Seed Dormancy Breaking and Germination in Bituminaria basaltica and B. bituminosa (Fabaceae)
Abstract
:1. Introduction
2. Results
2.1. Morphometric Analysis, Seed Coat Structure, and Water Imbibition Test
2.2. Effect of Dormancy-Breaking Treatments on Germination Behavior
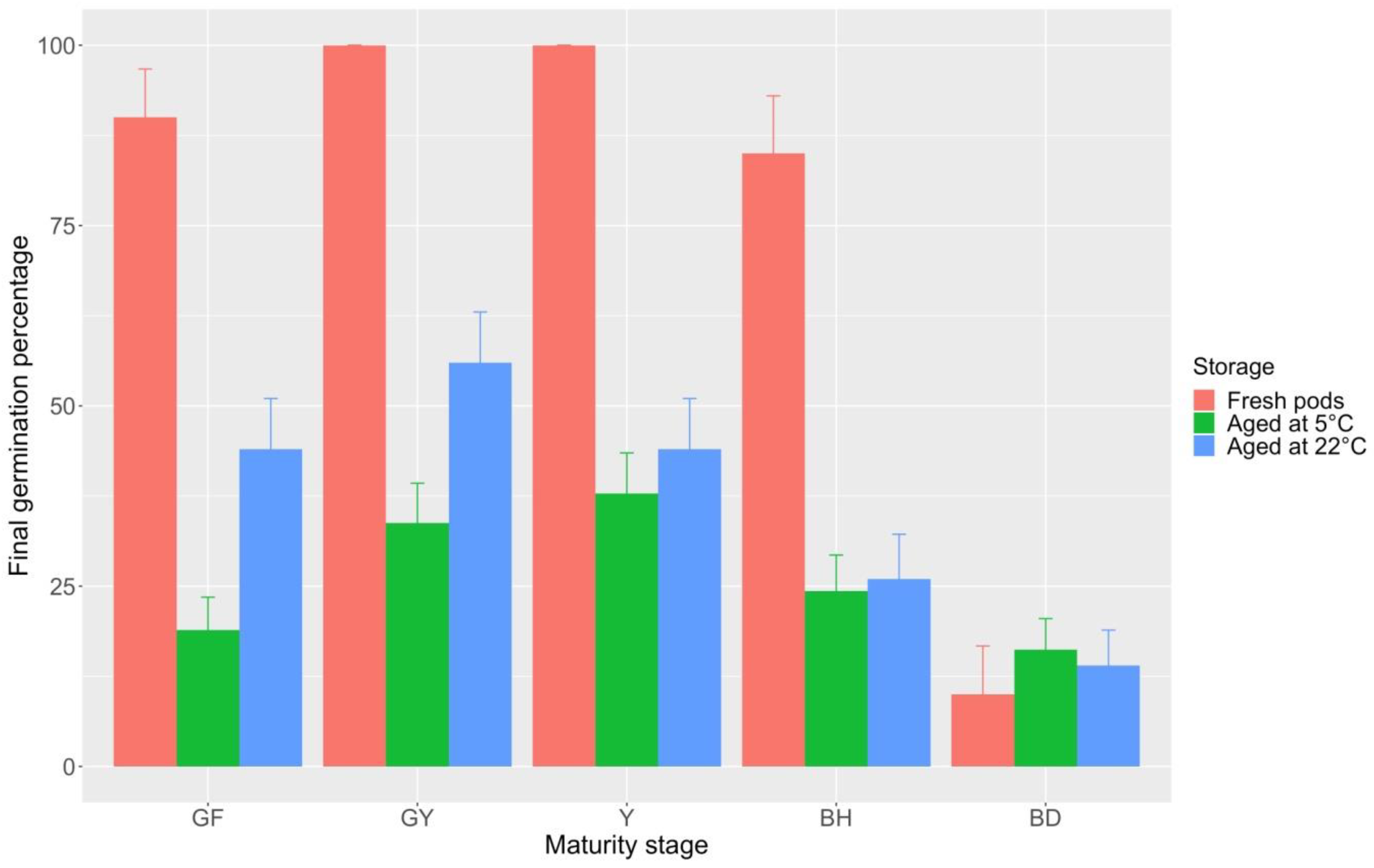
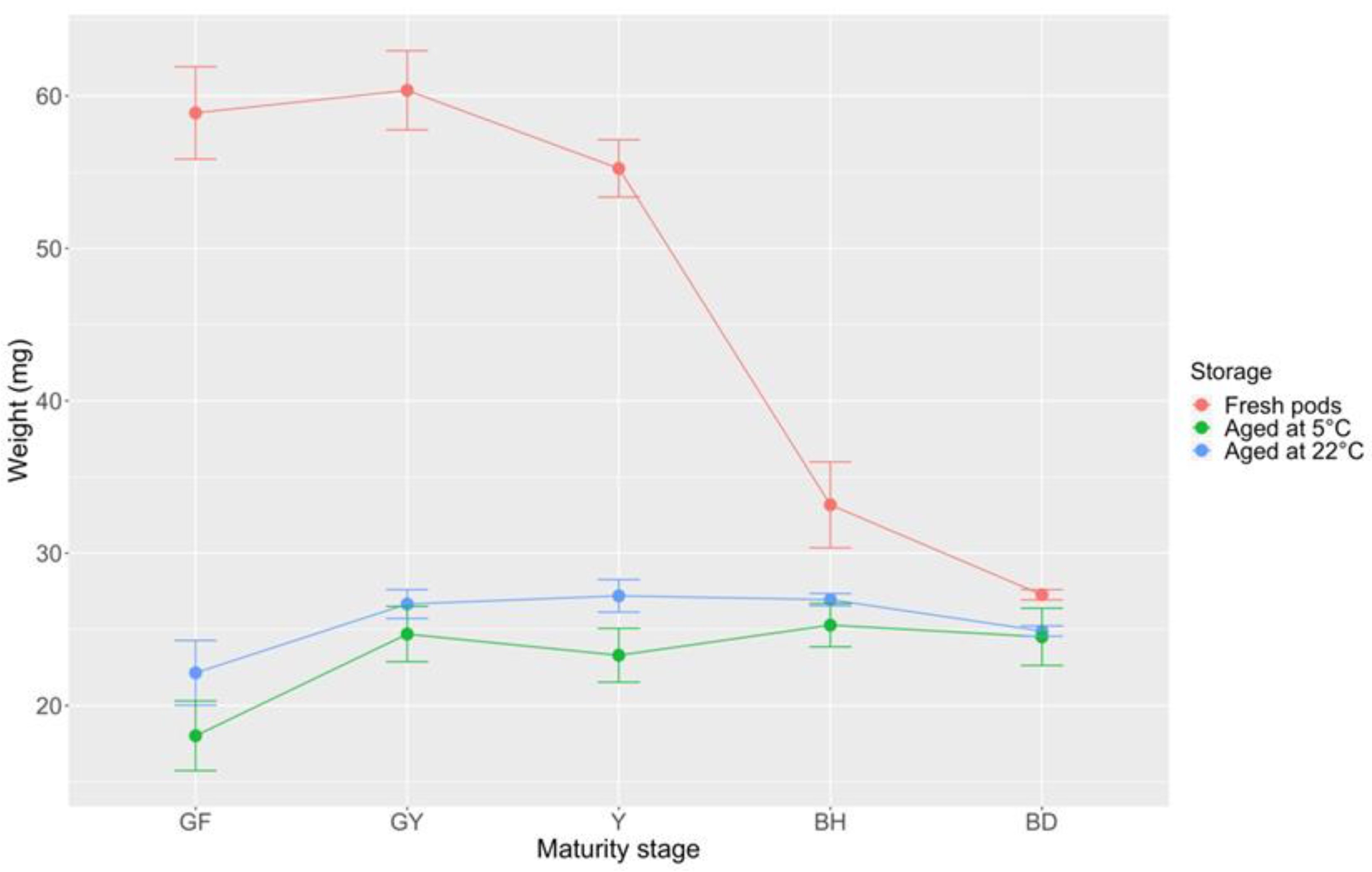
2.3. Pod Maturity Stages and Seed Germination Behavior over Time
3. Discussion
4. Materials and Methods
4.1. Study Species and Sampling Strategy
4.2. Morphometric Analysis and Seed Coat Anatomical Structure
4.3. Seed Dormancy-Breaking Treatments and Germination Assessment
4.4. Effect of Pod Maturity Stage and Storage Conditions on Germination Behavior
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maxted, N.; Bennett, S.J. Legume diversity in the Mediterranean region. In Plant Genetic Resources of Legumes in the Mediterranean; Maxted, N., Bennett, S.J., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 51–75. [Google Scholar] [CrossRef]
- Brígido, C.; Menéndez, E.; Paço, A.; Glick, B.R.; Belo, A.; Félix, M.R.; Oliveira, S.; Carvalho, M. Mediterranean native leguminous plants: A reservoir of endophytic bacteria with potential to enhance chickpea growth under stress conditions. Microorganisms 2019, 7, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazos-Navarro, M.; Dabauza, M.; Correal, E.; Walker, D.; del Río, J.A.; Ortuño, A.; Méndez, P.; Santos, A.; Ríos, S.; Martínez-Frances, V.; et al. Legumes for grazing and health: The Case of Bituminaria Bituminosa. In Legumes: Types, Nutritional Composition and Health Benefits; Satou, H., Nakamura, R., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2013; pp. 1–39. [Google Scholar]
- Melis, R.A.M.; Pecetti, L.; Annicchiarico, P.; Porqueddu, C. Legumes for rainfed Mediterranean farming systems. Legume Perspect. 2016, 12, 36–38. [Google Scholar]
- Melis, R.A.M.; Franca, A.; Re, G.A.; Porqueddu, C. Bio-agronomic characterization and implications on the potential use as forage of Bituminaria bituminosa and B. morisiana accessions. Grass Forage Sci. 2018, 73, 284–296. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant. Sci. 2014, 5, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Loi, A.; Cocks, P.S.; Howieson, J.G.; Carr, S.J. Hardseededness and the pattern of softening in Biserrula pelecinus L., Ornithopus compressus L., and Trifolium subterraneum L. seeds. Aust. J. Agric. Res. 1999, 50, 1073–1081. [Google Scholar] [CrossRef]
- Asci, O.O.; Acar, Z.; Ayan, I.; Basaran, U.; Mut, H. Effect of pretreatments on seed germination rate of red clover (Trifolium pratense L.) populations. Afr. J. Agric. Res. 2011, 6, 3055–3060. [Google Scholar] [CrossRef]
- Gresta, F.; Avola, G.; Onofri, A.; Anastasi, U.; Cristaudo, A. When does hard coat impose dormancy in legume seeds? Lotus and Scorpiurus case study. Crop Sci. 2011, 51, 1739–1747. [Google Scholar] [CrossRef]
- Gresta, F.; Avola, G.; Tuttobene, R.; Barrile, V.; Cristaudo, A.; Abbate, V. The effect of fire on the dormancy break of three annual legume seeds. Ital. J. Agron. 2011, 6, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.Â.; Ferreira, I.Q.; Freitas, S.L.; Pires, J.M.; Arrobas, M.P. Self-reseeding annual legumes for cover cropping in rainfed managed olive orchards. Span. J. Agric. Res. 2015, 13, e0302. [Google Scholar] [CrossRef]
- Renzi, J.P.; Duchoslav, M.; Brus, J.; Hradilová, I.; Pechanec, V.; Václavek, T.; Machalová, J.; Hron, K.; Verdier, J.; Smýkal, P. Physical dormancy release in Medicago truncatula seeds is related to environmental variations. Plants 2020, 9, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillikin, J.W.; Graham, J.S. Purification and developmental analysis of the major anionic peroxidase from the seed coat of Glycine max. Plant Physiol. 1991, 96, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Hyde, E.O.C.; Mc Leavey, A.M.; Harris, G.S. Seed development in ryegrass, and in red and white clover. N. Z. J. Agric. Res. 1959, 2, 947–952. [Google Scholar] [CrossRef]
- Taylor, G.B. Hardseededness in Mediterranean annual pasture legumes in Australia: A review. Aust. J. Agric. Res. 2005, 56, 645–661. [Google Scholar] [CrossRef]
- Renzi, J.P.; Lasa, J.C.; Cantamutto, M.A. Influence of maturity at harvest on the quality of alfalfa (Medicago sativa L.) seeds. Riarev. Investig. Agropecu. 2011, 37, 261–267. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA; London, UK; Waltham, MA, USA, 2014. [Google Scholar]
- Rusdy, M. A review on hardseedness and breaking dormancy in tropical forage legumes. Livest. Res. Rural Dev. 2017, 29, 237. Available online: http://www.lrrd.org/lrrd29/12/muhr29237.html (accessed on 22 May 2020).
- Kimura, E.; Islam, M.A. Seed Scarification Methods and their Use in Forage Legumes. Res. J. Seed Sci. 2012, 5, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Statwick, J.M. Germination pretreatments to break hard-seed dormancy in Astragalus cicer L. (Fabaceae). PeerJ 2016, 4, e2621. [Google Scholar] [CrossRef] [Green Version]
- Abbate, V.; Maugeri, G.; Cristaudo, A.; Gresta, F. Scorpiurus muricatus L. subsp. subvillosus (L.) Thell., a potential forage legume species for a Mediterranean environment: A review. Grass Forage Sci. 2010, 65, 2–10. [Google Scholar] [CrossRef]
- Guma, I.R.; Santos-Guerra, A.; Reyes-Betancort, J.A.; Padrón-Mederos, M.A.; Méndez, P.; González-Montelongo, R. Perennial forage legumes endemic to the Canary Islands: Collection and ex situ conservation. Genet. Resour. Crop. Evol. 2011, 58, 181–187. [Google Scholar] [CrossRef]
- Ventura, M.R.; Castanon, J.I.R.; Pieltain, M.C.; Flores, M.P. Nutritive value of forage shrubs: Bituminaria bituminosa, Rumex lunaria, Acacia salicina, Cassia sturtii and Adenocorpus foliosus. Small Rumin. Res. 2004, 52, 13–18. [Google Scholar] [CrossRef]
- Brullo, S.; Brullo, C.; Cambria, S.; Cristaudo, A.; Giusso del Galdo, G. Bituminaria antiatlantica (Psoraleeae, Fabaceae), a new species from Morocco. PhytoKeys 2017, 85, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Bogdanović, S.; Brullo, C.; Brullo, S.; Cambria, S.; Giusso del Galdo, G. Psoralea bituminosa var. Atropurpurea (Psoraleeae, Fabaceae) from Morocco recognised as a distinct species in Bituminaria. Phytotaxa 2020, 451, 195–205. [Google Scholar] [CrossRef]
- Foster, K.; Ryan, M.H.; Real, D.; Ramankutty, P.; Lambers, H. Seasonal and diurnal variation in the stomatal conductance and paraheliotropism of tedera (Bituminaria bituminosa var. albomarginata) in the field. Funct. Plant Biol. 2013, 40, 719–729. [Google Scholar] [CrossRef]
- Foster, K.; Lambers, H.; Real, D.; Ramankutty, P.; Cawthray, G.; Ryan, M. Drought resistance and recovery in mature Bituminaria bituminosa var. Albomarginata. Ann. Appl. Biol. 2015, 166, 154–169. [Google Scholar] [CrossRef]
- Real, D.; Verbyla, A.P. Maximizing genetic gains using a “plant” model in the Tedera (Bituminaria bituminosa var. Albomarginata and var. crassiuscula) breeding program in Australia. Options Méditerranéennessérie A 2010, 92, 87–96. Available online: https://om.ciheam.org/om/pdf/a92/00801221.pdf (accessed on 15 May 2020).
- Poschenrieder, C.; Bech, J.; Llugany, M.; Pace, A.; Fenés, E.; Barceló, J. Copper in plant species in a copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 2001, 230, 247–256. [Google Scholar] [CrossRef]
- del Río, M.; Font, R.; Almela, C.; Vélez, D.; Montoro, R.; De Haro Bailón, A. Heavy metals and arsenic uptake by wild vegetation in the Guadiamar river area after the toxic spill of the Aznalcóllar mine. J. Biotechnol. 2002, 98, 125–137. [Google Scholar] [CrossRef]
- Martínez, S.; Correal, E.; Real, D.; Ortuno, A.; del Río, J.A. Bituminaria bituminosa: A source of furanocoumarins of pharmaceutical interest. In Drug Plants. Recent Progress in Medicinal Plants; Awaad, A.S., Govil, J.N., Singh, V.K., Eds.; Studium Press LLC: Houston, TX, USA, 2010; pp. 307–322. [Google Scholar]
- Pistelli, L.; Noccioli, C.; Appendino, G.; Bianchi, F.; Sterner, O.; Ballero, M. Pterocarpans from Bituminaria morisiana and Bituminaria bituminosa. Phytochemistry 2003, 64, 595–598. [Google Scholar] [CrossRef]
- Martínez-Fernández, D.; Walker, D.J.; Romero-Espinar, P.; Flores, P.; del Río, J.A. Physiological responses of Bituminaria bituminosa to heavy metals. J. Plant Phys. 2011, 168, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Spínola, V.; Gouveia, S.; Castilhoa, P.C. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crop. Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Pecetti, L.; Mella, M.; Tava, A. Variation in herbage biochemical composition among pitch trefoil (Bituminaria bituminosa) populations from Elba Island, Italy. J. Agric. Food Chem. 2016, 64, 195–203. [Google Scholar] [CrossRef]
- Zennouhi, O.; El Mderssa, M.; Ibijbijen, J.; Bouiamrine, E.; Nassiri, L. The use of Bituminaria bituminosa (L.) Stirton and microbial biotechnologies for restoration of degraded pastoral lands: The case of the Middle Atlas of Morocco. Int. J. Sci. Res. Environ. Sci. 2019, 5, 1–6. [Google Scholar] [CrossRef]
- Correal, E.; Costa, J.; Hoyos, A.; Méndez, P.; Real, D.; Ríos, S.; Snowball, R. Seed Production of Bituminaria bituminosa: Size, production, retention and germination capacity of the legumes. Options Méditerranéennessérie A 2008, 79, 379–383. Available online: https://om.ciheam.org/om/pdf/a79/00800680.pdf (accessed on 22 May 2020).
- Beard, C.; Nichols, P.G.H.; Loo, C.; Michael, P. Establishment of Tedera (Bituminaria Bituminosa var. Albomarginata); Technical Report n. 13; Future Farm Industries CRC: Crawley, UK, 2014; pp. 1–50. [Google Scholar]
- Barberá, M.; Flores, M.; Castanon, J.; Diazavila, E.; Ventura, M. Germination characteristics of tedera (Bituminaria bituminosa var. bituminosa). Agrofor 2017, 2, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Herranz, J.M.; Ferrandis, P.; Martínez-Sánchez, J.J. Influence of heat on seed germination of seven Mediterranean Leguminosae species. Plant Ecol. 1998, 136, 95–103. [Google Scholar] [CrossRef]
- Zennouhi, O.; Rfaki, A.; El Mderssa, M.; Bouiamrine, E.H.; Ibijbijen, J.; Nassiri, L. Effect of salinity and temperature on the seed germination of Bituminaria bituminosa var. bituminosa. Int. J. Curr. Res. 2018, 10, 72610–72613. [Google Scholar]
- Reyes, O.; Trabaud, L. Germination behaviour of 14 Mediterranean species in relation to fire factors: Smoke and heat. Plant Ecol. 2009, 202, 113. [Google Scholar] [CrossRef]
- Minissale, P.; Brullo, C.; Brullo, S.; Giusso Del Galdo, G.; Sciandrello, S. Bituminaria basaltica (Fabaceae), a new species from Italy. Phytotaxa 2013, 98, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Gresta, F.; Cristaudo, A.; Onofri, A.; Restuccia, A.; Avola, G. Germination response of four pasture species to temperature, light, and post-harvest period. Plant Biosyst. 2010, 144, 888–895. [Google Scholar] [CrossRef]
- Gresta, F.; Cristaudo, A.; Trostle, C.; Anastasi, U.; Guarnaccia, P.; Catara, S.; Onofri, A. Germination of guar (Cyamopsis tetragonoloba (L.) Taub.) genotypes with reduced temperature requirements. Aust. J. Crop Sci. 2018, 12, 954–960. [Google Scholar] [CrossRef]
- Souza De, F.H.D.; Marcos-Filho, J. The seed coat as a modulator of seed-environment relationships in Fabaceae. Braz. J. Bot. 2001, 24, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. The development of desiccation tolerance and maximum seed quality during seed maturation in six Grain legumes. Ann. Bot. 1987, 59, 23–29. [Google Scholar] [CrossRef]
- Mai-Hong, T.; Hong, T.D.; Hien, N.T.; Ellis, R.H. Onset of germinability, desiccation tolerance and hardseededness in developing seeds of Peltophorum pterocarpum (DC) K. Heyne (Caesalpinioideae). Seed Sci. Res. 2003, 13, 323–327. [Google Scholar] [CrossRef]
- Duguma, B.; Kang, B.T.; Okali, D.U.U. Factors affecting germination of Leucaena leucocephala. Seed Sci. Res. 1988, 16, 489–500. [Google Scholar]
- Samarah, N.H.; Allataifeh, N.; Turk, M.; Tawaha, A.R. Effect of maturity stage on germination and dormancy of fresh and air-dried seeds of bitter vetch (Vicia ervilia L.). N. Z. J. Agric. Res. 2003, 46, 347–354. [Google Scholar] [CrossRef]
- Cristaudo, A.; Gresta, F.; Avola, G.; Miano, V. Germination capability of immature seeds of Lotus ornithopodioides L. and Scorpiurus subvillosus L. Options Méditerranéennessérie A 2008, 79, 289–292. Available online: https://om.ciheam.org/om/pdf/a79/00800663.pdf (accessed on 18 May 2020).
- Argel, P.J.; Paton, C.J. Overcoming legume hardseededness. In Forage Seed Production; Loch, D.S., Ferguson, J.E., Eds.; CABI: Wallingford, UK, 1999; Volume 2, pp. 247–265. [Google Scholar]
- Venier, P.; Funes, G.; Carrizo García, C. Physical dormancy and histological features of seeds of five Acacia species (Fabaceae) from xerophytic forests in central Argentina. Flora 2012, 207, 39–46. [Google Scholar] [CrossRef]
- Galussi, A.A.; Moya, M.E. Anatomical and chemical insights into the white clover (Trifolium repens L.) seed coat associated to water permeability. In Advances in Seed Biology; Jimenez-Lopez, J.C., Ed.; IntechOpen: London, UK, 2017; pp. 187–197. [Google Scholar] [CrossRef] [Green Version]
- Hradilová, I.; Duchoslav, M.; Brus, J.; Pechanec, V.; Hýbl, M.; Kopecký, P.; Smržová, L.; Štefelová, N.; Vaclávek, T.; Bariotakis, M.; et al. Variation in wild pea (Pisum sativum subsp. elatius) seed dormancy and its relationship to the environment and seed coat traits. PeerJ 2019, 7, e6263. [Google Scholar] [CrossRef] [Green Version]
- Russi, L.; Cocks, P.S.; Roberts, E.H. Coat thickness and hard-seededness in some Medicago and Trifolium species. Seed Sci. Res. 1992, 2, 243–249. [Google Scholar] [CrossRef]
- Marbach, I.; Mayer, A.M. Permeability of seed coats to water as related to drying conditions and metabolism phenolics. Plant Physiol. 1974, 54, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Werker, E.; Marbach, I.; Mayer, A.M. Relation between the anatomy of the testa, water permeability and the presence of phenolics in the genus Pisum. Ann. Bot. 1979, 43, 765–771. [Google Scholar] [CrossRef]
- Galussi, A.A.; Argüello, J.A.; Cerana, M.M.; Maximino, M.; Moya, M.E. Anatomical and chemical characteristics of the seed coat of Medicago sativa L. (alfalfa) cv. Baralfa 85 seeds and their association with seed dormancy. Phyton Int. J. Exp. Bot. 2016, 84, 163–175. [Google Scholar]
- Hu, X.W.; Wang, Y.R.; Wu, Y.P.; Baskin, C.C. Role of the lens in controlling water uptake in seeds of two Fabaceae (Papilonoideae) species treated with sulphuric acid and hot water. Seed Sci. Res. 2009, 19, 73–80. [Google Scholar] [CrossRef]
- Gama-Arachchige, N.S.; Baskin, J.M.; Geneve, R.L.; Baskin, C.C. Identification and characterization of ten new water-gaps in seeds and fruits with physical dormancy and classification of water-gap complexes. Ann. Bot. 2013, 112, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Yaklich, R.W.; Vigil, E.L.; Wergin, W. Pore development and seed coat permeability in soybean. Crop Sci. 1986, 26, 616–624. [Google Scholar] [CrossRef]
- Ragus, L.N. Role of water absorbing capacity in soybean germination and seedling vigour. Seed Sci. Technol. 1987, 15, 285–296. [Google Scholar]
- Souza De, F.H.D.; Marcos-Filho, J.; Nogueira, M.C.S. Características físicas das sementes de Calopogonium mucunoides associadas a absorção de água e qualidade fisiológica. I. Tamanho. Rev. Bras. Sementes 1996, 18, 33–40. [Google Scholar] [CrossRef]
- Rodrigues-Junior, A.G.; Mello, A.C.M.P.; Baskin, C.C.; Baskin, J.M.; Oliveira, D.M.T.; Garcia, Q.S. Why large seeds with physical dormancy become nondormant earlier than small ones. PLoS ONE 2018, 13, e0202038. [Google Scholar] [CrossRef]
- Efron, B. Nonparametric estimates of standard error: The Jackknife, the bootstrap and other methods. Biometrika 1981, 68, 589–599. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Version 4.0.2; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 22 June 2020).












| Species | Date | Site | Elevation m a.s.l. | Coordinates (WGS84) | |
|---|---|---|---|---|---|
| Latitude | Longitude | ||||
| Bituminaria basaltica | July 2015 | Filicudi (Messina, Italy) | 283 | 38° 34′ 07.70” | 14° 34′ 14.99” |
| Bituminaria bituminosa | July 2015 | Fiumefreddo di Sicilia (Catania, Italy) | 123 | 37° 48′ 12.56” | 15° 11′ 51.56” |
| Treatment | Maturity Stage | Seed Age | Storage Condition | Immersion Time | Incubation Temperature | |
|---|---|---|---|---|---|---|
| Untreated—B. basaltica | GF | 1 | - | - | 10, 15, 20, 25 °C | |
| GF | 180 | 5 ± 1 °C | - | 10, 15, 20, 25 °C | ||
| Untreated—B. bituminosa | BD | 1 | - | - | 20 °C | |
| BH | 1 | - | - | 20 °C | ||
| Y | 1 | - | - | 20 °C | ||
| GY | 1 | - | - | 20 °C | ||
| GF | 1 | - | - | 20 °C | ||
| BD | 420 | 5 ± 1 °C | - | 20 °C | ||
| BH | 420 | 5 ± 1 °C | - | 20 °C | ||
| Y | 420 | 5 ± 1 °C | - | 20 °C | ||
| GY | 420 | 5 ± 1 °C | - | 20 °C | ||
| GF | 420 | 5 ± 1 °C | - | 20 °C | ||
| BD | 420 | 22 ± 2 °C | - | 20 °C | ||
| BH | 420 | 22 ± 2 °C | - | 20 °C | ||
| Y | 420 | 22 ± 2 °C | - | 20 °C | ||
| GY | 420 | 22 ± 2 °C | - | 20 °C | ||
| GF | 420 | 22 ± 2 °C | - | 20 °C | ||
| GF | 180 | 5 ± 1 °C | - | 10, 15, 20, 25 °C | ||
| BD | 180 | 5 ± 1 °C | - | 10, 15, 20, 25 °C | ||
| Mechanical | needle pin | BD | 270 | 22 ± 2 °C | - | 15/10, 20/10, 20/15 25/20 °C |
| BD | 800 | 22 ± 2 °C | - | 15/10, 20/10, 20/15, 25/20 °C 10, 15, 20, 25 °C | ||
| Thermal | boiling water gradually cooling | BD | 300 | 22 ± 2 °C | 5, 10, 15, 30, 1440 min | 20/15, 25/20 °C |
| 70, 100 °C hot constant water | BD | 700 | 22 ± 2 °C | 2, 4, 6, 8, 10 min | 20/15, 25/20 °C 10, 15, 20, 25 °C | |
| 70, 80, 90, 100 °C hot constant water | BD | 840 | 22 ± 2 °C | 2, 4, 6, 8, 10 min | 25/20 °C | |
| Chemical | H2SO4 (98%) | BD | 450 | 22 ± 2 °C | 10, 20, 30, 40, 50 min | 20/15, 25/20 °C 15, 20, 25 °C |
| Control | BD | 270 | 22 ± 2 °C | - | 15/10, 20/10, 20/15, 25/20 °C | |
| BD | 700 | 22 ± 2 °C | - | 20/15, 25/20 °C 10, 15, 20, 25 °C | ||
| BD | 800 | 22 ± 2 °C | - | 15/10, 20/10, 20/15, 25/20 °C 10, 15, 20, 25 °C | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carruggio, F.; Onofri, A.; Impelluso, C.; Giusso del Galdo, G.; Scopece, G.; Cristaudo, A. Seed Dormancy Breaking and Germination in Bituminaria basaltica and B. bituminosa (Fabaceae). Plants 2020, 9, 1110. https://doi.org/10.3390/plants9091110
Carruggio F, Onofri A, Impelluso C, Giusso del Galdo G, Scopece G, Cristaudo A. Seed Dormancy Breaking and Germination in Bituminaria basaltica and B. bituminosa (Fabaceae). Plants. 2020; 9(9):1110. https://doi.org/10.3390/plants9091110
Chicago/Turabian StyleCarruggio, Francesca, Andrea Onofri, Carmen Impelluso, Gianpietro Giusso del Galdo, Giovanni Scopece, and Antonia Cristaudo. 2020. "Seed Dormancy Breaking and Germination in Bituminaria basaltica and B. bituminosa (Fabaceae)" Plants 9, no. 9: 1110. https://doi.org/10.3390/plants9091110
APA StyleCarruggio, F., Onofri, A., Impelluso, C., Giusso del Galdo, G., Scopece, G., & Cristaudo, A. (2020). Seed Dormancy Breaking and Germination in Bituminaria basaltica and B. bituminosa (Fabaceae). Plants, 9(9), 1110. https://doi.org/10.3390/plants9091110







