertilizers. J. AOAC Int. 2014, 97, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Akelah, A. Novel utilizations of conventional agrochemicals by controlled release formulations. Mater. Sci. Eng. C Biomimetic Mater. Sens. Syst. 1996, 4, 83–98. [Google Scholar] [CrossRef]
- Sui, B.A.; Feng, X.M.; Tian, G.L.; Hu, X.Y.; Shen, Q.R.; Guo, S.W. Optimizing nitrogen supply increases rice yield and nitrogen use efficiency by regulating yield formation factors. Field Crops Res. 2013, 150, 99–107. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Alva, A.K. Nitrogen uptake and growth of two citrus rootstock seedlings in a sandy soil receiving different controlled-release fertilizer sources. Biol. Fert. Soils 1998, 26, 169–172. [Google Scholar] [CrossRef]
- Wei, H.-Y.; Chen, Z.-F.; Xing, Z.-P.; Zhou, L.; Liu, Q.-Y.; Zhang, Z.-Z.; Jiang, Y.; Hu, Y.-J.; Zhu, J.-Y.; Cui, P.-Y.; et al. Effects of slow or controlled release fertilizer types and fertilization modes on yield and quality of rice. J. Integr. Agric. 2018, 17, 2222–2234. [Google Scholar] [CrossRef]
- Morikawa, C.K.; Saigusa, M.; Nishizawa, N.K.; Mori, S. Importance of contact between rice roots and co-situs applied fertilizer granules on iron absorption by paddy rice in a calcareous paddy soil. Soil Sci. Plant Nutr. 2008, 54, 467–472. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, G.; Ma, J.; Zhang, G.-B.; Xu, H. Effects of Urea and Controlled Release Urea Fertilizers on Methane Emission from Paddy Fields: A Multi-Year Field Study. Pedosphere 2014, 24, 662–673. [Google Scholar] [CrossRef]
- Garcia, C.; Vallejo, A.; Diez, J.A.; Garcia, L.; Cartagena, M.C. Nitrogen use efficiency with the application of controlled release fertilizers coated with kraft pine lignin. Soil Sci. Plant Nutr. 1997, 43, 443–449. [Google Scholar] [CrossRef]
- Treinyte, J.; Grazuleviciene, V.; Ostrauskaite, J. Biodegradable polymer composites with nitrogen- and phosphorus-containing waste materials as the fillers. Ecol. Chem. Eng. S 2014, 21, 515–528. [Google Scholar] [CrossRef]
- Birrenkott, B.A.; Craig, J.L.; McVey, G.R. A leach collection system to track the release of nitrogen from controlled-release fertilizers in container ornamentals. Hortscience 2005, 40, 1887–1891. [Google Scholar] [CrossRef]
- Cox, D.A. Reducing nitrogen leaching-losses from containerized plants—The effectiveness of controlled-release fertilizers. J. Plant Nutr. 1993, 16, 533–545. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yano, T.; Sugiura, M.; Nagasaki, Y. Effects of Controlled-Release Fertilizer on Leaf Area Index and Fruit Yield in High-Density Soilless Tomato Culture Using Low Node-Order Pinching. PLoS ONE 2014, 9, e113074. [Google Scholar] [CrossRef] [PubMed]
- Bufogle, A.; Bollich, P.K.; Kovar, J.L.; Lindau, C.W.; Macchiavellid, R.E. Comparison of ammonium sulfate and urea as nitrogen sources in rice production. J. Plant Nutr. 1998, 21, 1601–1614. [Google Scholar] [CrossRef]
- Gaudin, R.; Dupuy, J. Ammoniacal nutrition of transplanted rice fertilized with large urea granules. Agron. J. 1999, 91, 33–36. [Google Scholar] [CrossRef]
- Patrick, W.H.; Wyatt, R. Soil nitrogen loss as a result of alternate submergence and drying. Soil Sci. Soc. Am. J. 1964, 28, 647–653. [Google Scholar] [CrossRef]
- Chowdhury, M.A. The controlled release of bioactive compounds from lignin and lignin-based biopolymer matrices. Int. J. Biol. Macromol. 2014, 65, 136–147. [Google Scholar] [CrossRef]
- Majeed, Z.; Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69–96. [Google Scholar] [CrossRef]
- Nardi, S.; Ertani, A.; Francioso, O. Soil-root cross-talking: The role of humic substances. J. Plant Nutr. Soil Sci. 2017, 180, 5–13. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Feng, J.F.; Li, F.B.; Deng, A.X.; Feng, X.M.; Fang, F.P.; Zhang, W.J. Integrated assessment of the impact of enhanced-efficiency nitrogen fertilizer on N2O emission and crop yield. Agric. Ecosyst. Environ. 2016, 231, 218–228. [Google Scholar] [CrossRef]
- Linquist, B.A.; Adviento-Borbe, M.A.; Pittelkow, C.M.; van Kessel, C.; van Groenigen, K.J. Fertilizer management practices and greenhouse gas emissions from rice systems: A quantitative review and analysis. Field Crops Res. 2012, 135, 10–21. [Google Scholar] [CrossRef]
- Grunes, D.L. Effect of nitrogen on the availability of soil and fertilizer phosphorus to plants. Adv. Agron. 1959, 11, 369–396. [Google Scholar] [CrossRef]
- Hashim, M.M.; Yusop, M.K.; Othman, R.; Wahid, S.A. Characterization of Nitrogen Uptake Pattern in Malaysian Rice MR219 at Different Growth Stages Using N-15 Isotope. Rice Sci. 2015, 22, 250–254. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Olaetxea, M.; Mora, V.; Bacaicoa, E.; Baigorri, R.; Garnica, M.; Fuentes, M.; Zamarreño, A.M.; Spíchal, L.; García-Mina, J.M. Root ABA and H+-ATPase are key players in the root and shoot growth-promoting action of humic acids. Plant Direct. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Moore, P.A.; Gilmour, J.T.; Wells, B.R. Seasonal patterns of growth and soil-nitrogen uptake by rice. Soil Sci. Soc. Am. J. 1981, 45, 875–879. [Google Scholar] [CrossRef]
- Counce, P.A.; Wells, B.R. Rice plant population density effect on early-season nitrogen requirement. J. Prod. Agric. 1990, 3, 390–393. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; Canadian Society of Soil Science: Boca Raton, FL, USA; CRC Press: Boca Raton, FL, USA, 2008; pp. 1–1264. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A re-examination of relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Peng, S.; Garcia, F.V.; Gines, H.C.; Laza, R.C.; Samson, M.I.; Sanico, A.L.; Visperas, R.M.; Cassman, K.G. Nitrogen use efficiency of irrigated tropical rice established by broadcast wet-seeding and transplanting. Fertil. Res. 1996, 45, 123–134. [Google Scholar] [CrossRef]
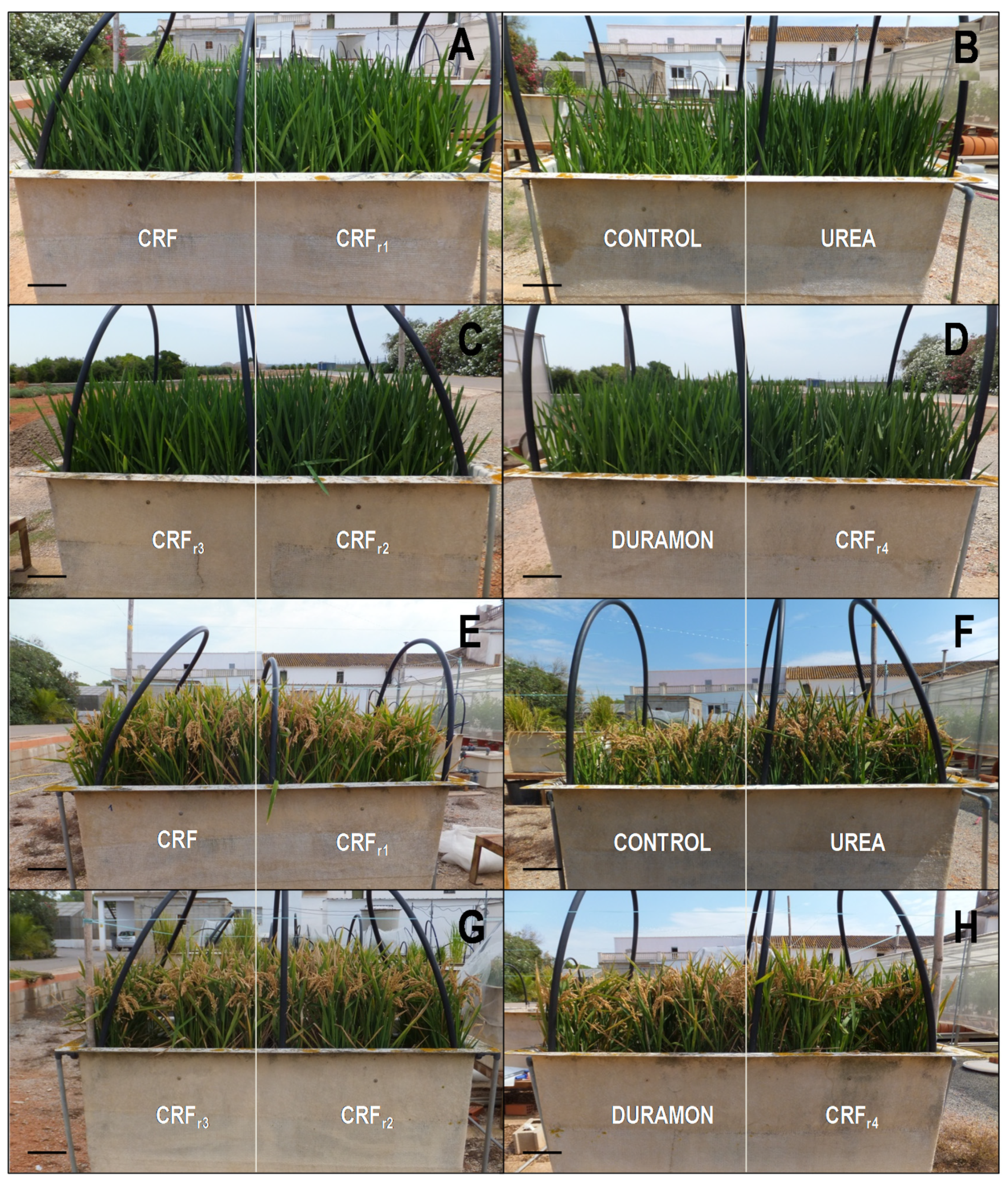

| Parameters | CRF | CRFr1 | CRFr2 | CRFr3 | CRFr4 | DURAMON® | UREA | CONTROL |
|---|---|---|---|---|---|---|---|---|
| ΦPSII | 0.71 ± 0.05 b | 0.69 ± 0.07 b | 0.70 ± 0.07 b | 0.69 ± 0.06 b | 0.67 ± 0.09 ab | 0.71 ± 0.07 b | 0.69 ± 0.04 b | 0.62 ± 0.02 a |
| Leaf greenness content (SPAD units) | 51.23 ± 8.07 b | 47.22 ± 6.04 ab | 47.80 ± 5.43 ab | 46.88 ± 3.48 ab | 45.76 ± 2.14 a | 48.18 ± 4.75 ab | 45.01 ± 3.65 a | 44.58 ± 6.54 a |
| N content (%) | 8.23 ± 1.02 c | 8.37 ± 0.51 c | 7.50 ± 0.66 bc | 7.30 ± 0.62 bc | 6.75 ± 0.68 b | 7.00 ± 1.68 bc | 6.55 ± 0.84 b | 4.23 ± 1.18 a |
| Total fresh weight (aerial part) (g) | 107.2 ± 26.6 ab | 134.8 ± 21.2 b | 135.2 ± 36.6 b | 127.8 ± 34.5 b | 129.6 ± 25.9 b | 128.0 ± 24.5 b | 115.6 ± 26.4 ab | 64.4 ± 6.2 a |
| Dry weight (aerial part) (%) | 23.90 ± 1.20 a | 27.10 ± 1.60 ab | 23.60 ± 0.70 a | 28.50 ± 2.60 b | 27.60 ± 2.20 ab | 28.30 ± 3.10 b | 30.10 ± 1.90 b | 29.20 ± 1.80 b |
| Total length (cm) | 69.70 ± 1.80 b | 71.50 ± 2.20 b | 67.10 ± 5.30 ab | 71.70 ± 3.20 b | 67.20 ± 30 ab | 70.80 ± 3.30 b | 70.40 ± 2.40 b | 62.30 ± 2.40 a |
| Primary stem length (cm) | 46.00 ± 2.90 a | 45.70 ± 2.80 a | 46.30 ± 2.80 a | 43.60 ± 2.70 a | 46.20 ± 2.80 a | 41.50 ± 7.60 a | 43.60 ± 1.90 a | 41.60 ± 6.90 a |
| Tillers number | 14.00 ± 3.10 ab | 13.40 ± 2.50 ab | 13.20 ± 3.10 ab | 11.80 ± 4.50 ab | 12.80 ± 1.30 ab | 15.80 ± 3.10 b | 13.40 ± 40 ab | 7.80 ± 1.90 a |
| Leaf weight (g) | 0.13 ± 0.01 ab | 0.16 ± 0.01 c | 0.15 ± 0.01 bc | 0.15 ± 0.01 c | 0.13 ± 0.01 abc | 0.15 ± 0.01 abc | 0.15 ± 0.01 abc | 0.13 ± 0.02 a |
| Leaf RWC (%) | 92.20 ± 4.70 b | 91.50 ± 3.80 b | 93.90 ± 3.20 b | 92.20 ± 2.10 b | 83.30 ± 2.90 ab | 92.60 ± 2.80 b | 92.00 ± 2.50 b | 82.80 ± 8.00 a |
| Total foliar area (cm2) | 10.80 ± 2.50 b | 12.50 ± 1.80 b | 12.00 ± 3.30 b | 11.10 ± 3.80 b | 10.60 ± 2.10 b | 12.10 ± 2.90 b | 11.60 ± 3.70 b | 3.40 ± 1.30 a |
| CRF | CRFr2 | DURAMON® | UREA | CONTROL | |
|---|---|---|---|---|---|
| IAA | 1.29 ± 0.04 a | 1.83 ± 0.07 a | 1.41 ± 0.06 a | 1.30 ± 0.06 a | 1.44 ± 0.06 a |
| JA | 0.29 ± 0.06 a | 0.42 ± 0.05 b | 0.55 ± 0.06 c | 0.85 ± 0.10 d | 0.27 ± 0.08 a |
| SA | 5499.5 ± 1758.2 a | 6841.8 ± 928.0 ab | 7841.6 ± 959.5 b | 7657.9 ± 714.3 b | 7255.2 ± 486.0 b |
| ABA | 15.27 ± 1.30 a | 14.43 ± 1.13 a | 19.48 ± 0.34 a | 19.91 ± 0.71 ab | 22.54 ± 1.12 b |
| iP | 0.18 ± 0.07 c | 0.05 ± 0.01 b | 0.02 ± 0.01 a | 0.03 ± 0.01 a | 0.02 ± 0.01 a |
| tZ | 0.47 ± 0.11 c | 0.46 ± 0.07 c | 0.10 ± 0.02 a | 0.24 ± 0.04 b | 0.20 ± 0.01 ab |
| Parameters | CRF | CRFr1 | CRFr2 | CRFr3 | CRFr4 | DURAMON® | UREA | CONTROL |
|---|---|---|---|---|---|---|---|---|
| Total fresh weight (aerial part *) (g) | 149.9 ± 46.0 c | 147.3 ± 41.9 bc | 122.6 ± 43.8 b | 152.6 ± 44.1 c | 128.5 ± 46.8 bc | 154.7 ± 43.4 c | 139.1 ± 42.4 bc | 88.4 ± 28.8 a |
| Dry weight (aerial part *) (%) | 18.4 ± 3.5 bc | 27.1 ± 6.9 d | 19.5 ± 3.1 b | 19.8 ± 3.3 c | 17.7 ± 2.3 b | 19.9 ± 1.9 c | 19.9 ± 3.6 bc | 19.8 ± 2.5 a |
| Primary stem length (cm) | 56.8 ± 2.6 d | 57.9 ± 2.7 d | 54.3 ± 3.4 c | 54.7 ± 3.2 c | 53.1 ± 3.2 bc | 53.9 ± 3.4 bc | 52.4 ± 3.7 b | 47.8 ± 1.9 a |
| Tillers number | 14.4 ± 3.7 abc | 15.9 ± 7.5 abc | 14.1 ± 5.7 ab | 16.1 ± 6.2 bc | 15.1 ± 6.9 abc | 19.0 ± 7.5 c | 15.8 ± 6.9 bc | 12.0 ± 4.9 a |
| Total panicle weight (g) | 51.5 ± 16.8 c | 38.8 ± 11.5 b | 35.9 ± 12.8 b | 38.0 ± 9.2 b | 37.7 ± 11.5 b | 37.1 ± 7.8 b | 33.1 ± 10.3 b | 21.2 ± 7.3 a |
| Panicle length (cm) | 12.8 ± 0.6 bc | 11.9 ± 1.0 ab | 11.9 ± 0.7 ab | 11.8 ± 0.4 ab | 12.7 ± 0.6 bc | 12.7 ± 0.7 bc | 13.6 ± 1.2 c | 11.0 ± 0.9 a |
| Panicle number | 12.9 ± 3.0 ab | 14.8 ± 6.8 b | 12.9 ± 4.8 ab | 14.1 ± 4.2 b | 14.1 ± 4.9 b | 14.3 ± 4.4 b | 11.1 ± 3.7 a | 10.3 ± 4.2 a |
| Mean panicle weight (g) | 4.0 ± 0.8 c | 3.1 ± 1.4 c | 2.9 ± 0.9 c | 2.8 ± 0.8 bc | 2.7 ± 0.4 a | 2.7 ± 0.6 ab | 3.1 ± 0.9 bc | 2.3 ± 1.0 bc |
| Weight of 1000 grains (g) | 31.0 ± 1.5 bc | 28.6 ± 1.5 ab | 30.3 ± 1.9 bc | 29.6 ± 1.4 abc | 30.1 ± 0.6 abc | 27.6 ± 3.8 a | 29.8 ± 1.7 abc | 31.4 ± 1.7 c |
| Grain number × 0.01 | 16.6 ± 5.4 c | 13.6 ± 4.0 b | 11.8 ± 4.2 b | 12.8 ± 3.1 b | 12.5 ± 3.8 b | 13.5 ± 2.8 b | 11.1 ± 3.5 b | 6.7 ± 2.3 a |
| Grain yield (g) | 50.5 ± 16.5 c | 38.1 ± 11.3 b | 35.2 ± 12.6 b | 37.2 ± 9.0 b | 36.8 ± 11.3 b | 36.4 ± 7.7 b | 32.3 ± 10.1 b | 20.6 ± 7.2 a |
| Parameters | CRF | CRFr2 | ACTIBION® | CONTROL |
|---|---|---|---|---|
| Biomass of the aerial part (t ha−1) | 18.31 ± 2.58 ab | 19.80 ± 4.00 ab | 21.23 ± 3.78 b | 15.14 ± 2.82 a |
| Panicle weight (t ha−1) | 5.85 ± 0.71 b | 6.85 ± 1.69 b | 6.32 ± 0.83 b | 3.74 ± 0.98 a |
| Grain weight (t ha−1) | 5.44 ± 0.78 b | 6.31 ± 1.43 b | 5.81 ± 0.61 b | 3.40 ± 0.88 a |
| Nitrogen Use Efficiency (kg kg−1 N) | 38.89 ± 5.60 a | 56.34 ± 12.74 b | 41.54 ± 4.33 a | - |
| Harvest Index | 0.48 ± 0.02 a | 0.51 ± 0.03 a | 0.49 ± 0.03 a | 0.46 ± 0.07 a |
| Parameters | Mean ± SD | |
|---|---|---|
| Microscale | Field | |
| Total nitrogen (g 100 g−1) | 0.12 ± 0.04 | 0.18 ± 0.02 |
| Total carbon (g 100 g−1) | 7.09 ± 0.08 | 6.74 ± 0.12 |
| Organic carbon (g 100 g−1) | 1.13 ± 0.18 | 2.03 ± 0.11 |
| pH | 8.5 ± 0.03 | 7.89 ± 0.04 |
| EC (µS cm−1) | 470.33 ± 34.12 | 645.25 ± 111.12 |
| P (g 100 g−1) | 0.051 ± 0.004 | 0.083 ± 0.007 |
| K (g 100 g−1) | 1.46 ± 0.12 | 0.81 ± 0.07 |
| Mg (g 100 g−1) | 1.87 ± 0.14 | 0.058 ± 0.04 |
| Ca (g 100 g−1) | 15.39 ± 1.06 | 11.77 ± 1.10 |
| Fe (g 100 g−1) | 2.43 ± 0.02 | 1.46 ± 0.07 |
| Cu (mg kg−1) | 32.54 ± 3.39 | 51.83 ± 2.96 |
| Mn (mg kg−1) | 339.57 ± 27.12 | 170.61 ± 11.25 |
| Zn (mg kg−1) | 43.19 ± 4.14 | 97.74 ± 6.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Ortiz, R.; Naranjo, M.Á.; Ruiz-Navarro, A.; Atares, S.; García, C.; Zotarelli, L.; San Bautista, A.; Vicente, O. Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice. Plants 2020, 9, 1183. https://doi.org/10.3390/plants9091183
Gil-Ortiz R, Naranjo MÁ, Ruiz-Navarro A, Atares S, García C, Zotarelli L, San Bautista A, Vicente O. Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice. Plants. 2020; 9(9):1183. https://doi.org/10.3390/plants9091183
Chicago/Turabian StyleGil-Ortiz, Ricardo, Miguel Ángel Naranjo, Antonio Ruiz-Navarro, Sergio Atares, Carlos García, Lincoln Zotarelli, Alberto San Bautista, and Oscar Vicente. 2020. "Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice" Plants 9, no. 9: 1183. https://doi.org/10.3390/plants9091183
APA StyleGil-Ortiz, R., Naranjo, M. Á., Ruiz-Navarro, A., Atares, S., García, C., Zotarelli, L., San Bautista, A., & Vicente, O. (2020). Enhanced Agronomic Efficiency Using a New Controlled-Released, Polymeric-Coated Nitrogen Fertilizer in Rice. Plants, 9(9), 1183. https://doi.org/10.3390/plants9091183






