Antagonistic Interaction between Phosphinothricin and Nepeta rtanjensis Essential Oil Affected Ammonium Metabolism and Antioxidant Defense of Arabidopsis Grown In Vitro
Abstract
1. Introduction
2. Results
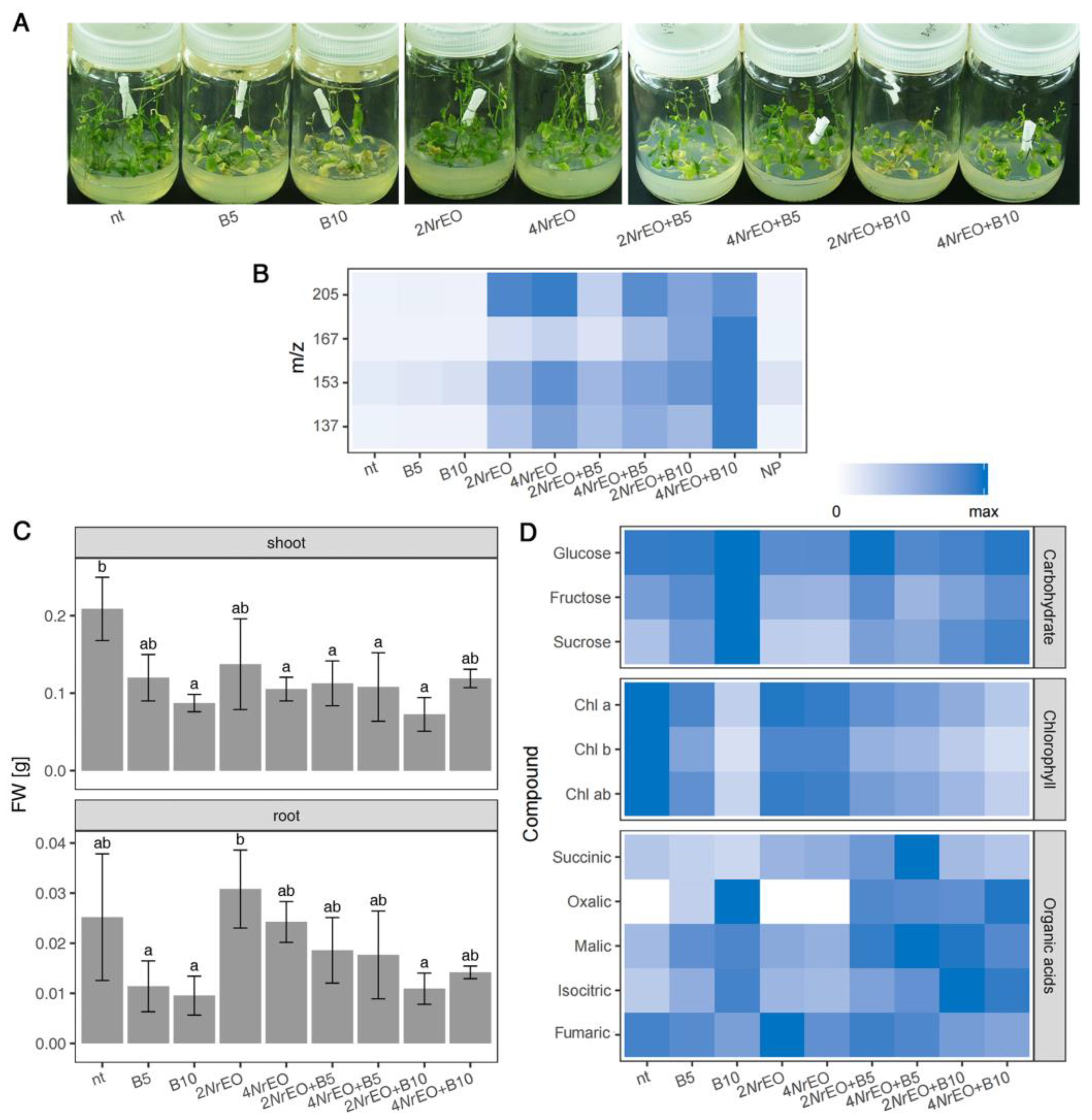
2.1. Growth and Metabolism of Arabidopsis as Influenced by BASTA and/or NrEO
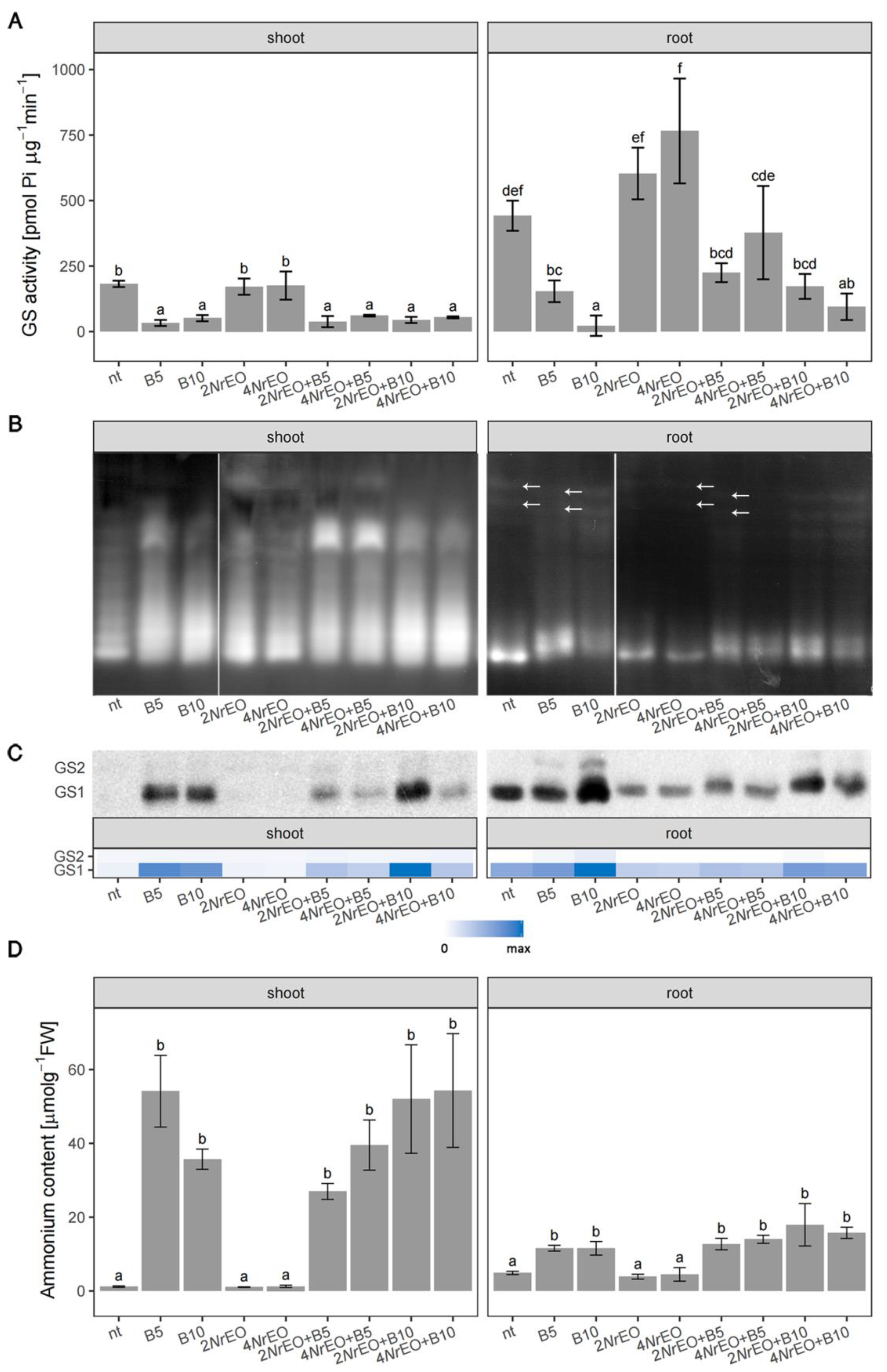
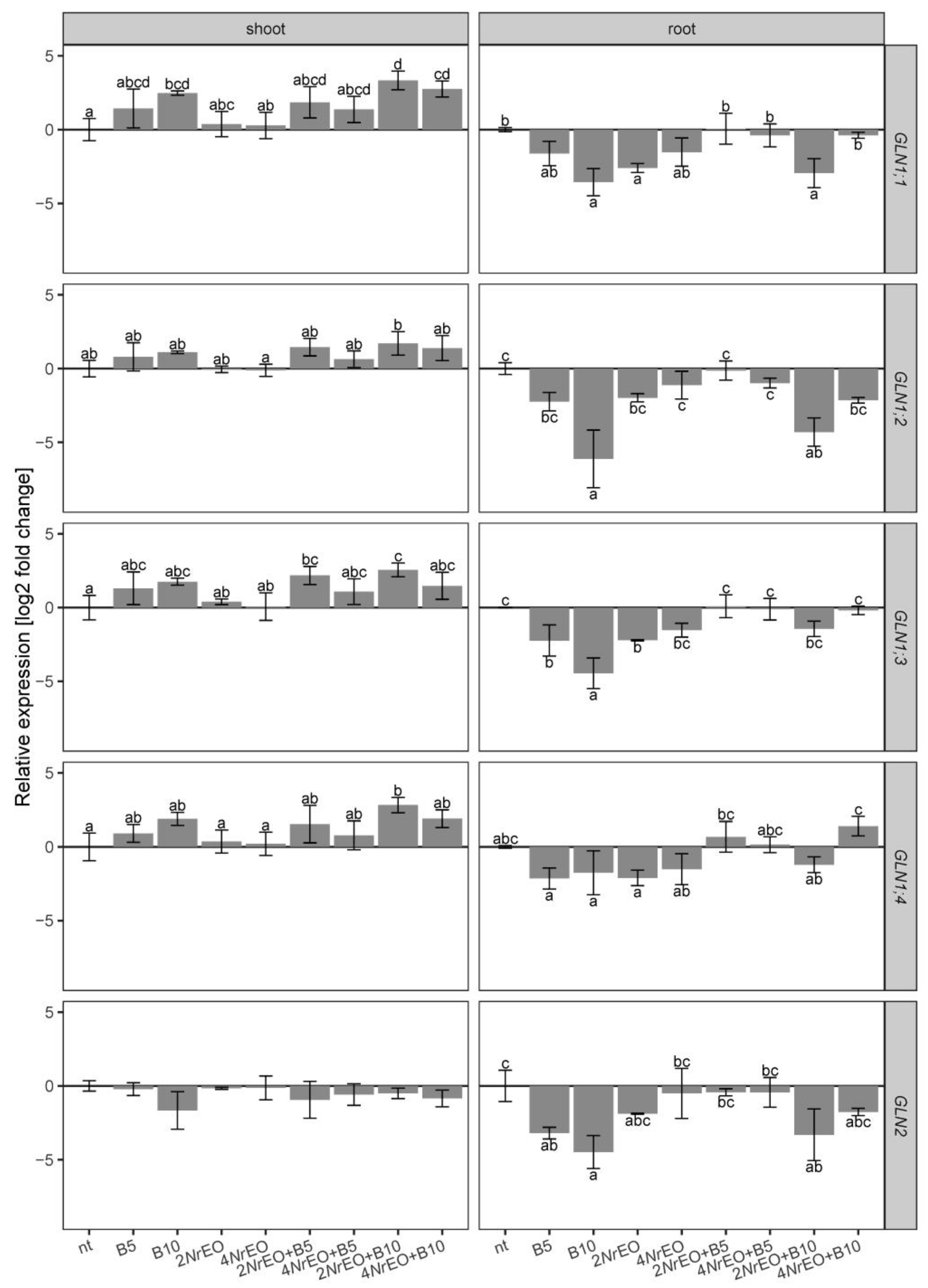
2.2. Ammonium Metabolism in Arabidopsis Shoots and Roots as Influenced by BASTA and/or NrEO
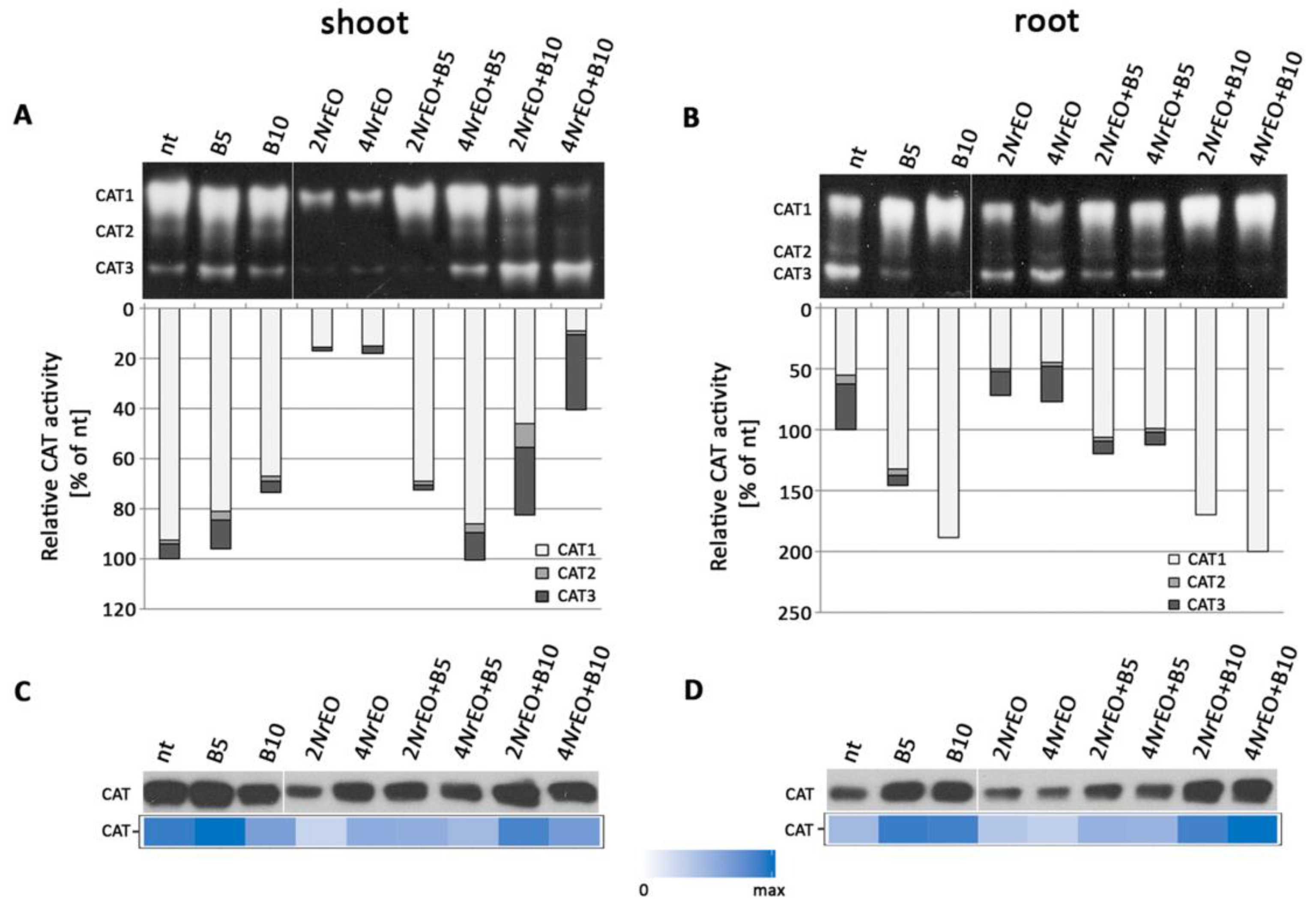
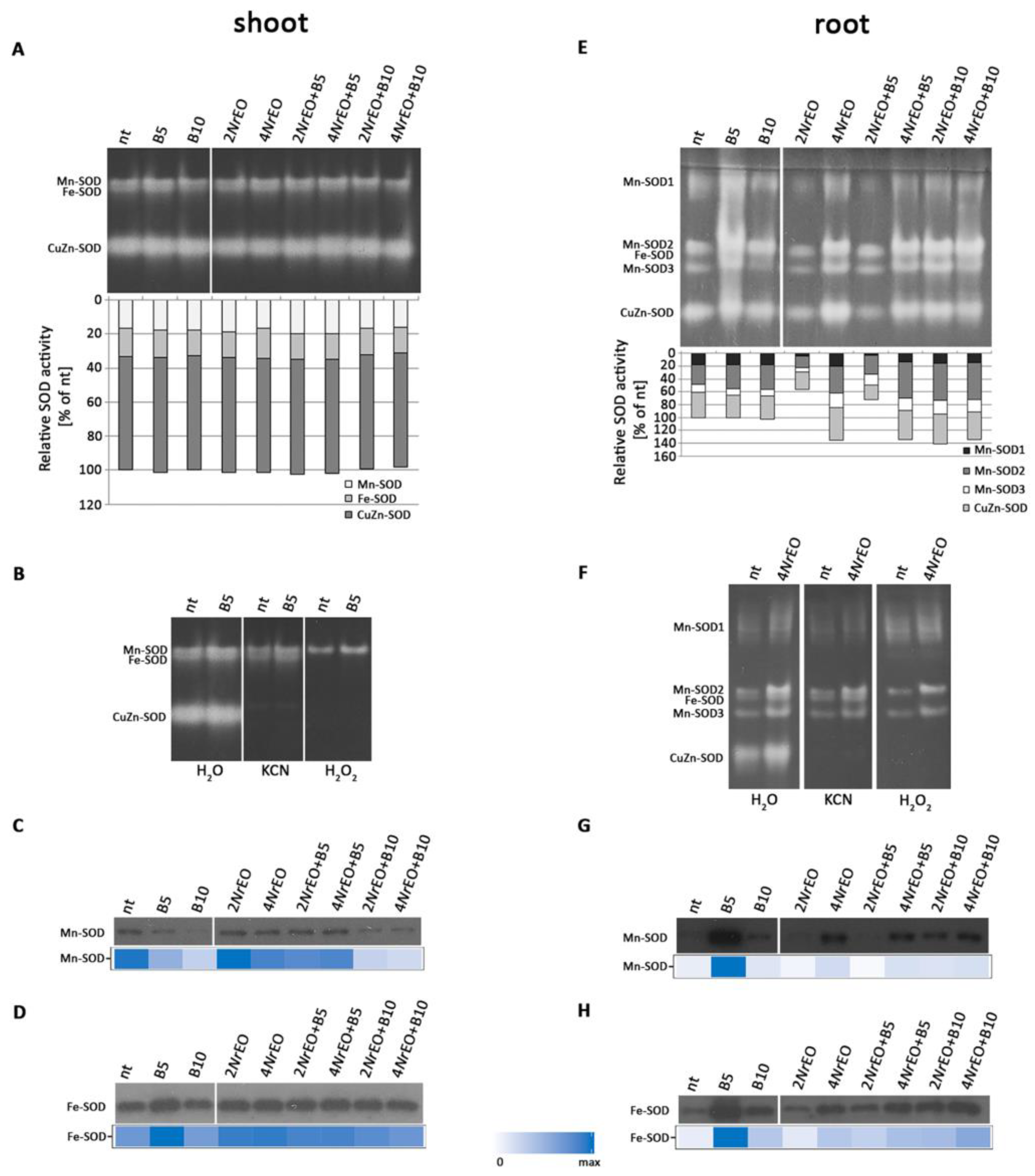
2.3. Antioxidant Enzymes Activity in Arabidopsis Shoots and Roots as Influenced by BASTA and/or NrEO
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Essential Oils
4.3. Joint Effect of BASTA via Roots and NrEO on A. thaliana Plants—In Vitro Phytotoxic Assay
4.4. Determination of Volatile Organic Compounds (VOCs) Concentration in the Atmosphere of Culture Vessels
4.5. Metabolic Profiling of Sugars, Organic Acids and Chlorophyll
4.6. Quantitative RT-PCR Analysis of GS-Coding Genes Expression
4.7. Determination of GS Activity and Ammonium Content
4.8. Determination of CAT, POX and SOD Activities
4.9. SDS-PAGE Electrophoresis and Immunoblotting
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Manderscheid, R.; Wild, A. Studies on the mechanism of inhibition by phosphinothricin of glutamine synthetase isolated from Triticum aestivum L. J. Plant Physiol. 1986, 123, 135–142. [Google Scholar] [CrossRef]
- Lydon, J.; Duke, S.O.; Singh, B.K. Inhibitors of glutamine biosynthesis. In Plant Amino Acids: Biochemistry and Biotechnology; BK Singh: New York, NY, USA, 1999; pp. 445–464. [Google Scholar]
- Wohlleben, W.; Arnold, W.; Broer, I.; Hillemann, D.; Strauch, E.; Punier, A. Nucleotide sequence of the phosphinothricin N-acetyltransferase gene from Streptomyces viridochromogenes Tü494 and its expression in Nicotiana tabacum. Gene 1988, 70, 25–37. [Google Scholar] [CrossRef]
- Dmitrović, S.; Dragićević, M.; Savić, J.; Milutinović, M.; Živković, S.; Maksimović, V.; Matekalo, D.; Mišić, D. Nepetalactone-rich essential oil mitigates phosphinothricin-induced ammonium toxicity in Arabidopsis thaliana (L.) Heynh. J. Plant Physiol. 2019, 237, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, R.; Zdravković-Korać, S.; Ninković, S.; Dragićević, M.; Miljuš-Đ, J.; Banović, B.; Bohanec, B.; Savić, J.; Mitić, N. Fertile transgenic Lotus corniculatus resistant to the non-selective herbicide phosphinothricin. Ann. Appl. Biol. 2013, 163, 475–493. [Google Scholar] [CrossRef]
- Eisenberg, D.; Gill, H.S.; Pfluegl, G.M.U.; Rotstein, S.H. Structure-function relationships of glutamine synthetase. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 2000, 1477, 122–145. [Google Scholar] [CrossRef]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant. Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming ammonium toxicity. Plant. Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef]
- Daniel-Vedele, F.; Filleur, S.; Caboche, M. Nitrate transport: A key step in nitrate assimilation. Curr. Opin. Plant. Biol. 1998, 1, 235–239. [Google Scholar] [CrossRef]
- Li, B.; Li, G.; Kronzucker, H.J.; Baluška, F.; Shi, W. Ammonium stress in Arabidopsis: Signaling, genetic loci, and physiological targets. Trends Plant. Sci. 2014, 19, 107–114. [Google Scholar] [CrossRef]
- Nimptsch, J.; Pflugmacher, S. Ammonia triggers the promotion of oxidative stress in the aquatic macrophyte Myriophyllum mattogrossense. Chemosphere 2007, 66, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Skopelitis, D.S.; Paranychianakis, N.V.; Paschalidis, K.A.; Pliakonis, E.D.; Delis, I.D.; Yakoumakis, D.I.; Kouvarakis, A.; Papadakis, A.K.; Stephanou, E.G.; Roubelakis-Angelakis, K.A. Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant. Cell 2006, 18, 2767–2781. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.D.A.; Rasmusson, A.G.; Escobar, M.A. Distinct signalling pathways and transcriptome response signatures differentiate ammonium-and nitrate-supplied plants. Plant. Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. A novel insight into the mode of action of glufosinate: How reactive oxygen species are formed. Photosynth. Res. 2020, 144, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.K.; Dayan, F.E. Glufosinate-ammonium: A review of the current state of knowledge. Pest Manag. Sci. 2020, 76, 3911–3925. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. Reactive oxygen species trigger the fast action of glufosinate. Planta 2019, 249, 1837–1849. [Google Scholar] [CrossRef]
- Shelp, B.J.; Swanton, C.J.; Hall, J.C. Glufosinate (phosphinothricin) mobility in young soybean shoots. J. Plant. Physiol. 1992, 139, 626–628. [Google Scholar] [CrossRef]
- Behrendt, H.; Matthies, M.; Gildemeister, H.; Görlitz, G. Leaching and transformation of glufosinate-ammonium and its main metabolite in a layered soil column. Environ. Toxicol. Chem. Int. J. 1990, 9, 541–549. [Google Scholar] [CrossRef]
- Gallina, M.A.; Stephenson, G.R. Dissipation of [14C] glufosinate ammonium in two Ontario soils. J. Agric. Food Chem. 1992, 40, 165–168. [Google Scholar] [CrossRef]
- Ismail, B.S.; Ahmad, A.R. Attenuation of the herbicidal activities of glufosinate-ammonium and imazapyr in two soils. Agric. Ecosyst. Environ. 1994, 47, 279–285. [Google Scholar] [CrossRef]
- Jia, G.; Xu, J.; Long, X.; Ge, S.; Chen, L.; Hu, D.; Zhang, Y. Enantioselective degradation and chiral stability of glufosinate in soil and water samples and formation of 3-methylphosphinicopropionic acid and N-acetyl-glufosinate metabolites. J. Agric. Food Chem. 2019, 67, 11312–11321. [Google Scholar] [CrossRef] [PubMed]
- Dorn, E.; Görlitz, G.; Heusel, R.; Stumpf, K. Verhalten von Glufosinat-ammonium in der Umwelt--Abbau im und Einflußauf das Ökosystem. Z. PflKrankh. PflSchutz Sonderh 1992, 13, 459–468. [Google Scholar]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F.E. Physiological factors affecting uptake and translocation of glufosinate. J. Agric. Food Chem. 2020, 68, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Craigmyle, B.D.; Ellis, J.M.; Bradley, K.W. Influence of weed height and glufosinate plus 2,4-D combinations on weed control in soybean with resistance to 2,4-D. Weed Technol. 2013. [Google Scholar] [CrossRef]
- Merchant, R.M.; Sosnoskie, L.M.; Culpepper, A.S.; Steckel, L.E.; York, A.C.; Braxton, L.B.; Ford, J.C. Weed response to 2,4-D, 2,4-DB, and dicamba applied alone or with glufosinate. J. Cotton Sci. 2013, 17, 212–218. [Google Scholar]
- Ganie, Z.A.; Jhala, A.J. Interaction of 2,4-D or dicamba with glufosinate for control of glyphosate-resistant giant ragweed (Ambrosia trifida L.) in glufosinate-resistant maize (Zea mays L.). Front. Plant Sci. 2017. [Google Scholar] [CrossRef]
- Koger, C.H.; Burke, I.C.; Miller, D.K.; Kendig, J.A.; Reddy, K.N.; Wilcut, J.W. MSMA antagonizes glyphosate and glufosinate efficacy on broadleaf and grass weeds. Weed Technol. 2007, 21, 159–165. [Google Scholar] [CrossRef]
- Dmitrović, S.; Perišić, M.; Stojić, A.; Živković, S.; Boljević, J.; Nestorović Živković, J.; Aničić, N.; Ristić, M.; Mišić, D. Essential oils of two Nepeta species inhibit growth and induce oxidative stress in ragweed (Ambrosia artemisiifolia L.) shoots in vitro. Acta Physiol. Plant. 2015. [Google Scholar] [CrossRef]
- Nestorović Živković, J.; Dmitrović, S.; Jovanović, V.; Živković, S.; Božić, D.; Aničić, N.; Mišić, D. Allelopathic potential of essential oil of Nepeta rtanjansis. Allelopath. J. 2016, 37, 207–219. [Google Scholar]
- Skorić, M.; Gligorijević, N.; Čavić, M.; Todorović, S.; Janković, R.; Ristić, M.; Mišić, D.; Radulović, S. Cytotoxic activity of Nepeta rtanjensis Diklić & Milojević essential oil and its mode of action. Ind. Crop. Prod. 2017, 100, 163–170. [Google Scholar] [CrossRef]
- Sparks, J.T.; Bohbot, J.D.; Ristić, M.; Mišić, D.; Skorić, M.; Mattoo, A.; Dickens, J.C. Chemosensory responses to the repellent Nepeta essential oil and its major component nepetalactone by Aedes aegypti (Diptera: Culicidae), a vector of Zika virus. J. Med. Entomol. 2017, 54, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Aničić, N.; Matekalo, D.; Skorić, M.; Pećinar, I.; Brkušanin, M.; Nestorović Živković, J.; Dmitrović, S.; Dajić Stevanović, Z.; Schulz, H.; Mišić, D. Trichome-specific and developmentally regulated biosynthesis of nepetalactones in leaves of cultivated Nepeta rtanjensis plants. Ind. Crops Prod. 2018, 117, 347–358. [Google Scholar] [CrossRef]
- Ishiyama, K.; Inoue, E.; Watanabe-Takahashi, A.; Obara, M.; Yamaya, T.; Takahashi, H. Kinetic properties and ammonium-dependent regulation of cytosolic isoenzymes of glutamine synthetase in arabidopsis. J. Biol. Chem. 2004, 279, 16598–16605. [Google Scholar] [CrossRef] [PubMed]
- Nestorović Živković, J. Antioxidative, Antimicrobial and Allelopathic Effects of Three Endemic Nepeta Species (Lamiaceae). Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2013. [Google Scholar]
- Hirel, B.; Gadal, P. Glutamine synthetase in rice: A comparative study of the enzymes from roots and leaves. Plant Physiol. 1980, 66, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; de Bang, T.C.; Pedersen, C.; Schjoerring, J.K. Cytosolic glutamine synthetase Gln1;2 is the main isozyme contributing to GS1 activity and can be up-regulated to relieve ammonium toxicity. Plant. Physiol. 2016, 171, 1921–1933. [Google Scholar] [CrossRef]
- Lothier, J.; Gaufichon, L.; Sormani, R.; Lemaître, T.; Azzopardi, M.; Morin, H.; Chardon, F.; Reisdorf-Cren, M.; Avice, J.C.; Masclaux-Daubresse, C. The cytosolic glutamine synthetase GLN1;2 plays a role in the control of plant growth and ammonium homeostasis in Arabidopsis rosettes when nitrate supply is not limiting. J. Exp. Bot. 2011, 62, 1375–1390. [Google Scholar] [CrossRef]
- Debouba, M.; Dguimi, H.M.; Ghorbel, M.; Gouia, H.; Suzuki, A. Expression pattern of genes encoding nitrate and ammonium assimilating enzymes in Arabidopsis thaliana exposed to short term NaCl stress. J. Plant. Physiol. 2013. [Google Scholar] [CrossRef]
- Logusch, E.W.; Walker, D.M.; McDonald, J.F.; Franz, J.E. Inhibition of plant glutamine synthetases by substituted phosphinothricins. Plant. Physiol. 1991, 95, 1057–1062. [Google Scholar] [CrossRef][Green Version]
- Forlani, G.; Obojska, A.; Berlicki, Ł.; Kafarski, P. Phosphinothricin analogues as inhibitors of plant glutamine synthetases. J. Agric. Food Chem. 2006, 54, 796–802. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, D.G.; Alam, I.; Kim, P.J.; Lee, J.J.; Ahn, Y.O.; Kwak, S.S.; Lee, I.J.; Bahk, J.D.; Kang, K.Y.; et al. Comparative proteomic study of arsenic-induced differentially expressed proteins in rice roots reveals glutathione plays a central role during as stress. Proteomics 2008, 8, 3561–3576. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant. Physiol. 2007, 164, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cui, Q.; Yue, S.; Lu, Z.; Zhao, M. Enantioselective effect of glufosinate on the growth of maize seedlings. Environ. Sci. Pollut. Res. 2019, 26, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Savić, J.; Platiša, J.; Dragićević, M.; Nikolić, R.; Mitić, N.; Cingel, A.; Vinterhalter, B. The activity of peroxidases and superoxide dismutases in transgenic phosphinothricin-resistant Lotus corniculatus shoots. Arch. Biol. Sci. 2010, 62, 1063–1070. [Google Scholar] [CrossRef]
- Qian, H.; Chen, W.; Sun, L.; Jin, Y.; Liu, W.; Fu, Z. Inhibitory effects of paraquat on photosynthesis and the response to oxidative stress in Chlorella vulgaris. Ecotoxicology 2009, 18, 537–543. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef]
- Mutlu, S.; Atici, Ö.; Esim, N.; Mete, E. Essential oils of catmint (Nepeta meyeri Benth.) induce oxidative stress in early seedlings of various weed species. Acta Physiol. Plant. 2011, 33, 943–951. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Taipale, R.; Ruuskanen, T.M.; Rinne, J.; Kajos, M.K.; Hakola, H.; Pohja, T.; Kulmala, M. Technical note: Quantitative long-term measurements of VOC concentrations by PTR-MS—Measurement, calibration, and volume mixing ratio calculation methods. Atmos. Chem. Phys. 2008, 8, 6681–6698. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta (BBA)-Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Dragićević, M.; Todorović, S.; Bogdanović, M.; Filipović, B.; Mišić, D.; Simonović, A. Knockout mutants as a tool to identify the subunit composition of Arabidopsis glutamine synthetase isoforms. Plant. Physiol. Biochem. 2014. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; Version R 3.5.0; R Foundation for Statistical Computing: Vienna, Austria, 2017; ISBN 3-900051-07-0. Available online: https://www.R-project.org (accessed on 1 May 2018).
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. Ser. B (Methodol.) 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Tree-based methods. In Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; pp. 251–269. [Google Scholar]
- Lenth, R.; Buerkner, P.; Herve, M.; LOve, J.; Riebl, H.; Singmann, H. emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.2.2 2018. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 1 July 2018).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrović, S.; Dragićević, M.; Savić, J.; Milutinović, M.; Živković, S.; Maksimović, V.; Matekalo, D.; Perišić, M.; Mišić, D. Antagonistic Interaction between Phosphinothricin and Nepeta rtanjensis Essential Oil Affected Ammonium Metabolism and Antioxidant Defense of Arabidopsis Grown In Vitro. Plants 2021, 10, 142. https://doi.org/10.3390/plants10010142
Dmitrović S, Dragićević M, Savić J, Milutinović M, Živković S, Maksimović V, Matekalo D, Perišić M, Mišić D. Antagonistic Interaction between Phosphinothricin and Nepeta rtanjensis Essential Oil Affected Ammonium Metabolism and Antioxidant Defense of Arabidopsis Grown In Vitro. Plants. 2021; 10(1):142. https://doi.org/10.3390/plants10010142
Chicago/Turabian StyleDmitrović, Slavica, Milan Dragićević, Jelena Savić, Milica Milutinović, Suzana Živković, Vuk Maksimović, Dragana Matekalo, Mirjana Perišić, and Danijela Mišić. 2021. "Antagonistic Interaction between Phosphinothricin and Nepeta rtanjensis Essential Oil Affected Ammonium Metabolism and Antioxidant Defense of Arabidopsis Grown In Vitro" Plants 10, no. 1: 142. https://doi.org/10.3390/plants10010142
APA StyleDmitrović, S., Dragićević, M., Savić, J., Milutinović, M., Živković, S., Maksimović, V., Matekalo, D., Perišić, M., & Mišić, D. (2021). Antagonistic Interaction between Phosphinothricin and Nepeta rtanjensis Essential Oil Affected Ammonium Metabolism and Antioxidant Defense of Arabidopsis Grown In Vitro. Plants, 10(1), 142. https://doi.org/10.3390/plants10010142








