Plukenetia huayllabambana Fruits: Analysis of Bioactive Compounds, Antibacterial Activity and Relative Action Mechanisms
Abstract
:1. Introduction
2. Results and Discussion
2.1. GC–MS Profiling of Potential Bioactive Phytoconstituents
2.2. Antibacterial Activity
2.3. Antibacterial Action Mechanisms
2.3.1. Antimicrobial Efficacy Testing
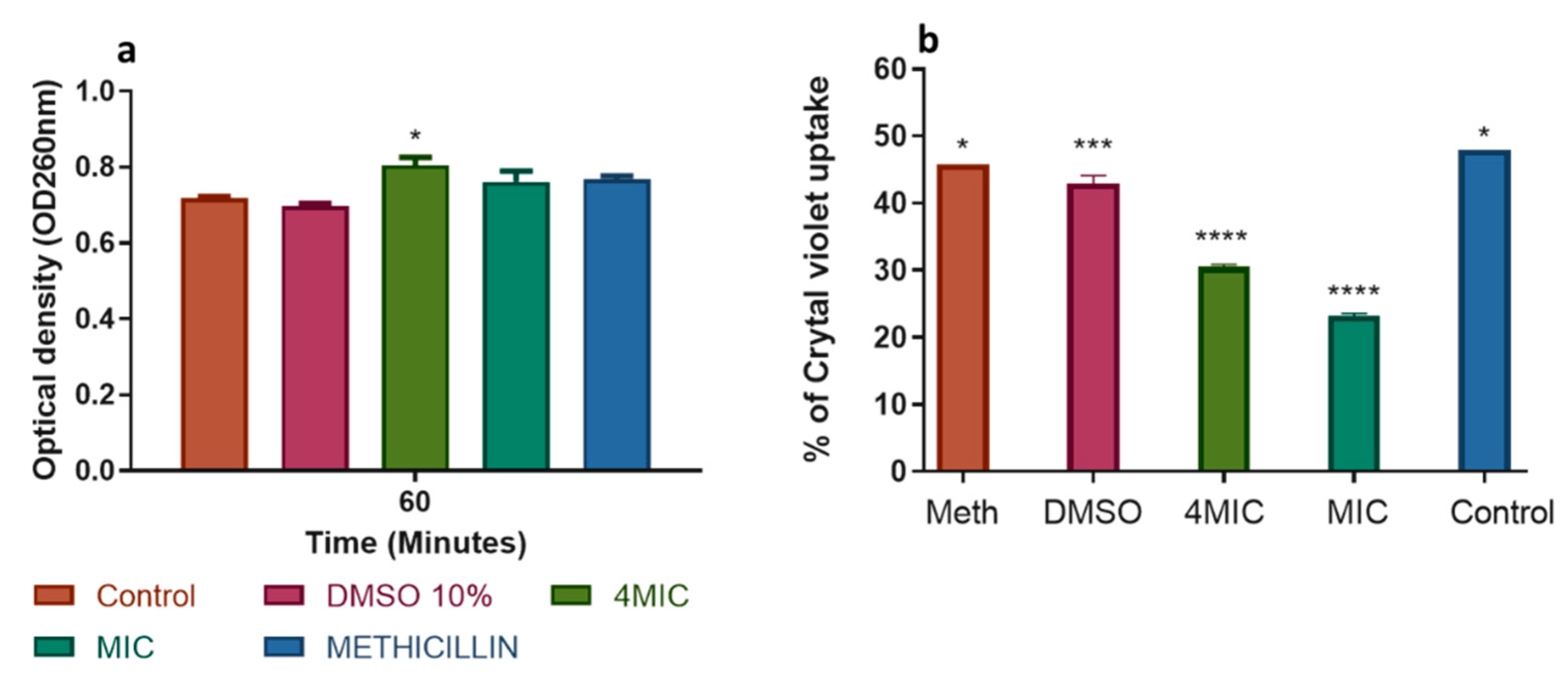
2.3.2. Action on the Cell Membrane
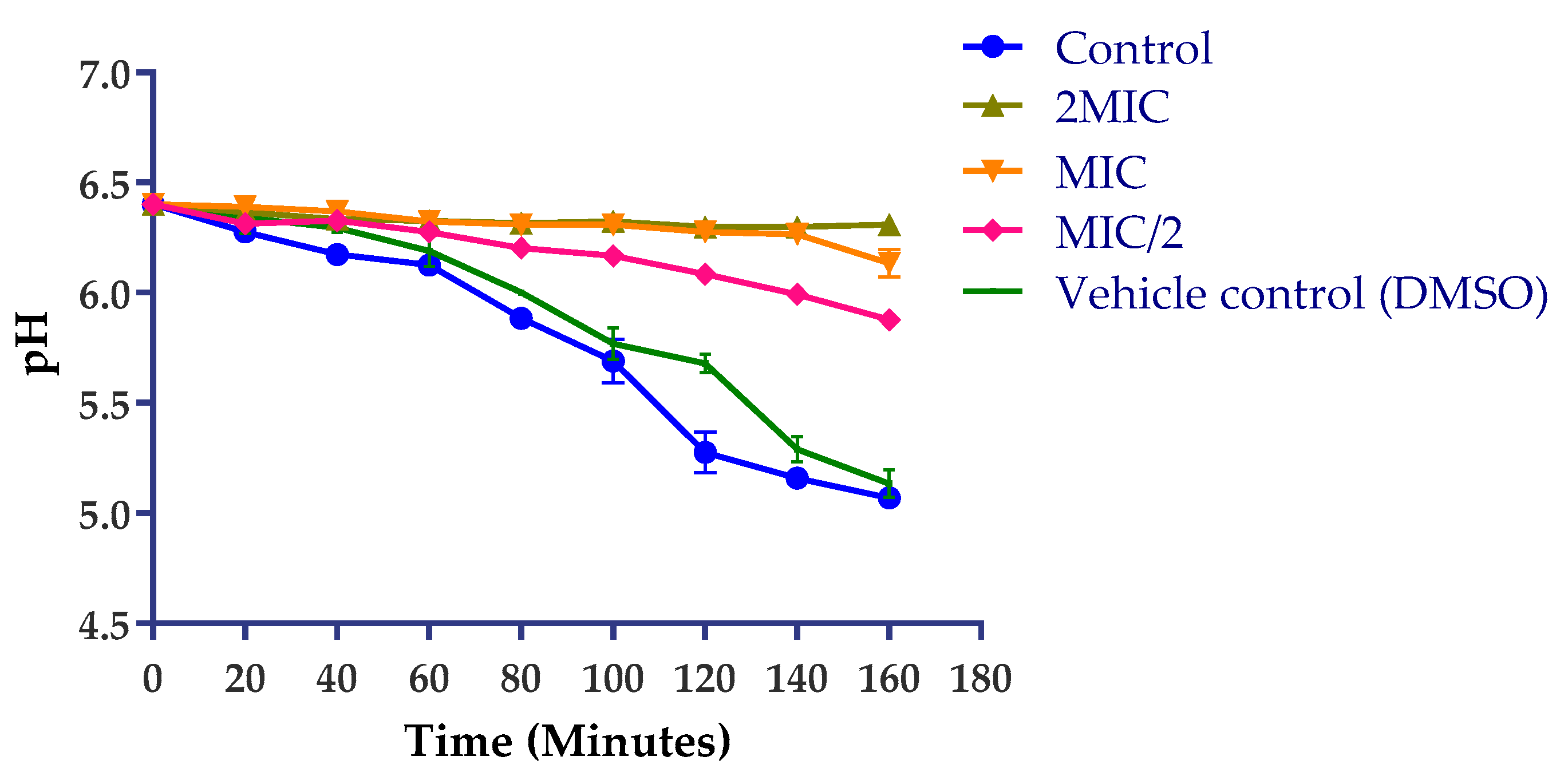
2.3.3. Action on H+-ATPase-Mediated Proton Pumping
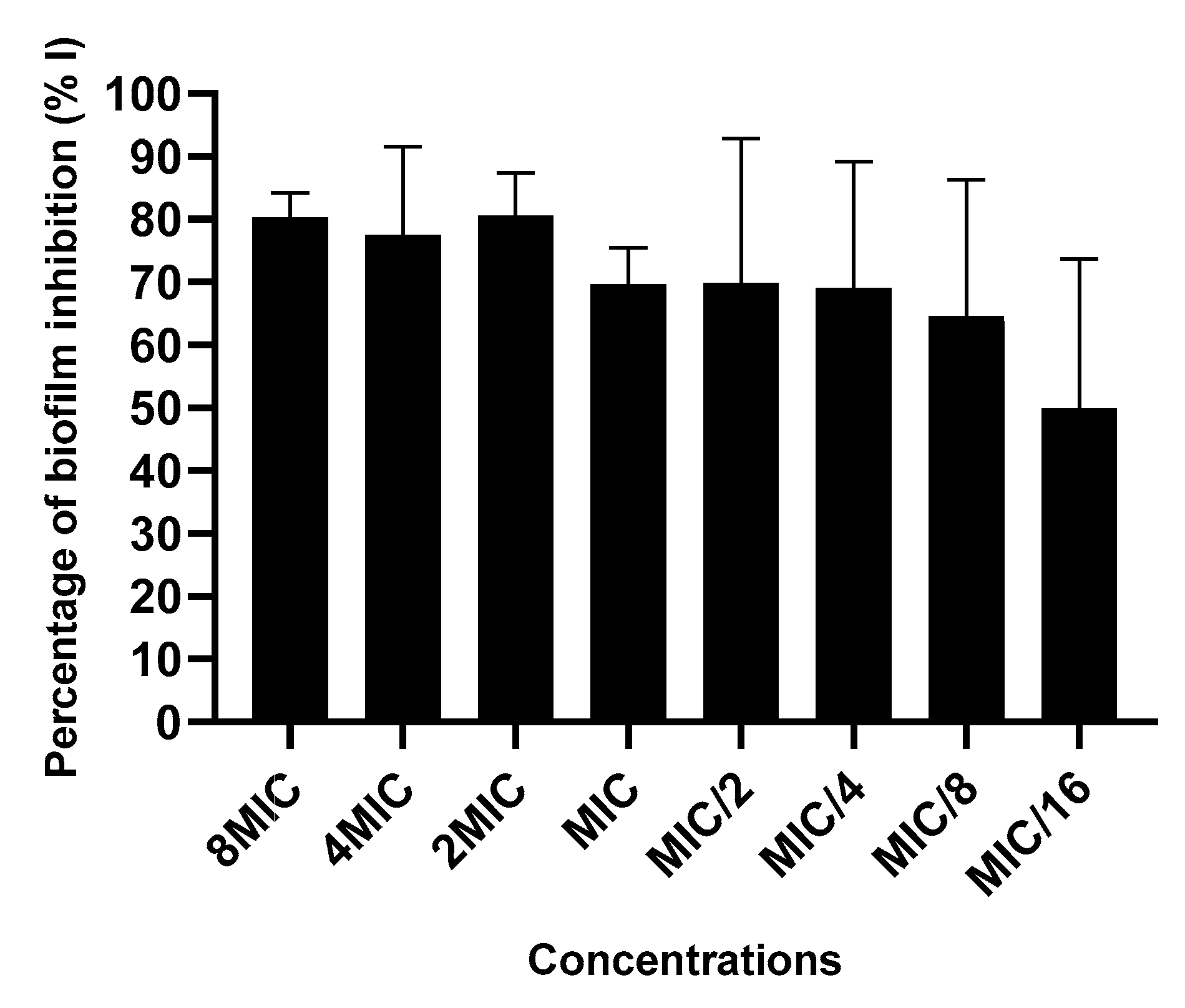
2.3.4. Action on Biofilm Formation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Extraction Process of Plant Material
3.4. Gas Chromatography-Mass Spectrometry (GC–MS) Analysis
3.5. Bacteria Strains
3.6. INT Colorimetric Assay for MIC and MBC Determination
3.7. Action Mechanisms
3.7.1. Antimicrobial Efficacy Testing
3.7.2. Action on Cell Membrane Integrity: Measurement of Intracellular Components (DNA/RNA)
3.7.3. Action on Membrane Permeability
3.7.4. Action on H+-ATPase-Mediated Proton Pumping
3.7.5. Action on Biofilm Formation
3.8. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization [WHO]. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017; WHO/EMP/IAU/2017.12. [Google Scholar]
- Dadgostar, P. Antimicrobial Resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. 2016. Available online: http://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf. (accessed on 1 September 2019).
- Kumar, M.; Yash, P.; Anand, V.K. An ethnobotanical study of medicinal plants used by the locals in Kishtwar, Jammu and Kashmir, India. Ethnobot. Leafl. 2009, 13, 1240–1256. [Google Scholar]
- Silva, N.C.C.; Fernandes, J.A. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Belyagoubi-Benhammou, N.; Belyagoubi, L.; Gismondi, A.; Di Marco, G.; Canini, A.; Bekkara, F.A. GC/MS analysis, and antioxidant and antimicrobial activities of alkaloids extracted by polar and apolar solvents from the stems of Anabasis articulata. Med. Chem. Res. 2019, 28, 754–767. [Google Scholar] [CrossRef]
- Seukep, A.J.; Fankam, A.G.; Djeussi, D.E.; Voukeng, I.K.; Tankeo, S.B.; Noumedem, J.A.K.; Kuete, A.H.; Kuete, V. Antibacterial activities of the methanol extracts of seven Cameroonian dietary plants against bacteria expressing MDR phenotypes. Springerplus 2013, 2, 363. [Google Scholar] [CrossRef] [Green Version]
- Seukep, A.J.; Ngadjui, B.T.; Kuete, V. Antibacterial activities of Fagara macrophylla, Canarium schweinfurthii, Myrianthus arboreus, Dischistocalyx grandifolius and Tragia benthamii against multi-drug resistant Gram-negative bacteria. Springerplus 2015, 4, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seukep, A.J.; Sandjo, L.P.; Ngadjui, B.T.; Kuete, V. Antibacterial and antibiotic-resistance modifying activity of the extracts and compounds from Nauclea pobeguinii against Gram-negative multi-drug resistant phenotypes. BMC Complement. Altern Med. 2016, 16, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seukep, A.J.; Sandjo, L.P.; Ngadjui, B.T.; Kuete, V. Antibacterial activities of the methanol extracts and compounds from Uapaca togoensis against Gram-negative multi-drug resistant phenotypes. S. Afr. J. Bot. 2016, 103, 1–5. [Google Scholar] [CrossRef]
- Manekeng, H.T.; Mbaveng, A.T.; Nguenang, G.S.; Seukep, J.A.; Wamba, E.N.B.; Nayim, P.; Yinkfu, N.R.; Fankam, A.G.; Kuete, V. Anti-staphylococcal and antibiotic-potentiating activities of seven Cameroonian edible plants against resistant phenotypes. Invest. Med. Chem. Pharmacol. 2018, 1, 7. [Google Scholar]
- Kuete, V. Medicinal Spices and Vegetables from Africa: Therapeutic Potential against Metabolic, Inflammatory, Infectious and Systemic Diseases, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; p. 697. ISBN 978-0-12-809286-6. [Google Scholar]
- Seukep, A.J.; Zhang, Y.-L.; Xu, Y.-B.; Guo, M.-Q. In vitro antibacterial and antiproliferative potential of Echinops lanceolatus Mattf. (Asteraceae) and identification of potential bioactive compounds. Pharmaceuticals 2020, 13, 59. [Google Scholar] [CrossRef] [Green Version]
- Seukep, A.J.; Kuete, V.; Nahar, L.; Sarker, S.D.; Guo, M. Plant-derived secondary metabolites as the main source of efflux pump inhibitors and methods for identification. J. Pharm. Anal. in press. [CrossRef]
- Mwine, J.T.; van Damme, P. Why do Euphorbiaceae tick as medicinal plants? A review of Euphorbiaceae family and its medicinal features. J. Med. Plants Res. 2011, 5, 652–662. [Google Scholar]
- Secco, R.D.S.; Cordeiro, I., II; de Senna-Vale, L.; de Sales, M.F.; de Lima, L.R.; Medeiros, D.; de Sá Haiad, B.; de Oliveira, A.S.; Caruzo, M.B.R.; Carneiro-Torres, D.; et al. An overview of recent taxonomic studies on Euphorbiaceae s.l. in Brazil. Rodriguesia 2012, 63, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhub, F.; Kakudac, Y. Sacha inchi (Plukenetia volubilis L.): Nutritional composition, biological activity, and uses. Food Chem. 2018, 265, 316–328. [Google Scholar] [CrossRef]
- Chirinos, R.; Zorrilla, D.; Aguilar-Galvez, A.; Pedreschi, R.; Campos, D. Impact of roasting on fatty acids, tocopherols, phytosterols, and phenolic compounds present in Plukenetia huayllabambana seed. J. Chem. 2016, 2016, 10. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Aspajo, G.; Belkhelfa, H.; Haddioui-Hbabi, L.; Bourdy, G.; Deharo, E. Sacha Inchi oil (Plukenetia volubilis L.) effect on adherence of Staphylococcus aureus to human skin explant and keratinocytes in vitro. J. Ethnopharmacol. 2015, 171, 330–334. [Google Scholar] [CrossRef]
- Aguilar, J.L.; Dávila, P.S.R.; Lozada-Requena, I.A.; Cabello, M.A. Comparative evaluation of the anti-inflammatory effect of two varieties of “Sacha Inchi” (Plukenetia volubilis and Plukenetia huayllabambana) in mice. Front. Immunol. 2015. [Google Scholar] [CrossRef]
- Nascimento, A.K.L.; Silveira, R.F.M.; Santos, N.D.; Fernandes, J.M.; Zucolotto, S.M.; Rocha, H.A.O.; Scortecci, K.C. Antioxidant and antiproliferative activities of leaf extracts from Plukenetia volubilis Linneo (Euphorbiaceae). Evid. Based Complement. Alternat. Med. 2013, 2013, 10. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, A.M.; Alvarado-Ruíz, C.; Castañeda, B.; Caparó, F.L.; Mendozac, E.B.; Lucero, L.C.; Cèspedes, E.M. Estúdio nutricional de Plukenetia huayllabambana sp. nov. (Text in spanish). Rev. Soc. Quim. Peru. 2013, 79, 47–56. [Google Scholar]
- Stein, S.E. An integrated method for spectrum extraction and compound identification from GC/MS data. J. Am. Soc. Mass. Spectr. 1999, 10, 770–781. [Google Scholar] [CrossRef] [Green Version]
- Schulz, S.; Peram, P.S.; Menke, M.; Hötling, S.; Röpke, R.; Melnik, K.; Poth, D.; Mann, F.; Henrichsen, S.; Dreyer, K. Mass spectrometry of aliphatic macrolides, important semiochemicals or pheromones. J. Nat. Prod. 2017, 80, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Escudero, F.; Muñoz, A.M.; Ramos-Escudero, M.; Viñas-Ospino, A.; Morales, M.T.; Asuero, A.G. Characterization of commercial Sacha inchi oil according to its composition: Tocopherols, fatty acids, sterols, triterpene and aliphatic alcohols. J. Food Sci. Technol. 2019, 56, 4503–4515. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.C.O.; Santana, E.F.; Saraiva, A.M.; Coutinho, F.N.; Castro, R.H.A.; Pisciottano, M.N.C.; Amorim, E.L.C.; Albuquerque, U.P. Which approach is more effective in the selection of plants with antimicrobial activity? Evid. Based Complement. Alternat. Med. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Kuete, V.; Poumale, H.M.P.; Guedem, A.N.; Shiono, Y.; Randrianasolo, R.; Ngadjui, B.T. Antimycobacterial, antibacterial and antifungal activities of the methanol extract and compounds from Thecacoris annobonae (Euphorbiaceae). S. Afr. J. Bot. 2010, 76, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Nikaido, H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science 1994, 264, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Ono, T.; Kashimura, M.; Suzuki, K.; Oyauchi, R.; Miyachi, J.; Ikuta, H.; Kawauchi, H.; Akashi, T.; Asaka, T.; Morimoto, S. In vitro and in vivo antimicrobial activities of tricyclic ketolide Te-802 and its analogs. J. Antibiot. 2004, 57, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Dzoyem, J.P.; Guru, S.K.; Pieme, C.A.; Kuete, V.; Sharma, A.; Khan, I.A.; Saxena, A.K.; Vishwakarma, R.A. Cytotoxic and antimicrobial activity of selected Cameroonian edible plants. BMC Complement. Altern. Med. 2013, 13, 78. [Google Scholar] [CrossRef] [Green Version]
- McGaw, L.J.; Jäger, A.K.; van Staden, J. Antibacterial effects of fatty acids and related compounds from plants. S. Afr. J. Bot. 2002, 68, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Semwal, P.; Painuli, S.; Badoni, H.; Bacheti, R.K. Screening of phytoconstituents and antibacterial activity of leaves and bark of Quercus leucotrichophora A. Camus from Uttarakhand Himalaya. Clin. Phytosci. 2018, 4, 30. [Google Scholar] [CrossRef]
- Dilika, F.; Bremner, P.D.; Meyer, J.J. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef]
- Huang, C.B.; Altimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.K. Phytosterols: Wide-spectrum antibacterial agents. Bioor. Chem. 1993, 21, 49–60. [Google Scholar] [CrossRef]
- Sen, A.; Dhavan, P.; Shukla, K.K.; Singh, S.; Tejovathi, G. Analysis of IR, NMR and antimicrobial activity of β-sitosterol isolated from Momordica charantia. Sci. Secure J. Biotech. 2012, 1, 9–13. [Google Scholar]
- Pejin, B.; Savic, A.; Sokovic, M.; Glamoclija, J.; Ciric, A.; Nikolic, M.; Radotic, K.; Mojovic, M. Further in vitro evaluation of antiradical and antimicrobial activities of phytol. Nat. Prod. Res. 2014, 28, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Kobayashi, H. A proton-translocating ATPase regulates pH of the bacterial cytoplasm. J. Biol. Chem. 1985, 260, 72–76. [Google Scholar]
- Lu, C.; Kirsch, B.; Zimmer, C.; de Jong, J.C.; Henn, C.; Maurer, C.K.; Müsken, M.; Häussler, S.; Steinbach, A.; Hartmann, R.W. Discovery of antagonists of PqsR, a key player in 2-alkyl-4-quinolone- dependent quorum sensing in Pseudomonas aeruginosa. Chem. Biol. 2012, 19, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Asfour, H.Z. Anti-quorum sensing natural compounds. J. Microsc. Ultrastruct. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Parsaee, H.; Asilib, J.; Mousavic, S.H.; Soofi, H.; Emami, S.A.; Tayarani-Najarane, Z. Apoptosis induction of Salvia chorassanica root extract on human cervical cancer cell line. Iran. J. Pharm. Res. 2013, 12, 75–83. [Google Scholar]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [Green Version]
- Appiah, T.; Boakye, Y.D.; Agyare, C. Antimicrobial activities and time-kill kinetics of extracts of selected Ghanaian mushrooms. Evid. Based Complement. Alternat. Med. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manavathu, E.K.; Dimmock, J.R.; Vashishtha, S.C.; Chandrasekar, P.H. Inhibition of H+-ATPase-mediated proton pumping in Cryptococcus neoformans by a novel conjugated styryl keton. J. Antimicrob. Chemother. 2001, 47, 491–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Etame, R.E.; Mouokeu, R.S.; Pouaha, C.C.L.; Voukeng, K.I.; Tchientcheu, R.; Assam, A.J.P.; Poundeu, M.F.S.; Tchinda, T.A.; Etoa, F.X.; Kuiate, J.R.; et al. Effect of fractioning on antibacterial activity of Enantia chlorantha Oliver (Annonaceae) methanol extract and mode of action. Evid. Based Complement. Alternat. Med. 2018, 2018, 13. [Google Scholar] [CrossRef] [Green Version]
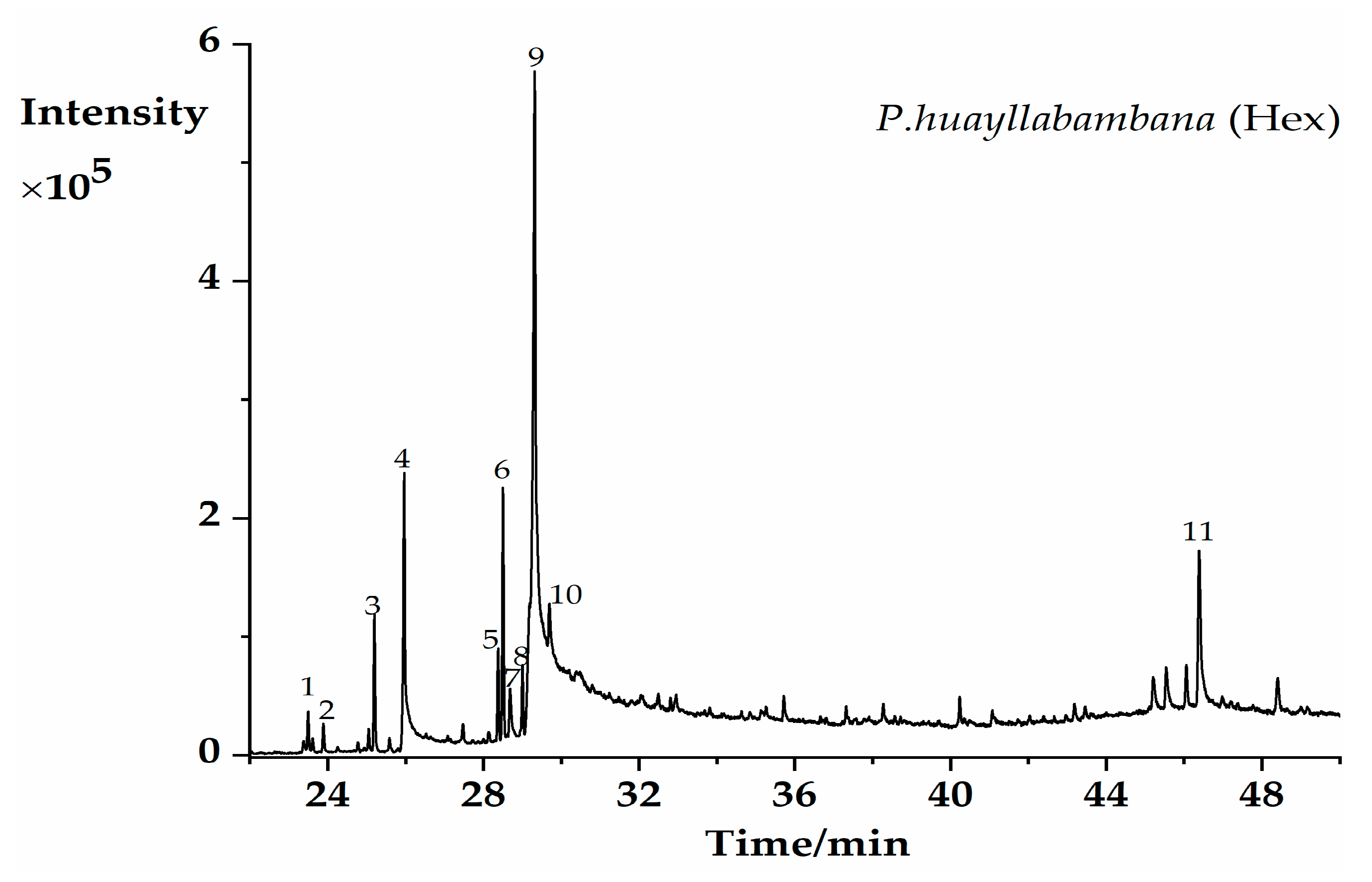


| PK | RT (min) | Chemical Formula | Compounds | Area% | Match % ** |
|---|---|---|---|---|---|
| 1 | 23.503 | C18H36O | Hexahydrofarnesyl acetone | 0.9 | 90.2 |
| 2 | 23.89 | C16H22O4 | Diisobutyl phthalate | 0.69 | 85.7 |
| 3 | 25.198 | C17H34O2 | Methyl palmitate | 3.05 | 92.6 |
| 4 | 25.967 | C16H32O2 | palmitic acid | 11.41 | 93.6 |
| 5 | 28.375 | C19H34O2 | Methyl linoleate | 1.97 | 90.4 |
| 6 | 28.507 | C19H36O2 | Methyl oleate | 5.36 | 92.4 |
| 7 | 28.682 | C20H40O | Phytol | 1.96 | 89.5 |
| 8 | 29.001 | C19H38O2 | Methyl stearate | 1.57 | 88.1 |
| 9 | 29.314 | C18H34O2 | Oleic acid | 46.55 | 92.4 |
| 10 | 29.695 | C18H34O2 | Octadeca-11-enoic acid | 7.79 | 87.4 |
| 11 | 46.384 | C29H50O | beta-Sitosterol | 8.3 | 87.3 |
| Bacteria Strains | MICs (mg/mL) of Crude MeOH Extract and Fractions * of P. huayllabambana | METH | STR | |||||
|---|---|---|---|---|---|---|---|---|
| MeOH | n-Hex | DCM | EA | n-BuOH | ||||
| Gram-negative | S. enterica | 0.125 | 0.25 | 0.25 | 0.5 | 1 | 0.064 | 0.004 |
| E. cloacae | 1 | 1 | >1 | 1 | >1 | >0.256 | 0.064 | |
| K. pneumoniae | 1 | 1 | >1 | 1 | >1 | >0.256 | >0.256 | |
| Gram-positive | S. aureus | 0.5 | 0.5 | 1 | 1 | >1 | 0.125 | 0.004 |
| E. faecalis | 0.25 | 0.5 | 0.5 | 1 | >1 | 0.032 | 0.125 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seukep, A.J.; Fan, M.; Sarker, S.D.; Kuete, V.; Guo, M.-Q. Plukenetia huayllabambana Fruits: Analysis of Bioactive Compounds, Antibacterial Activity and Relative Action Mechanisms. Plants 2020, 9, 1111. https://doi.org/10.3390/plants9091111
Seukep AJ, Fan M, Sarker SD, Kuete V, Guo M-Q. Plukenetia huayllabambana Fruits: Analysis of Bioactive Compounds, Antibacterial Activity and Relative Action Mechanisms. Plants. 2020; 9(9):1111. https://doi.org/10.3390/plants9091111
Chicago/Turabian StyleSeukep, Armel Jackson, Minxia Fan, Satyajit Dey Sarker, Victor Kuete, and Ming-Quan Guo. 2020. "Plukenetia huayllabambana Fruits: Analysis of Bioactive Compounds, Antibacterial Activity and Relative Action Mechanisms" Plants 9, no. 9: 1111. https://doi.org/10.3390/plants9091111
APA StyleSeukep, A. J., Fan, M., Sarker, S. D., Kuete, V., & Guo, M.-Q. (2020). Plukenetia huayllabambana Fruits: Analysis of Bioactive Compounds, Antibacterial Activity and Relative Action Mechanisms. Plants, 9(9), 1111. https://doi.org/10.3390/plants9091111






