Genetic Architecture of Cereal Leaf Beetle Resistance in Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Genotypic Analysis and Association Mapping
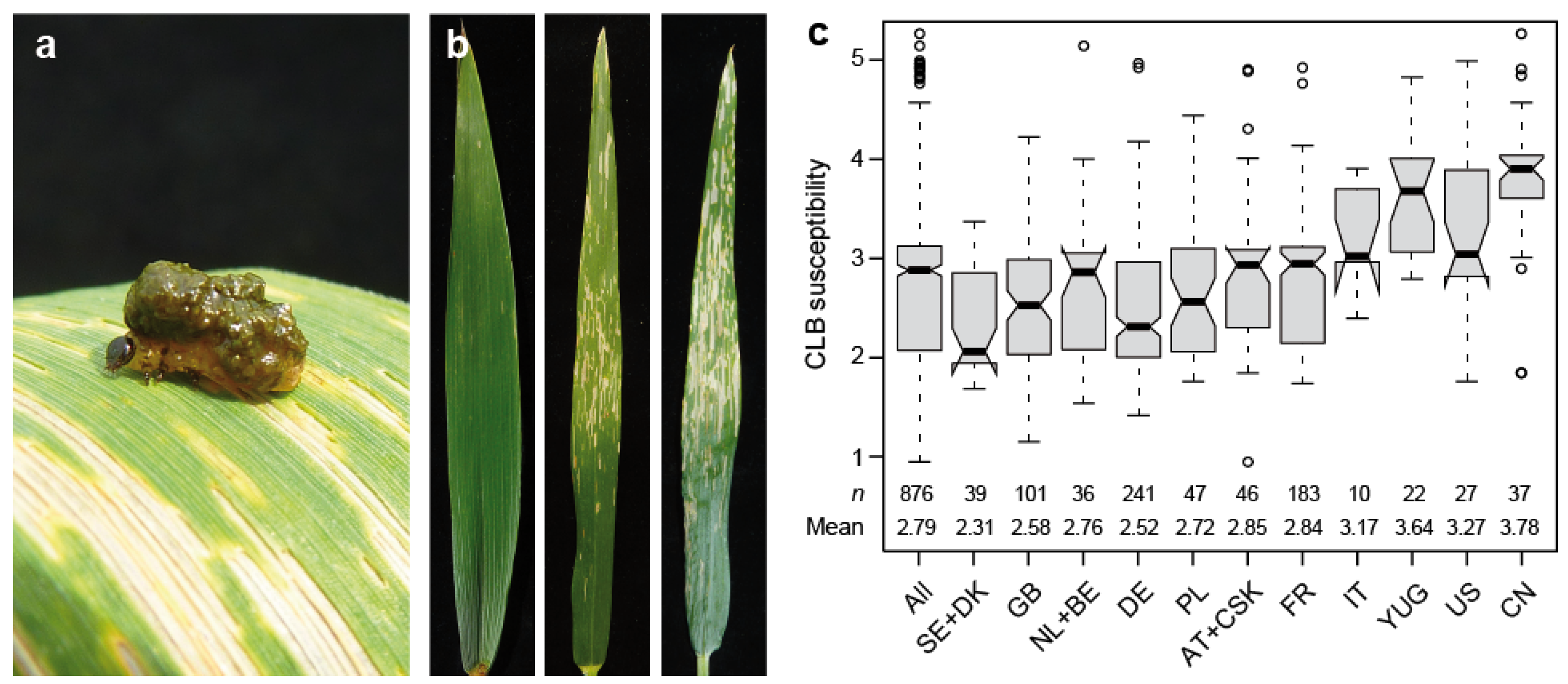
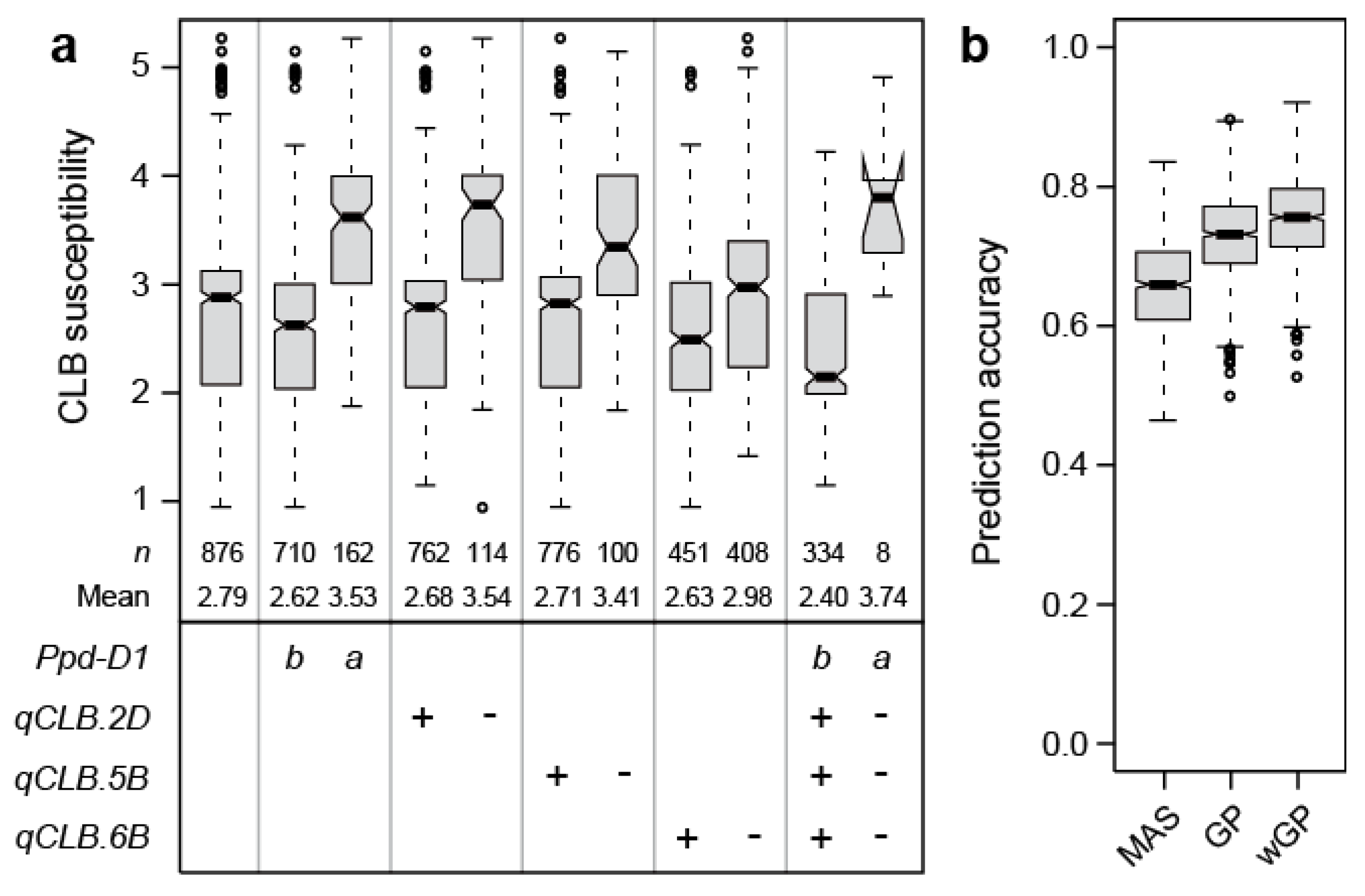
3. Results
4. Discussion
4.1. Phenotypic Variation of Cereal Leaf Beetle Resistance
4.2. Plant Characteristics Affecting Cereal Leaf Beetle Resistance
4.3. Deciphering the Genetic Architecture Underlying Cereal Leaf Beetle Resistance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Buntin, G.D.; Flanders, K.L.; Slaughter, R.W.; DeLamar, Z.D. Damage loss assessment and control of the cereal leaf beetle (Coleoptera: Chrysomelidae) in winter wheat. J. Econ. Entomol. 2004, 97, 374–382. [Google Scholar] [CrossRef]
- Philips, C.R.; Herbert, D.A.; Kuhar, T.P.; Reisig, D.D.; Thomason, W.E.; Malone, S. Fifty years of cereal leaf beetle in the U.S.: An update on its biology, management, and current research. J. Integr. Pest Mngmt. 2011, 2, 2011. [Google Scholar] [CrossRef]
- Herbert, D.A., Jr.; Van Duyn, J.W.; Bryan, M.D.; Karren, J.B. Cereal Leaf Beetle. In Handbook of Small Grain Insects; Buntin, G.D., Pike, K.S., Weiss, M.J., Webster, J.A., Eds.; Entomological Society of America: Lanham, MD, USA, 2007; p. 120. [Google Scholar]
- Papp, M.; Mesterházy, Á. Resistance of winter wheat to cereal leaf beetle (Coleoptera: Chrysomelidae) and bird cherry-oat aphid (Homoptera: Aphidiae). J. Econ. Entomol. 1996, 89, 1649–1657. [Google Scholar] [CrossRef]
- Joukhadar, R.; El-Bouhssini, M.; Jighly, A.; Ogbonnaya, F.C. Genome-wide association mapping for five major pest resistances in wheat. Mol. Breed. 2013, 32, 943–960. [Google Scholar] [CrossRef]
- Boeven, P.H.G.; Longin, C.F.H.; Würschum, T. A unified framework for hybrid breeding and the establishment of heterotic groups in wheat. Theor. Appl. Genet. 2016, 129, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Würschum, T.; Langer, S.M.; Longin, C.F.H.; Tucker, M.R.; Leiser, W.L. A modern Green Revolution gene for reduced height in wheat. Plant J. 2017, 92, 892–903. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.; Piepho, H.-P.; Whitaker, D. Augmented p-rep designs. Biom. J. 2011, 53, 19–27. [Google Scholar] [CrossRef]
- Würschum, T.; Langer, S.M.; Longin, C.F.H.; Tucker, M.R.; Leiser, W.L. Refining the genetic architecture of flag leaf glaucousness in wheat. Theor. Appl. Genet. 2020, 133, 981–991. [Google Scholar] [CrossRef] [Green Version]
- Piepho, H.P.; Möhring, J. Computing heritability and selection response from unbalanced plant breeding trials. Genetics 2007, 177, 1881–1888. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: http://www.R-project.org (accessed on 28 August 2020).
- Gilmour, A.R.; Gogel, B.; Cullis, B.; Thompson, R. ASReml User Guide Release 3.0; VSN International Ltd.: Hemel Hempstead, UK, 2009. [Google Scholar]
- Li, H.; Vikram, P.; Singh, R.P.; Kilian, A.; Carling, J.; Song, J.; Burgueño-Ferreira, J.; Bhavani, S.; Huerta-Espino, J.; Payne, T.; et al. A high density GBS map of bread wheat and its application for dissecting complex disease resistance traits. BMC Genom. 2015, 16, 216. [Google Scholar] [CrossRef] [Green Version]
- Beales, J.; Turner, A.; Griffiths, S.; Snape, J.W.; Laurie, D.A. A Pseudo-Response Regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 115, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Utz, H.F.; Melchinger, A.E.; Schön, C.C. Bias and sampling error of the estimated proportion of genotypic variance explained by quantitative trait loci determined from experimental data in maize using cross validation and validation with independent samples. Genetics 2000, 154, 1839–1849. [Google Scholar] [PubMed]
- Bernays, E.A.; Chapman, R.L. Host-Plant Selection by Phytophagous Insects; Chapman and Hall: New York, NY, USA, 1994. [Google Scholar]
- Würschum, T.; Boeven, P.H.G.; Langer, S.M.; Longin, C.F.H.; Leiser, W.L. Multiply to conquer: Copy number variations at Ppd-B1 and Vrn-A1 facilitate global adaptation in wheat. BMC Genet. 2015, 16, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Würschum, T.; Langer, S.M.; Longin, C.F.H.; Tucker, M.R.; Leiser, W.L. A three-component system incorporating Ppd-D1, copy number variation at Ppd-B1, and numerous small-effect QTL facilitates adaptation of heading time in winter wheat cultivars of worldwide origin. Plant Cell Environ. 2018, 41, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.D.; Weaver, D.K.; Hofland, M.L.; Sing, S.E.; Buteler, M.; Lanning, S.P.; Naruoka, Y.; Crutcher, F.; Blake, N.K.; Martin, J.M.; et al. Identification of novel QTL for sawfly resistance in wheat. Crop Sci. 2010, 50, 73–86. [Google Scholar] [CrossRef]
- Langer, S.M.; Longin, C.F.H.; Würschum, T. Flowering time control in European winter wheat. Front. Plant Sci. 2014, 5, 537. [Google Scholar] [CrossRef] [Green Version]
- Schillinger, J.A., Jr.; Gallun, R.L. Leaf pubescence of wheat as a deterrent to the cereal leaf beetle, Oulema melanopus. Ann. Entomol. Soc. Am. 1968, 61, 900–903. [Google Scholar] [CrossRef]
- Eagles, H.A.; Cane, K.; Kuchel, H.; Hollamby, G.J.; Vallance, N.; Eastwood, R.F.; Gororo, N.N.; Martin, P.J. Photoperiod and vernalization gene effects in southern Australian wheat. Crop Pasture Sci. 2010, 61, 721–730. [Google Scholar] [CrossRef]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar]
- Heffner, E.L.; Sorrells, M.E.; Jannink, J.-L. Genomic Selection for Crop Improvement. Crop Sci. 2009, 49, 1–12. [Google Scholar] [CrossRef]
- Reddy, A.; Gadi, V.P.; Guerrero, A. Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Markovič, D.; Bohinc, T.; Trdan, S. Susceptibility of 20 cabbage genotypes to flea beetles attack under field conditions. J. Food Agric. Environ. 2014, 12, 1356–1361. [Google Scholar]
- Bohinc, T.; Devetak, M.; Trdan, S. Quantity of glucosinolates in 10 cabbage genotypes and their impact on the feeding of Mamestra brassicae caterpillars. Arch. Biol. Sci. 2014, 66, 867–876. [Google Scholar] [CrossRef] [Green Version]

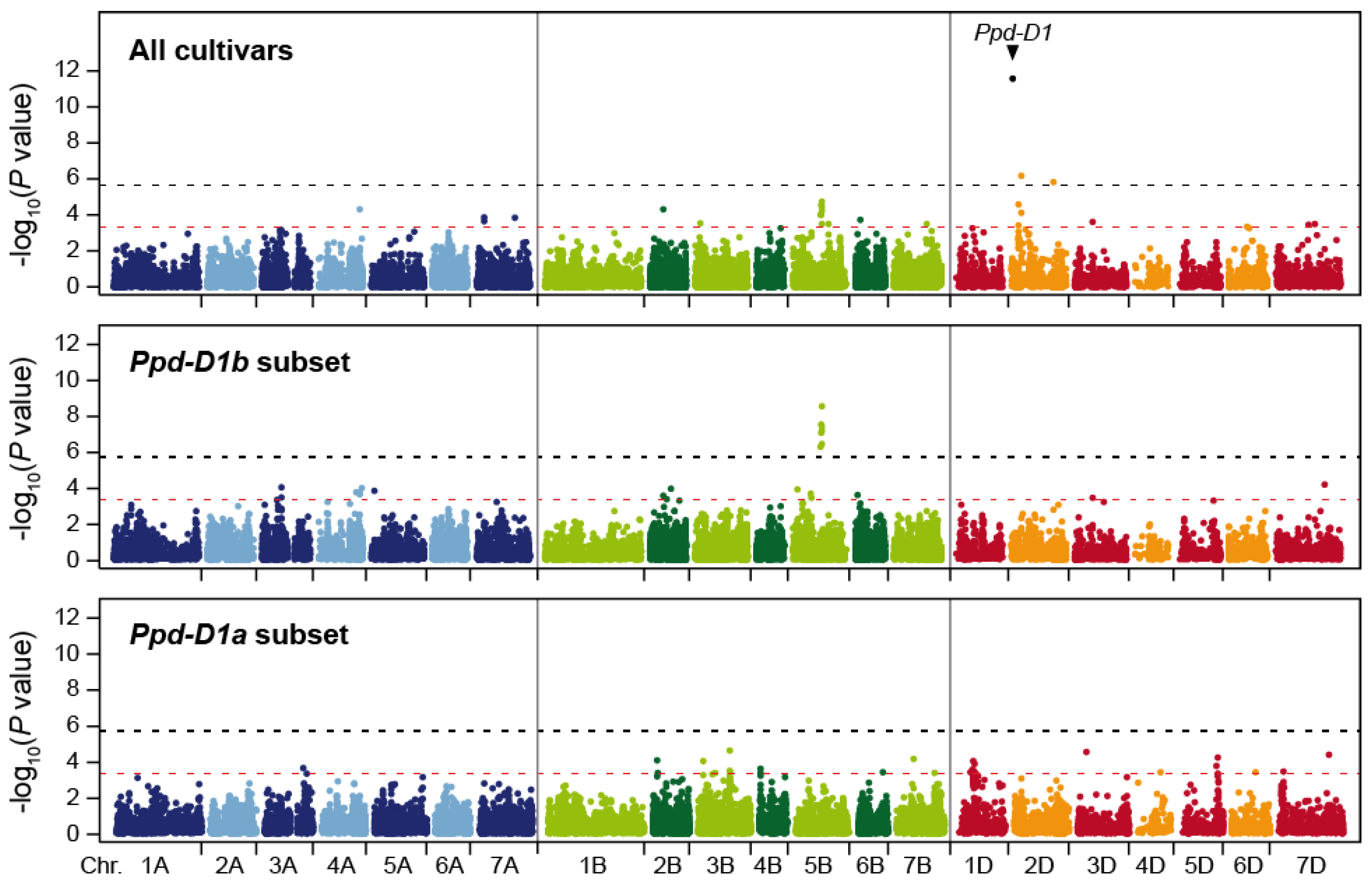
| Gene/Marker | Chr. | Pos. (cM) | Pos. (bp) a | P Value | pG | Effect | pb |
|---|---|---|---|---|---|---|---|
| Ppd-D1 | 2D | ~51 | 2.7 × 10−6 | 35.3 | 0.46 | 0.81 | |
| D1104237 | 2D | 244.8 | 568.757.425 | 1.57 × 10−6 | 6.2 | 0.43 | 0.87 |
| Additional putative quantitative trait loci (QTL) c | |||||||
| D2255871 | 4A | 236.8 | 720.048.127 | 5.17 × 10−5 | 2.4 | 0.15 | 0.09 |
| S1100606 | 7A | 46.4 | 34.920.176 | 1.47 × 10−4 | 0.6 | 0.34 | 0.88 |
| D1208731 | 2B | 76.7 | 65.468.079 | 5.17 × 10−5 | 2.6 | 0.13 | 0.85 |
| D1062313 | 3B | 28.7 | 13.410.285 | 3.07 × 10−4 | 0.7 | −0.37 | 0.94 |
| D1233649 | 5B | 161.7 | 579.076.944 | 1.8 × 10−5 | 6.5 | 0.34 | 0.89 |
| S1027735 | 6B | 27.2 | 34.050.222 | 1.9 × 10−4 | 4.0 | 0.17 | 0.53 |
| D977492 | 7B | 188.8 | 688.130.100 | 3.2 × 10−4 | 2.5 | −0.22 | 0.70 |
| D1301286 | 3D | 101.0 | 100.414.776 | 2.5 × 10−4 | 1.1 | 0.27 | 0.72 |
| D1109181 | 6D | 103.8 | 157.373.073 | 4.7 × 10−4 | 0.1 | −0.32 | 0.79 |
| D1129811 | 7D | 188.5 | 207.189.065 | 3.5 × 10−4 | 1.5 | 0.28 | 0.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Würschum, T.; Leiser, W.L.; Langer, S.M.; Tucker, M.R.; Miedaner, T. Genetic Architecture of Cereal Leaf Beetle Resistance in Wheat. Plants 2020, 9, 1117. https://doi.org/10.3390/plants9091117
Würschum T, Leiser WL, Langer SM, Tucker MR, Miedaner T. Genetic Architecture of Cereal Leaf Beetle Resistance in Wheat. Plants. 2020; 9(9):1117. https://doi.org/10.3390/plants9091117
Chicago/Turabian StyleWürschum, Tobias, Willmar L. Leiser, Simon M. Langer, Matthew R. Tucker, and Thomas Miedaner. 2020. "Genetic Architecture of Cereal Leaf Beetle Resistance in Wheat" Plants 9, no. 9: 1117. https://doi.org/10.3390/plants9091117





