Aerobic Barley Mg-protoporphyrin IX Monomethyl Ester Cyclase is Powered by Electrons from Ferredoxin
Abstract
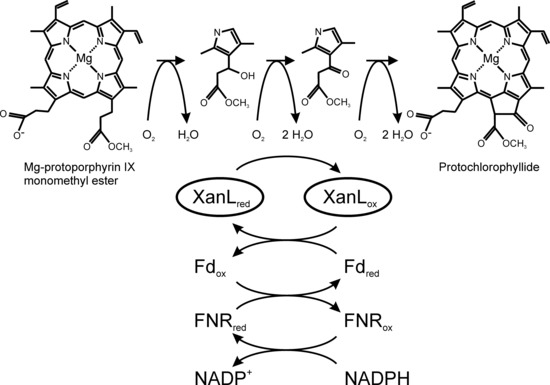
:1. Introduction
2. Results
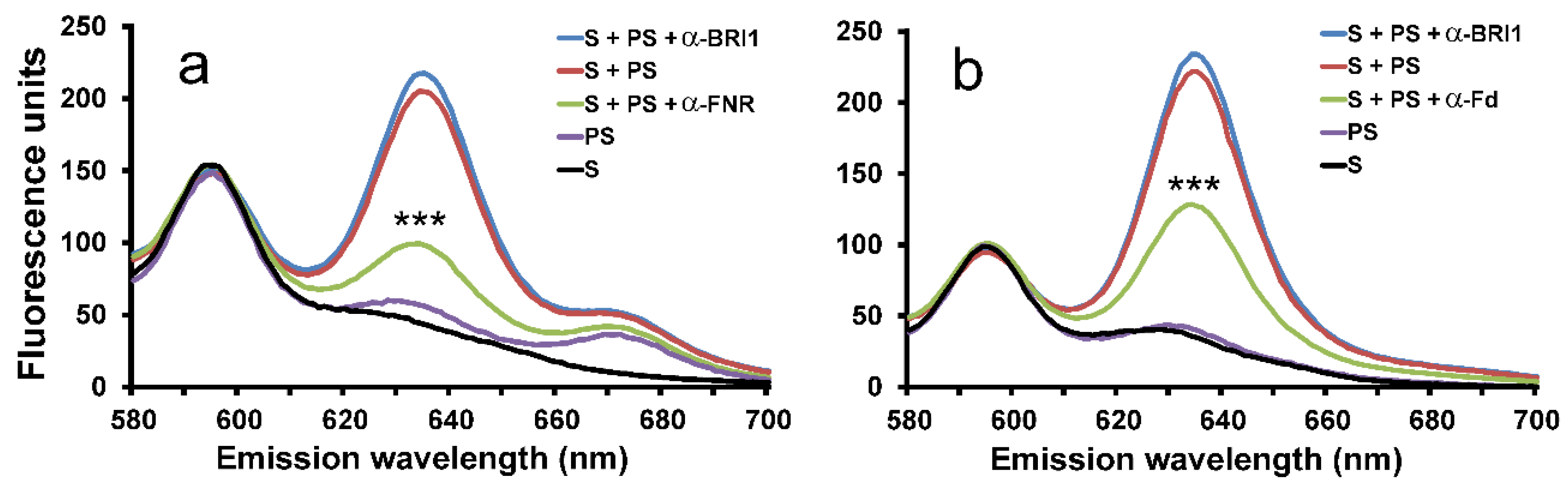
2.1. Inhibition of Cyclase Activity with Antibodies
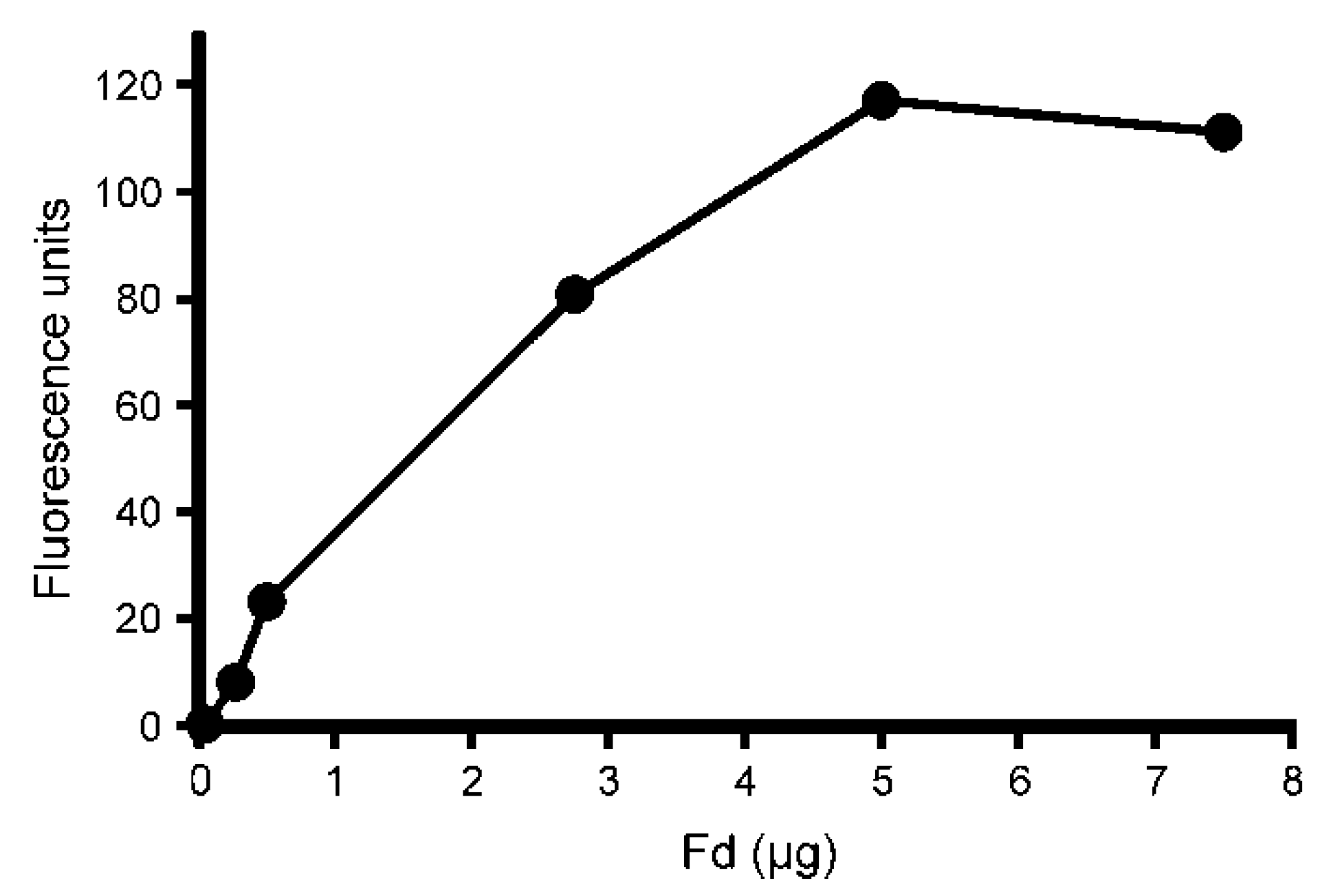
2.2. Development of a Recombinant Cyclase Assay
3. Discussion
4. Materials and Methods
4.1. Barley Plastid Preparation
4.2. Cyclase Assays
4.3. Antibodies Used
4.4. Plasmid Constructs
4.5. Protein Production
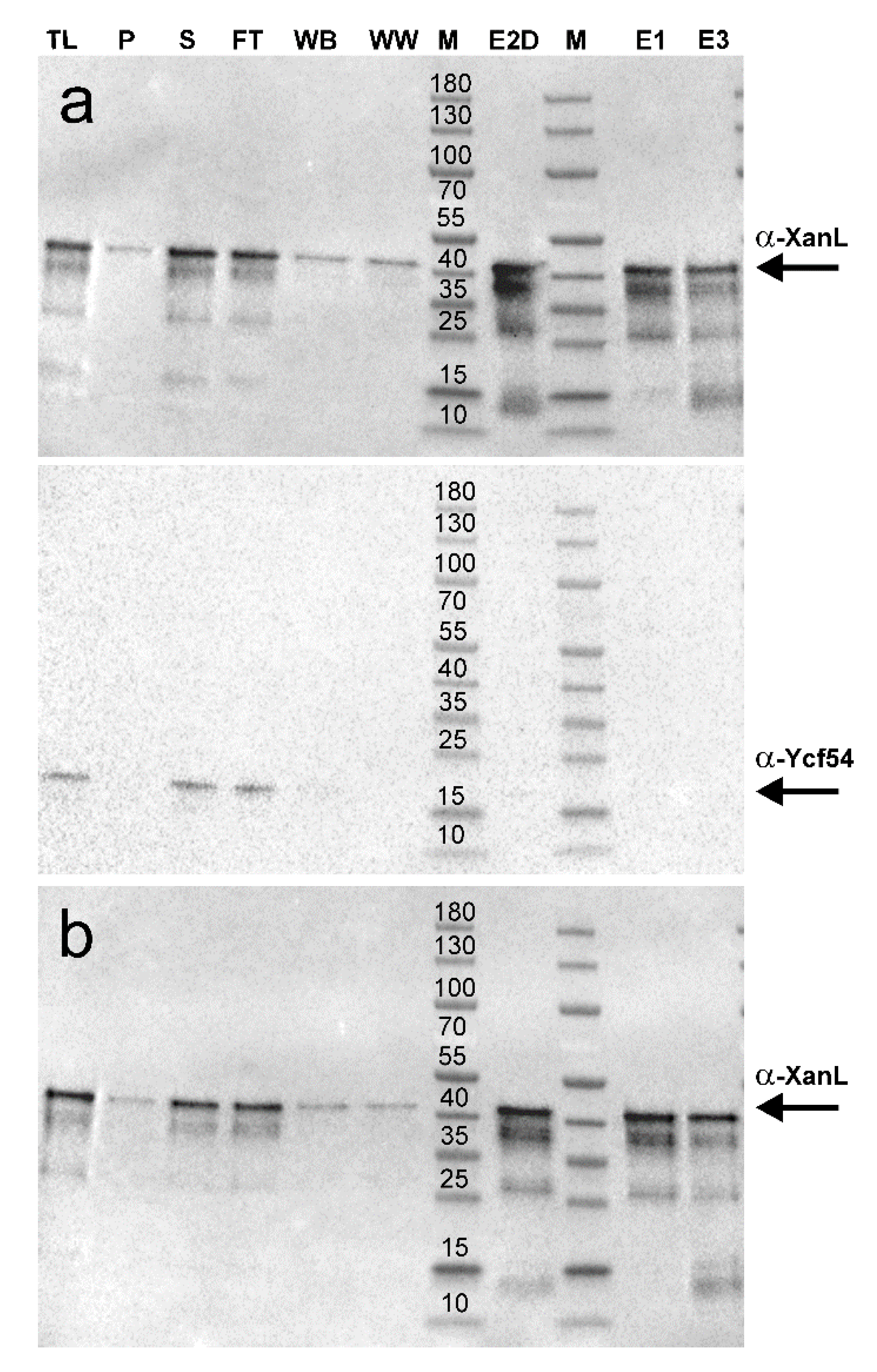
4.6. Protein Purification
4.7. SDS-PAGE and Immunoblotting
Author Contributions
Funding
Conflicts of Interest
References
- Tanaka, R.; Tanaka, A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Albus, C.A.; Salinas, A.; Czarnecki, O.; Kahlau, S.; Rothbart, M.; Thiele, W.; Lein, W.; Bock, R.; Grimm, B.; Schottler, M.A. LCAA, a novel factor required for magnesium protoporphyrin monomethylester cyclase accumulation and feedback control of aminolevulinic acid biosynthesis in tobacco. Plant Physiol. 2012, 160, 1923–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.E.; Canniffe, D.P.; Barnett, S.F.H.; Hollingshead, S.; Brindley, A.A.; Vasilev, C.; Bryant, D.A.; Hunter, C.N. Complete enzyme set for chlorophyll biosynthesis in Escherichia coli. Sci. Adv. 2018, 4, eaaq1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.E.; Canniffe, D.P.; Hunter, C.N. Three classes of oxygen-dependent cyclase involved in chlorophyll and bacteriochlorophyll biosynthesis. Proc. Natl. Acad. Sci. USA 2017, 114, 6280–6285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chereskin, B.M.; Castelfranco, P.A. Effects of iron and oxygen on chlorophyll biosynthesis. II. Observations on the biosynthetic pathway in isolated etiochloroplasts. Plant Physiol. 1982, 69, 112–116. [Google Scholar] [CrossRef]
- Pinta, V.; Picaud, M.; Reiss-Husson, F.; Astier, C. Rubrivivax gelatinosus acsF (previously orf358) codes for a conserved, putative binuclear-iron-cluster-containing protein involved in aerobic oxidative cyclization of Mg-protoporphyrin IX monomethylester. J. Bacteriol. 2002, 184, 746–753. [Google Scholar] [CrossRef] [Green Version]
- Rzeznicka, K.; Walker, C.J.; Westergren, T.; Kannangara, C.G.; von Wettstein, D.; Merchant, S.; Gough, S.P.; Hansson, M. Xantha-l encodes a membrane subunit of the aerobic Mg-protoporphyrin IX monomethyl ester cyclase involved in chlorophyll biosynthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 5886–5891. [Google Scholar] [CrossRef] [Green Version]
- Steccanella, V.; Hansson, M.; Jensen, P.E. Linking chlorophyll biosynthesis to a dynamic plastoquinone pool. Plant Physiol. Biochem. 2015, 97, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Tottey, S.; Block, M.A.; Allen, M.; Westergren, T.; Albrieux, C.; Scheller, H.V.; Merchant, S.; Jensen, P.E. Arabidopsis CHL27, located in both envelope and thylakoid membranes, is required for the synthesis of protochlorophyllide. Proc. Natl. Acad. Sci. USA 2003, 100, 16119–16124. [Google Scholar] [CrossRef] [Green Version]
- Walker, C.J.; Mansfield, K.E.; Smith, K.M.; Castelfranco, P.A. Incorporation of atmospheric oxygen into the carbonyl functionality of the protochlorophyllide isocyclic ring. Biochem. J. 1989, 257, 599–602. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.S.; Castelfranco, P.A.; Goff, D.A.; Smith, K.M. Intermediates in the formation of the chlorophyll isocyclic ring. Plant Physiol. 1985, 79, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J.; Schafer, W.; Gadón, N.; Katheder, I.; Drews, G.; Scheer, H. Origin of the two carbonyl oxygens of bacteriochlorophyll a. Demonstration of two different pathways for the formation of ring E in Rhodobacter sphaeroides and Roseobacter denitrificans, and a common hydratase mechanism for 3-acetyl group formation. Eur. J. Biochem. 1996, 239, 85–92. [Google Scholar] [CrossRef]
- Ouchane, S.; Steunou, A.S.; Picaud, M.; Astier, C. Aerobic and anaerobic Mg-protoporphyrin monomethyl ester cyclases in purple bacteria: A strategy adopted to bypass the repressive oxygen control system. J. Biol. Chem. 2004, 279, 6385–6394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, M.; Moseley, J.L.; Tottey, S.; Del Campo, J.A.; Quinn, J.; Kim, Y.; Merchant, S. Genetic dissection of nutritional copper signaling in Chlamydomonas distinguishes regulatory and target genes. Genetics 2004, 168, 795–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moseley, J.; Quinn, J.; Eriksson, M.; Merchant, S. The Crd1 gene encodes a putative di-iron enzyme required for photosystem I accumulation in copper deficiency and hypoxia in Chlamydomonas reinhardtii. EMBO J. 2000, 19, 2139–2151. [Google Scholar] [CrossRef] [Green Version]
- Minamizaki, K.; Mizoguchi, T.; Goto, T.; Tamiaki, H.; Fujita, Y. Identification of two homologous genes, chlAI and chlAII, that are differentially involved in isocyclic ring formation of chlorophyll a in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 2008, 283, 2684–2692. [Google Scholar] [CrossRef] [Green Version]
- Peter, E.; Salinas, A.; Wallner, T.; Jeske, D.; Dienst, D.; Wilde, A.; Grimm, B. Differential requirement of two homologous proteins encoded by sll1214 and sll1874 for the reaction of Mg protoporphyrin monomethylester oxidative cyclase under aerobic and micro-oxic growth conditions. Biochim. Biophys. Acta 2009, 1787, 1458–1467. [Google Scholar] [CrossRef] [Green Version]
- Wettstein, D.V.; Kahn, A.; Nielsen, O.F.; Gough, S. Genetic regulation of chlorophyll synthesis analyzed with mutants in barley. Science 1974, 184, 800–802. [Google Scholar] [CrossRef]
- Berthold, D.A.; Stenmark, P. Membrane-bound diiron carboxylate proteins. Annu. Rev. Plant Biol. 2003, 54, 497–517. [Google Scholar] [CrossRef]
- Walker, C.J.; Castelfranco, P.A.; Whyte, B.J. Synthesis of divinyl protochlorophyllide. Enzymological properties of the Mg-protoporphyrin IX monomethyl ester oxidative cyclase system. Biochem. J. 1991, 276, 691–697. [Google Scholar] [CrossRef]
- Wong, Y.S.; Castelfranco, P.A. Resolution and reconstitution of Mg-protoporphyrin IX monomethyl ester (oxidative) cyclase, the enzyme system responsible for the formation of the chlorophyll isocyclic ring. Plant Physiol. 1984, 75, 658–661. [Google Scholar] [CrossRef] [PubMed]
- Bollivar, D.W.; Beale, S.I. The chlorophyll biosynthetic enzyme Mg-protoporphyrin IX monomethyl ester (oxidative) cyclase. Characterization and partial purification from Chlamydomonas reinhardtii and Synechocystis sp. PCC 6803. Plant Physiol. 1996, 112, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollivar, D.; Braumann, I.; Berendt, K.; Gough, S.P.; Hansson, M. The Ycf54 protein is part of the membrane component of Mg-protoporphyrin IX monomethyl ester cyclase from barley (Hordeum vulgare L.). FEBS J. 2014, 281, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Hollingshead, S.; Kopecna, J.; Jackson, P.J.; Canniffe, D.P.; Davison, P.A.; Dickman, M.J.; Sobotka, R.; Hunter, C.N. Conserved chloroplast open-reading frame ycf54 is required for activity of the magnesium protoporphyrin monomethylester oxidative cyclase in Synechocystis PCC 6803. J. Biol. Chem. 2012, 287, 27823–27833. [Google Scholar] [CrossRef] [Green Version]
- Ellsworth, R.K.; Aronoff, S. Investigations of the biogenesis of chlorophyll a. IV. Isolation and partial characterization of some biosynthetic intermediates between Mg-protoporphine IX monomethyl ester and Mg-vinylpheoporphine a5, obtained from Chlorella mutants. Arch. Biochem. Biophys. 1969, 130, 374–383. [Google Scholar] [CrossRef]
- Granick, S. The structural and functional relationships between heme and chlorophyll. Harvey Lect. 1948, 44, 220–245. [Google Scholar]
- Warshawsky, I.; Hortin, G.L. Effect of substrate size on immunoinhibition of amylase activity. J. Clin. Lab. Anal. 2001, 15, 64–70. [Google Scholar] [CrossRef]
- Schmidt, H.; Heinz, E. Involvement of ferredoxin in desaturation of lipid-bound oleate in chloroplasts. Plant Physiol. 1990, 94, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Dockter, C.; Gruszka, D.; Braumann, I.; Druka, A.; Druka, I.; Franckowiak, J.; Gough, S.P.; Janeczko, A.; Kurowska, M.; Lundqvist, J.; et al. Induced variations in brassinosteroid genes define barley height and sturdiness, and expand the green revolution genetic toolkit. Plant Physiol. 2014, 166, 1912–1927. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.E.; Hunter, C.N. Protochlorophyllide synthesis by recombinant cyclases from eukaryotic oxygenic phototrophs and the dependence on Ycf54. Biochem. J. 2020, 477, 2313–2325. [Google Scholar] [CrossRef]
- Berthold, D.A.; Andersson, M.E.; Nordlund, P. New insight into the structure and function of the alternative oxidase. Biochim. Biophys. Acta 2000, 1460, 241–254. [Google Scholar] [CrossRef]
- Shinohara, F.; Kurisu, G.; Hanke, G.; Bowsher, C.; Hase, T.; Kimata-Ariga, Y. Structural basis for the isotype-specific interactions of ferredoxin and ferredoxin: NADP+ oxidoreductase: An evolutionary switch between photosynthetic and heterotrophic assimilation. Photosynth. Res. 2017, 134, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Herbst, J.; Girke, A.; Hajirezaei, M.R.; Hanke, G.; Grimm, B. Potential roles of YCF54 and ferredoxin-NADPH reductase for magnesium protoporphyrin monomethylester cyclase. Plant J. 2018, 94, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, C.J.; Mansfield, K.E.; Rezzano, I.N.; Hanamoto, C.M.; Smith, K.M.; Castelfranco, P.A. The magnesium-protoporphyrin IX (oxidative) cyclase system. Studies on the mechanism and specificity of the reaction sequence. Biochem. J. 1988, 255, 685–692. [Google Scholar] [PubMed]
- Oster, U.; Tanaka, R.; Tanaka, A.; Rudiger, W. Cloning and functional expression of the gene encoding the key enzyme for chlorophyll b biosynthesis (CAO) from Arabidopsis thaliana. Plant J. 2000, 21, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Hollingshead, S.; Kopecna, J.; Armstrong, D.R.; Bucinska, L.; Jackson, P.J.; Chen, G.E.; Dickman, M.J.; Williamson, M.P.; Sobotka, R.; Hunter, C.N. Synthesis of chlorophyll-binding proteins in a fully segregated Dycf54 strain of the cyanobacterium Synechocystis PCC 6803. Front. Plant Sci. 2016, 7, 292. [Google Scholar] [CrossRef]
- Furuya, T.; Hayashi, M.; Semba, H.; Kino, K. The mycobacterial binuclear iron monooxygenases require a specific chaperonin-like protein for functional expression in a heterologous host. FEBS J. 2013, 280, 817–826. [Google Scholar] [CrossRef]
- Izzo, V.; Leo, G.; Scognamiglio, R.; Troncone, L.; Birolo, L.; Di Donato, A. PHK from phenol hydroxylase of Pseudomonas sp. OX1. Insight into the role of an accessory protein in bacterial multicomponent monooxygenases. Arch. Biochem. Biophys. 2011, 505, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, V.S.; Scheller, H.V.; Møller, B.L. The photosystem I mutant viridis-zb63 of barley (Hordeum vulgare) contains low amounts of active but unstable photosystem I. Physiol. Plant. 1996, 98, 637–644. [Google Scholar] [CrossRef]
- Gough, S.P.; Rzeznicka, K.; Peterson Wulff, R.; de Cruz Francisco, J.; Hansson, A.; Jensen, P.E.; Hansson, M. A new method for isolating physiologically active Mg-protoporphyrin monomethyl ester, the substrate of the cyclase enzyme of the chlorophyll biosynthetic pathway. Plant Physiol. Biochem. 2007, 45, 932–936. [Google Scholar] [CrossRef]
- Andersen, B.; Koch, B.; Scheller, H.V. Structural and functional analysis of the reducing side of photosystem I. Physiol. Plant. 1992, 84, 154–161. [Google Scholar] [CrossRef]
- Andersen, B.; Scheller, H.V.; Møller, B.L. The PSI-E subunit of photosystem I binds ferredoxin:NADP+ oxidoreductase. FEBS Lett. 1992, 311, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Hansson, M.D.; Rzeznicka, K.; Rosenbäck, M.; Hansson, M.; Sirijovski, N. PCR-mediated deletion of plasmid DNA. Anal. Biochem. 2008, 375, 373–375. [Google Scholar] [CrossRef] [PubMed]



| Name | Sequence | Description |
|---|---|---|
| Duet_XanL_TEV_Forward | 5′-TGAGAATCTTTATTTTCAGGGCAA GCCGGGTAGCCCGAAGAAACGTGGCA | Forward primer to add TEV site to pETDuet-1HvXanL constructs |
| Duet_XanL_TEV_Reverse | 5′-CCCTGAAAATAAAGATTCTCAAG CTTGTCGACCTGCAGGCGCGCCGAGCT | Reverse primer to add TEV site to pETDuet-1HvXanL constructs |
| DuetTEV2x His_Forward | 5′-CACCACCATCATAGCCAGGATCC GAATTCGAGCTCGGCGCGCCTGCAGGT | Forward primer to add 2 extra Histidine residues to make a 8x His tag |
| DuetTEV2x His_Reverse | 5′-GGATCCTGGCTATGATGGTGGTG ATGATGGTGATGGCTGCTGCCCATGGT | Reverse primer to add 2 extra Histidine residues to make a 8x His tag |
| DuetTEV4x His_Reverse | 5′-GGATCCTGGCTATGATGGTGGTGATG ATGGTGATGATGATGGCTGCTGCCCATGGT | Reverse primer to add 2 extra Histidine residues to make a 10x His Tag. This primer was run with DuetTEV2xHis_Forward |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuart, D.; Sandström, M.; Youssef, H.M.; Zakhrabekova, S.; Jensen, P.E.; Bollivar, D.W.; Hansson, M. Aerobic Barley Mg-protoporphyrin IX Monomethyl Ester Cyclase is Powered by Electrons from Ferredoxin. Plants 2020, 9, 1157. https://doi.org/10.3390/plants9091157
Stuart D, Sandström M, Youssef HM, Zakhrabekova S, Jensen PE, Bollivar DW, Hansson M. Aerobic Barley Mg-protoporphyrin IX Monomethyl Ester Cyclase is Powered by Electrons from Ferredoxin. Plants. 2020; 9(9):1157. https://doi.org/10.3390/plants9091157
Chicago/Turabian StyleStuart, David, Malin Sandström, Helmy M. Youssef, Shakhira Zakhrabekova, Poul Erik Jensen, David W. Bollivar, and Mats Hansson. 2020. "Aerobic Barley Mg-protoporphyrin IX Monomethyl Ester Cyclase is Powered by Electrons from Ferredoxin" Plants 9, no. 9: 1157. https://doi.org/10.3390/plants9091157