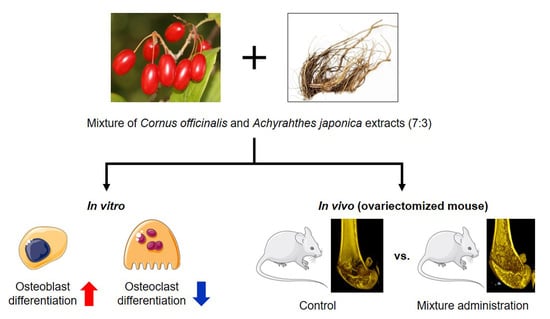
Anti-Osteoporotic Effects of the Herbal Mixture of Cornus officinalis and Achyranthes japonica In Vitro and In Vivo
Abstract
:1. Introduction
2. Results and Discussion
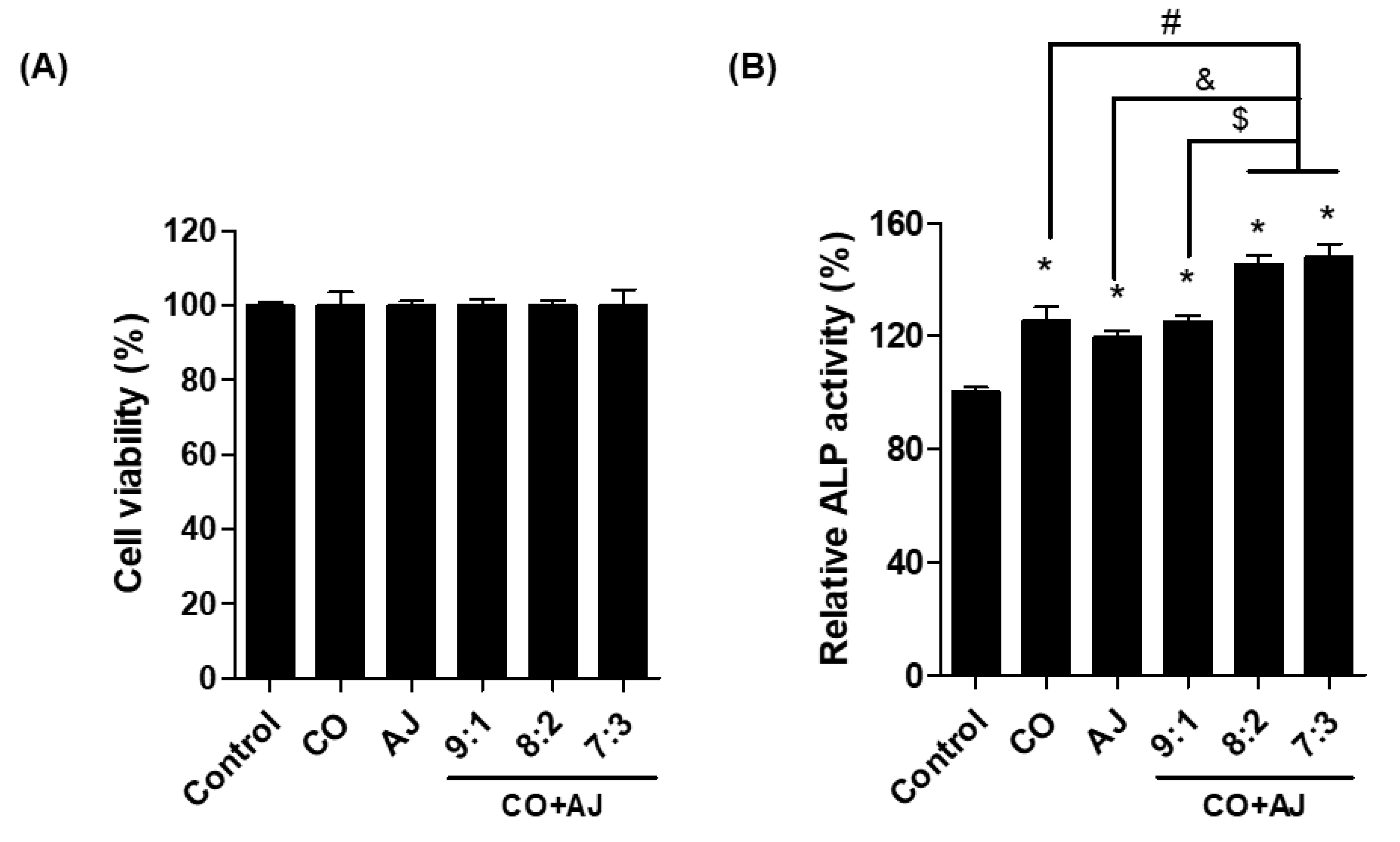
2.1. CO and AJ Mixtures Induced an Increase in Osteoblast Differentiation Compared to Individual Treatment
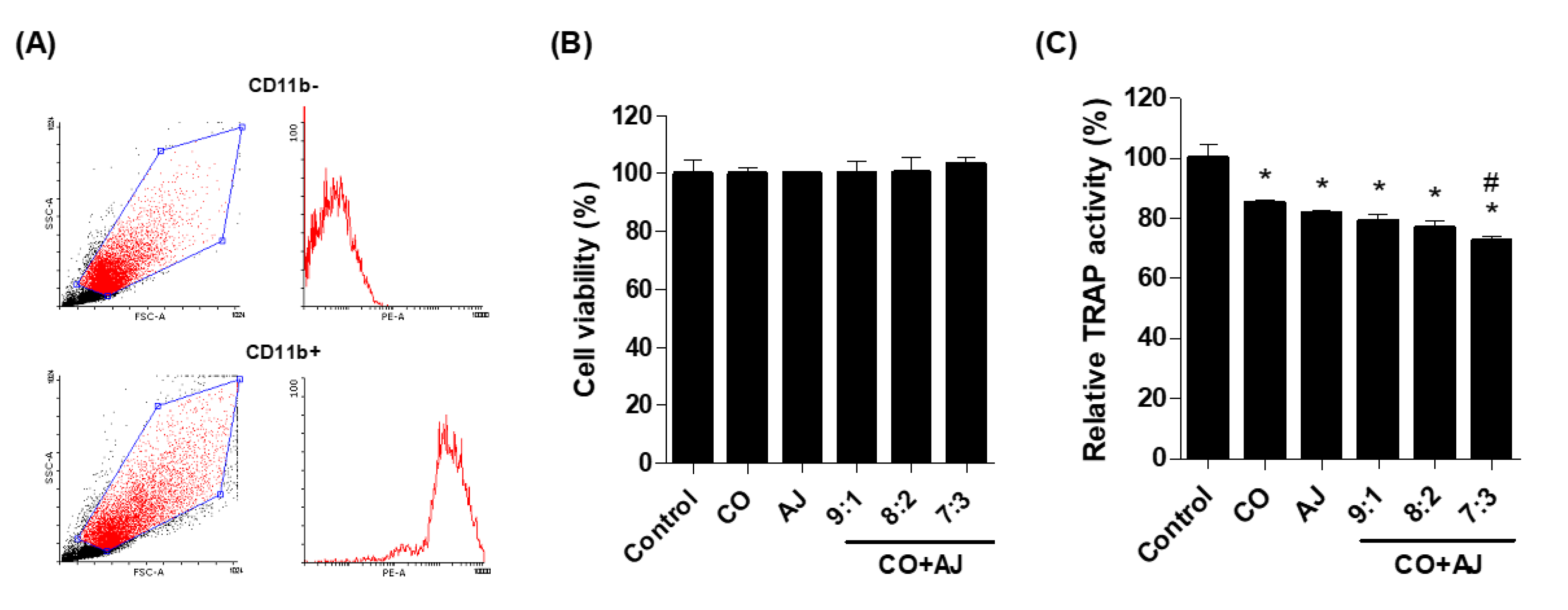
2.2. CO and AJ Mixture Inhibited Osteoclast Differentiation Compared to Individual Treatment
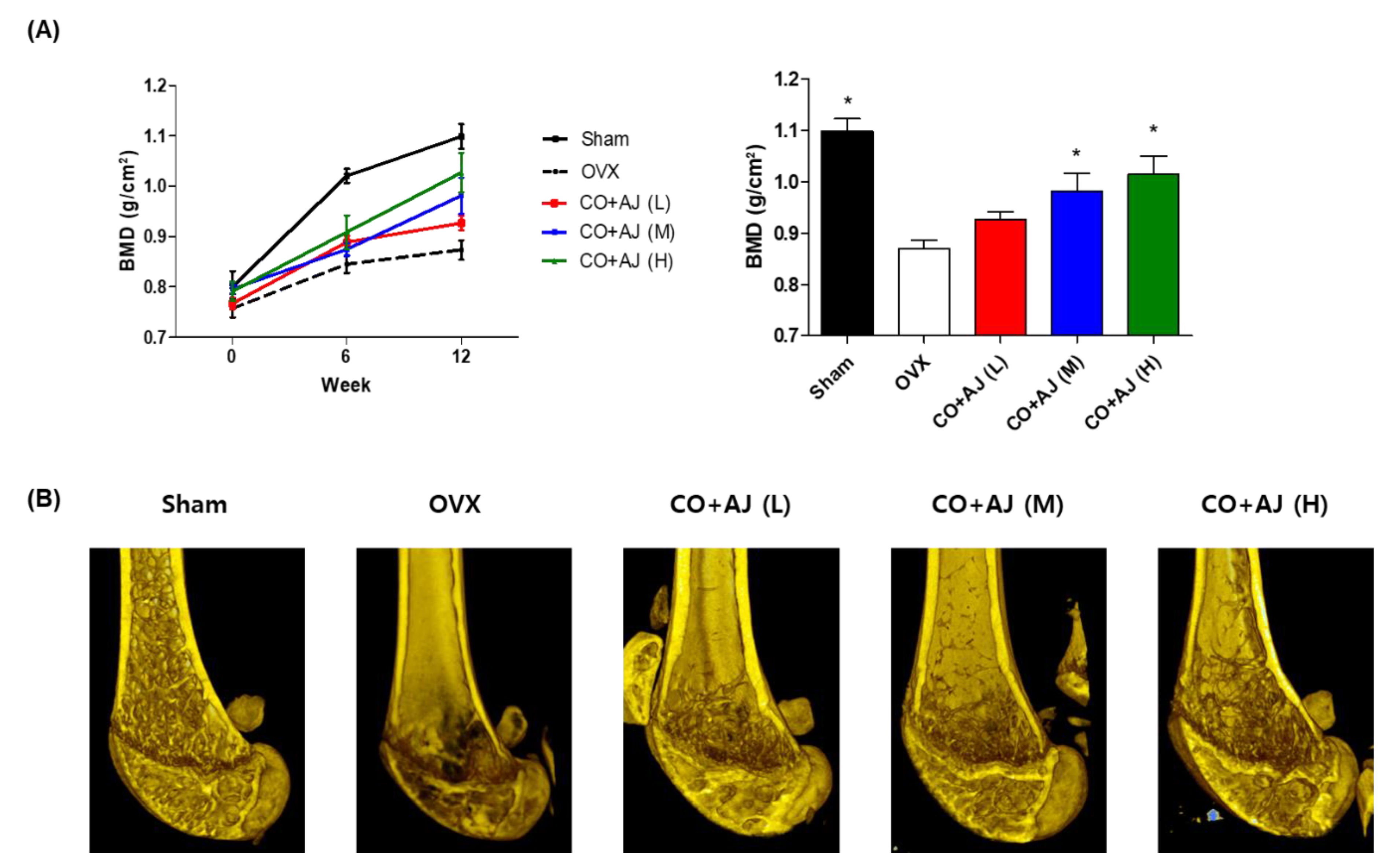
2.3. CO and AJ Mixture Showed an Anti-Osteoporotic Effect on OVX-Induced Osteoporosis in Mice
3. Materials and Methods
3.1. Extraction and Sample Preparation
3.2. Osteoblast and Osteoclast Differentiation
3.3. Measurement of ALP and TRAP Activity
3.4. Cell Viability
3.5. Quantitative Reverse-Transcription PCR (qRT-PCR)
3.6. Ovariectomized Osteoporotic Model Mice Experiment
3.7. Analysis of Bone Marrow Density (BMD) and Transverse Micro-CT Imaging in Bone Tissues
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weatherholt, A.M.; Fuchs, R.K.; Warden, S.J. Specialized connective tissue: Bone, the structural framework of the upper extremity. J. Hand Ther. 2012, 25, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Su, N.; Yang, J.; Xie, Y.; Du, X.; Chen, H.; Zhou, H.; Chen, L. Bone function, dysfunction and its role in diseases including critical illness. Int. J. Biol. Sci. 2019, 15, 776–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T. Regulation of bone homeostasis by bone cells. Clin. Calcium 2013, 23, 218–228. [Google Scholar]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef] [Green Version]
- Wright, N.C.; Saag, K.G.; Dawson-Hughes, B.; Khosla, S.; Siris, E.S. The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the USA. Osteoporos. Int. 2017, 28, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, T.; Danks, L.; Sabokbar, A.; Athanasou, N.A. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology (Oxford) 2002, 41, 1232–1239. [Google Scholar] [CrossRef] [Green Version]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2016-Executive Summary. Endocr. Pract. 2016, 22, 1111–1118. [Google Scholar] [CrossRef] [Green Version]
- Jalleh, R.; Basu, G.; Le Leu, R.; Jesudason, S. Denosumab-Induced Severe Hypocalcaemia in Chronic Kidney Disease. Case Rep. Nephrol. 2018, 2018, 7384763. [Google Scholar] [CrossRef]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Su, D.F. Defining pharmacology of natural products in the 21st century-challenge on multiple fronts. Front. Pharmacol. 2010, 1, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Lawson, D.; Kras, A.; Ryan, D. The use of preventive strategies for bone loss. Am. J. Chin. Med. 2005, 33, 299–306. [Google Scholar] [CrossRef] [PubMed]
- HemaIswarya, S.; Doble, M. Potential synergism of natural products in the treatment of cancer. Phytother. Res. 2006, 20, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Wohlfarth, C.; Efferth, T. Natural products as promising drug candidates for the treatment of hepatitis B and C. Acta Pharmacol. Sin. 2009, 30, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Shin, J.S.; Lee, W.S.; Shim, H.Y.; Park, J.M.; Jang, D.S.; Lee, K.T. Chikusetsusaponin IVa Methyl Ester Isolated from the Roots of Achyranthes japonica Suppresses LPS-Induced iNOS, TNF-alpha, IL-6, and IL-1beta Expression by NF-kappaB and AP-1 Inactivation. Biol. Pharm. Bull. 2016, 39, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Hwang, K.A.; Hwang, Y.J.; Song, J. Antioxidant activities and oxidative stress inhibitory effects of ethanol extracts from Cornus officinalis on raw 264.7 cells. BMC Complement Altern. Med. 2016, 16, 196. [Google Scholar] [CrossRef] [Green Version]
- Telang, N.T.; Li, G.; Sepkovic, D.W.; Bradlow, H.L.; Wong, G.Y. Anti-proliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor-positive clinical breast cancer. Mol. Med. Rep. 2012, 5, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kim, Y.K.; Choi, M.K.; Oh, J.; Kwak, H.B.; Kim, J.J. Effect of Cornus Officinalis on Receptor Activator of Nuclear Factor-kappaB Ligand (RANKL)-induced Osteoclast Differentiation. J. Bone Metab. 2012, 19, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.-Z.; Lee, S.-Y.; Yu, S.-J.; Kim, B.-O. Effect of Cornus officinalis extract on the differentiation of MC3T3-E1 osteoblast-like cells. Tissue Eng. Regen. Med. 2015, 12, 113–121. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.W.; Kim, S.K.; Na, S.W.; Kim, Y.O. Osteoprotective effect of extract from Achyranthes japonica in ovariectomized rats. J. Exerc. Rehabil. 2014, 10, 372–377. [Google Scholar] [CrossRef] [Green Version]
- He, G.; Guo, W.; Lou, Z.; Zhang, H. Achyranthes bidentata saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK MAPK signaling pathway. Cell Biochem. Biophys. 2014, 70, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [PubMed] [Green Version]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tariq, S.; Tariq, S.; Lone, K.P.; Khaliq, S. Alkaline phosphatase is a predictor of Bone Mineral Density in postmenopausal females. Pak. J. Med. Sci. 2019, 35, 749–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Otto, F.; Thornell, A.P.; Crompton, T.; Denzel, A.; Gilmour, K.C.; Rosewell, I.R.; Stamp, G.W.; Beddington, R.S.; Mundlos, S.; Olsen, B.R.; et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 1997, 89, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.R.; Brennan-Speranza, T.C.; Levinger, I.; Byrnes, E.; Lim, E.M.; Blekkenhorst, L.C.; Sim, M.; Hodgson, J.M.; Zhu, K.; Lim, W.H.; et al. Effects of calcium supplementation on circulating osteocalcin and glycated haemoglobin in older women. Osteoporos. Int. 2019, 30, 2065–2072. [Google Scholar] [CrossRef]
- Boyce, B.F.; Rosenberg, E.; de Papp, A.E.; Duong, L.T. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur. J. Clin. Invest. 2012, 42, 1332–1341. [Google Scholar] [CrossRef]
- Feng, X.; Teitelbaum, S.L. Osteoclasts: New Insights. Bone Res. 2013, 1, 11–26. [Google Scholar]
- Ek-Rylander, B.; Andersson, G. Osteoclast migration on phosphorylated osteopontin is regulated by endogenous tartrate-resistant acid phosphatase. Exp. Cell Res. 2010, 316, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, J.; Yeo, S.; Lim, E.; Choi, C.W.; Choi, S.; Li, W.Y.; Lee, J.W.; Park, J.H.; Huh, D.; et al. Anti-Osteoporotic Effects of Combined Extract of Lycii Radicis Cortex and Achyranthes japonica in Osteoblast and Osteoclast Cells and Ovariectomized Mice. Nutrients 2019, 11, 2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inada, M.; Matsumoto, C.; Miyaura, C. Animal models for bone and joint disease. Ovariectomized and orchidectomized animals. Clin. Calcium 2011, 21, 164–170. [Google Scholar] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.; Lee, C.G.; Kim, J.; Lim, E.; Hwang, S.; Yun, S.H.; Yong, Y.; Jeong, H.; Kim, J.A.; Jin, H.-S.; et al. Anti-Osteoporotic Effects of the Herbal Mixture of Cornus officinalis and Achyranthes japonica In Vitro and In Vivo. Plants 2020, 9, 1114. https://doi.org/10.3390/plants9091114
Park E, Lee CG, Kim J, Lim E, Hwang S, Yun SH, Yong Y, Jeong H, Kim JA, Jin H-S, et al. Anti-Osteoporotic Effects of the Herbal Mixture of Cornus officinalis and Achyranthes japonica In Vitro and In Vivo. Plants. 2020; 9(9):1114. https://doi.org/10.3390/plants9091114
Chicago/Turabian StylePark, Eunkuk, Chang Gun Lee, Jeonghyun Kim, Eunguk Lim, Seokjin Hwang, Seung Hee Yun, Yoonjoong Yong, Hyesoo Jeong, Ji Ae Kim, Hyun-Seok Jin, and et al. 2020. "Anti-Osteoporotic Effects of the Herbal Mixture of Cornus officinalis and Achyranthes japonica In Vitro and In Vivo" Plants 9, no. 9: 1114. https://doi.org/10.3390/plants9091114