Insights into the Role of Streptomyces hydrogenans as the Plant Growth Promoter, Photosynthetic Pigment Enhancer and Biocontrol Agent against Meloidogyne incognita in Solanum lycopersicum Seedlings
Abstract
:1. Introduction
2. Results
2.1. Morphological Parameters
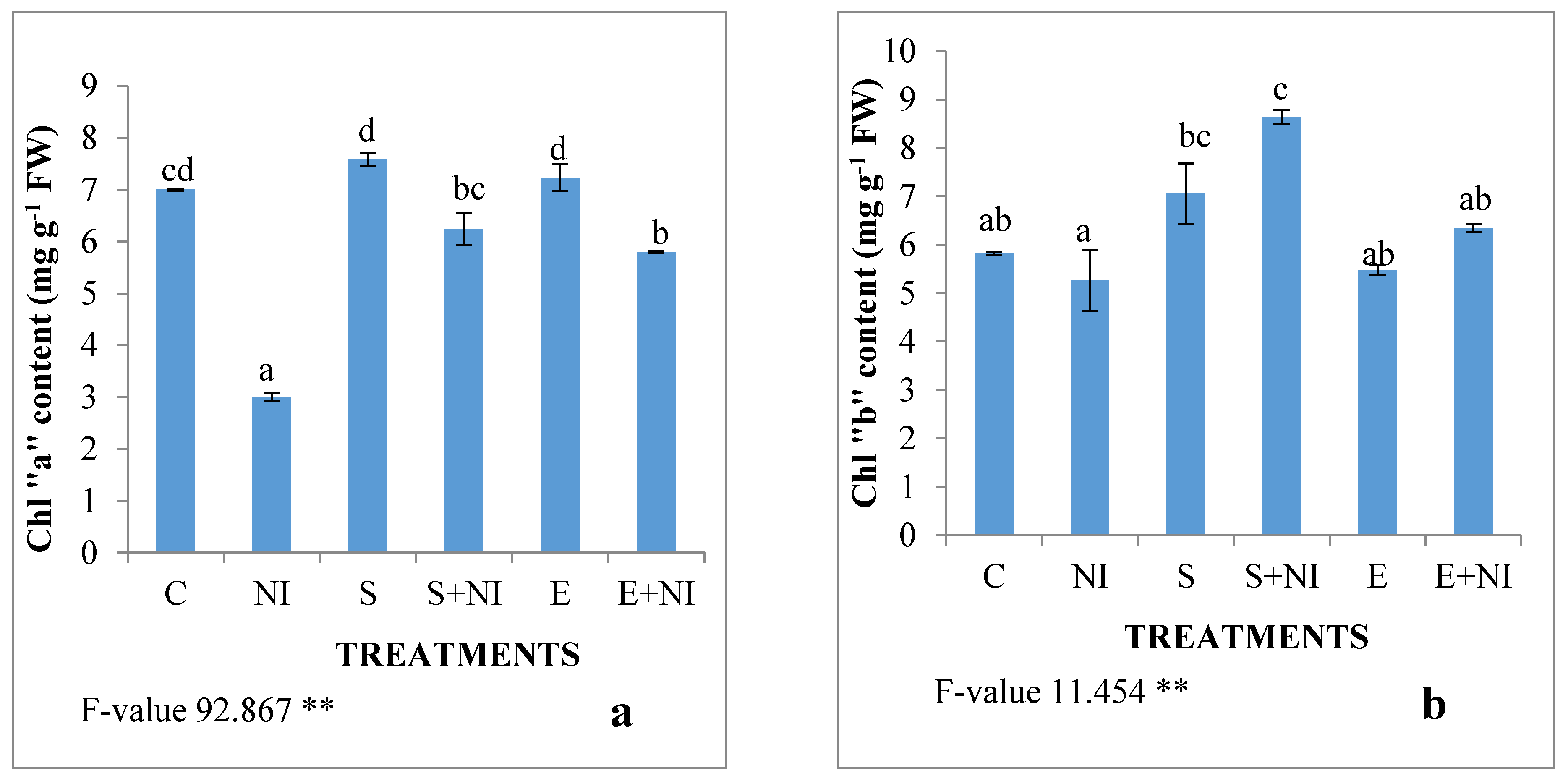
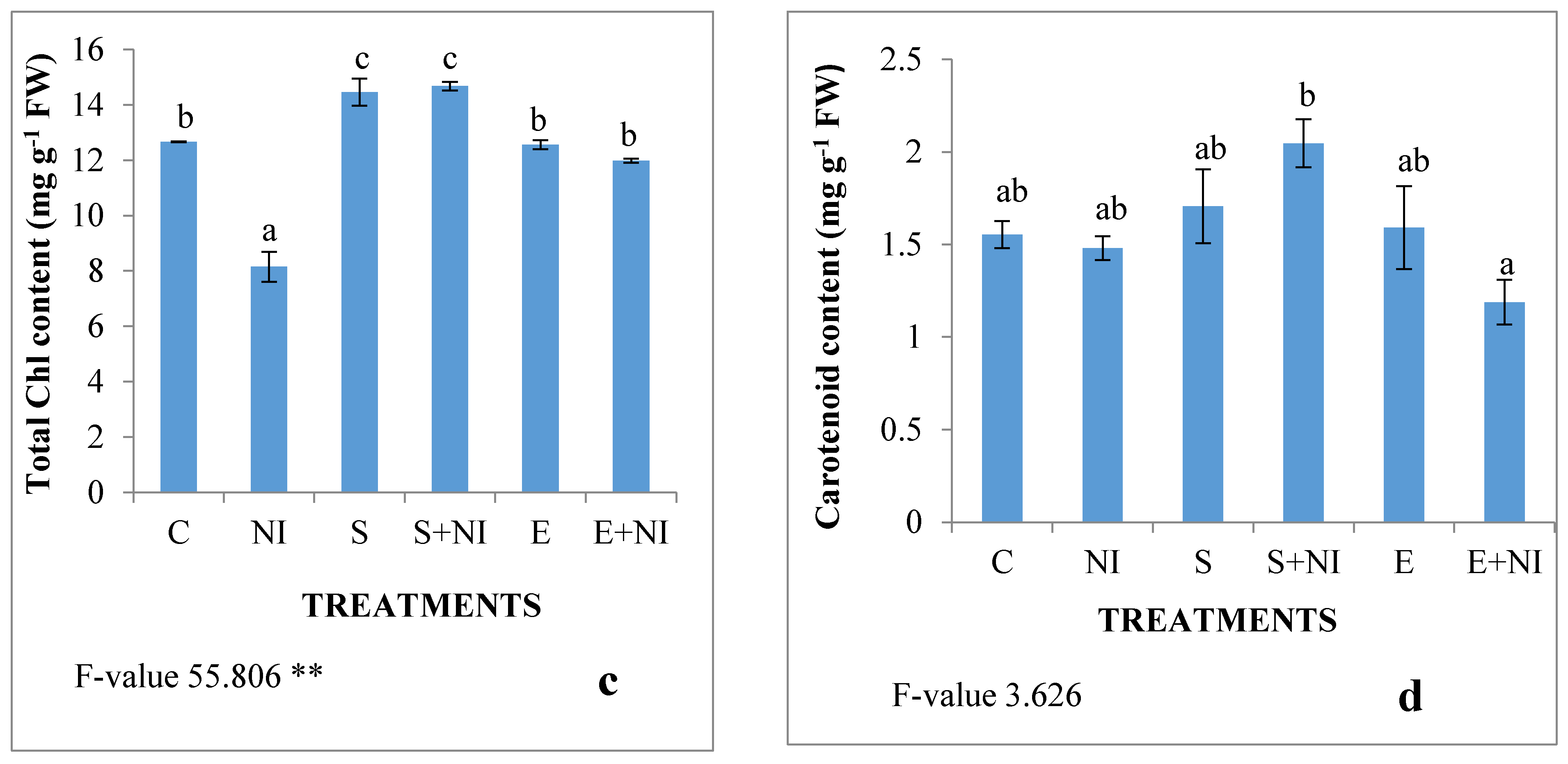
2.2. Effect of Biometabolites Produced by S. hydrogenans Strain DH-16 on the Photosynthetic Pigment of S. lycopersicum Seedlings 7 DAI
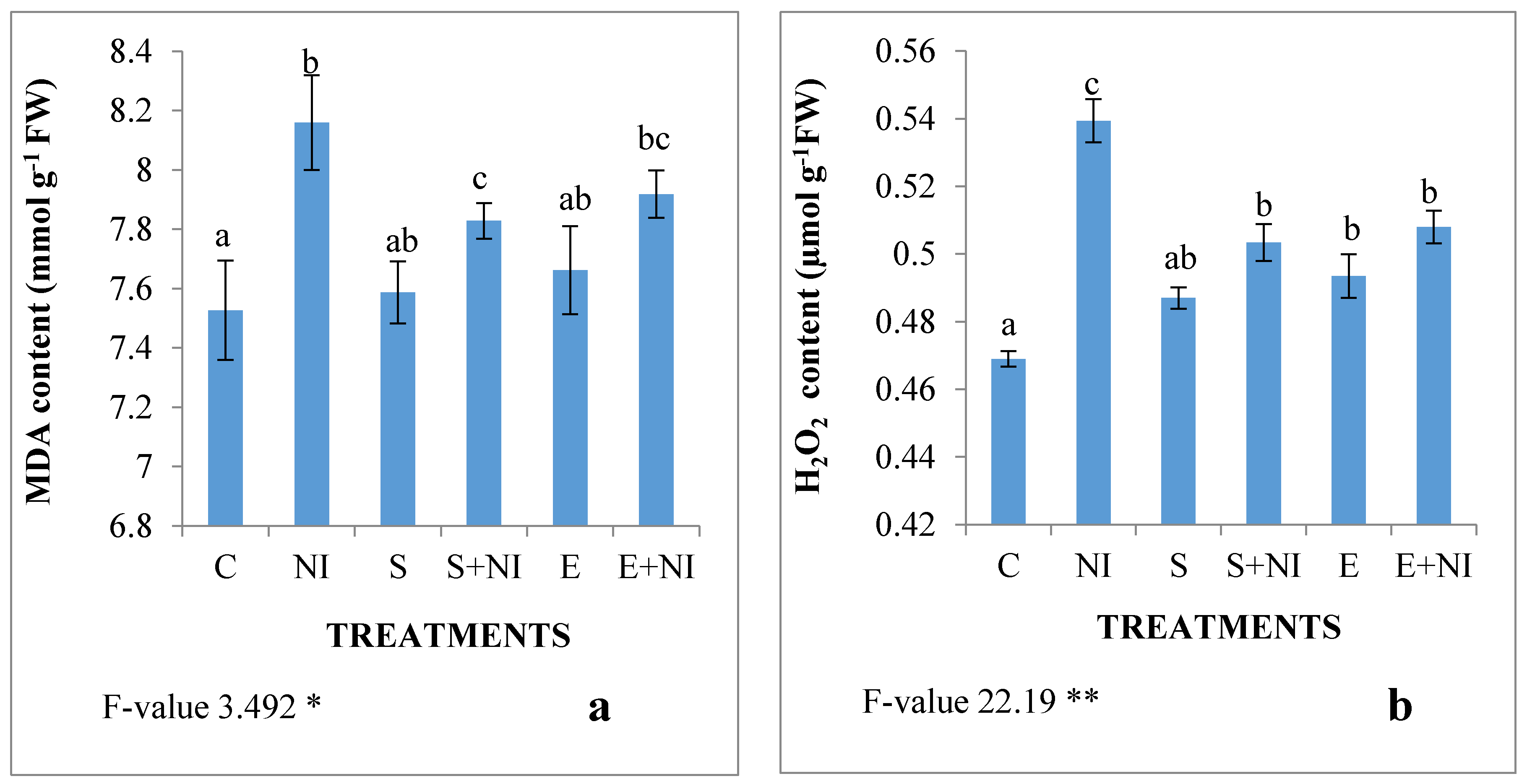
2.3. Malondialdehyde (MDA) and H2O2 Content
2.4. Antioxidative Enzyme Activities
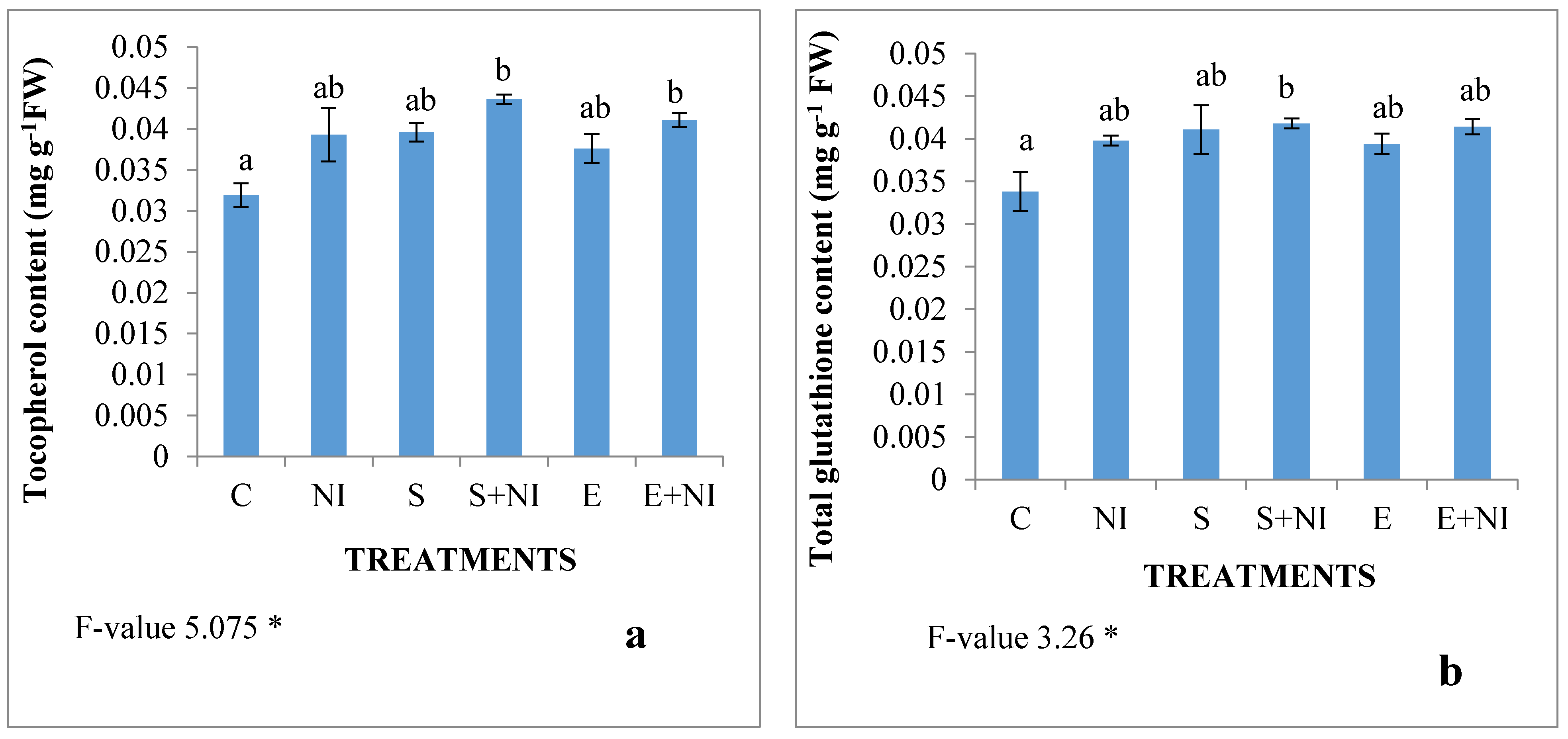
2.5. Non-Enzymatic Antioxidants
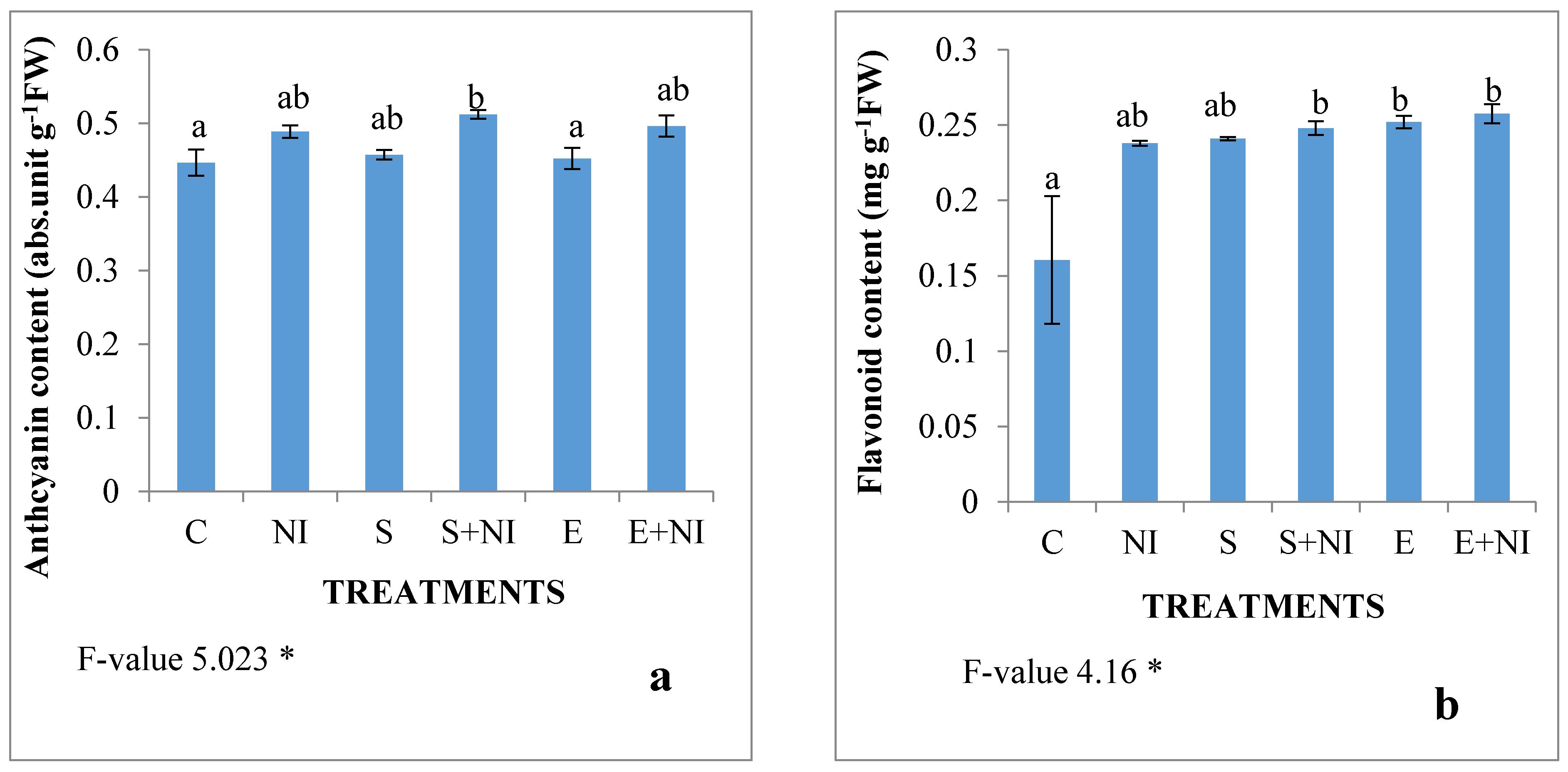
2.6. Phenolic Compounds
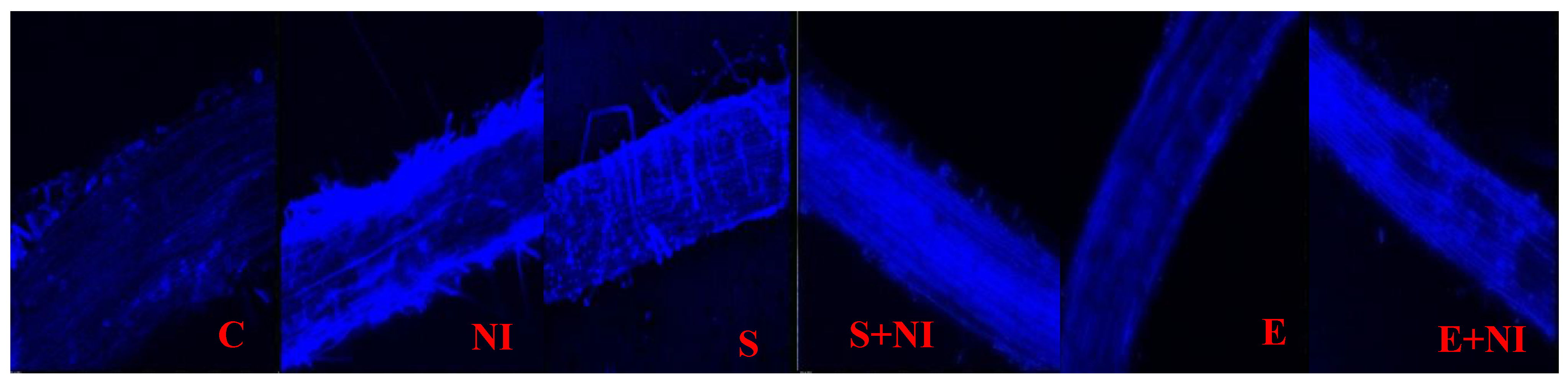
2.7. Confocal Microscopy
3. Discussion
4. Materials and Methods
4.1. Production of Secondary Metabolites
4.2. Plant Material and Treatments
4.3. Morphological Parameters
4.4. Determination of Carotenoid and Chlorophyll Contents
4.5. Determination of Malondialdehyde (MDA) Content and H2O2 Content
4.6. Protein Content and Antioxidative Enzymes
4.7. Non Enzymatic Antioxidants
4.8. Estimation of Phenolic Compounds
4.9. Confocal Microscopy
4.10. Statictical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferraz, L.C.; Brown, D.J. An Introduction to Nematodes: Plant Nematology; Pensoft, Sofia-Moscow: Sofia, Bulgaria, 2002. [Google Scholar]
- Sikora, R.A.; Fernandez, E. Nematode parasites of vegetables. Plant Parasit. Nematodes Subtrop. Trop. Agric. 2005, 2, 319–392. [Google Scholar]
- Nicol, J.; Turner, S.; Coyne, D.; Den Nijs, L.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. [Google Scholar]
- Hussain, M.A.; Mukhtar, T.; Kayani, M.Z. Characterization of susceptibility and resistance responses to root-knot nematode (Meloidogyne incognita) infection in okra germplasm. Pak. J. Agric. Sci. 2014, 51, 309–314. [Google Scholar]
- Singh, A.; Jain, A.; Sarma, B.K.; Upadhyay, R.S.; Singh, H.B. Rhizosphere competent microbial consortium mediates rapid changes in phenolic profiles in chickpea during Sclerotium rolfsii infection. Microbiol. Res. 2014, 169, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Khan, A.; Khan, I.; Rizvi, R.; Saquib, M. Morphological and biochemical resposes of cow pea (cv. pusa barsati) grown on fly ash amended soil in presence and absence of Meloidogyne Javanica and Rhizobium leguminosarum. Ecoprint Int. J. Ecol. 2010, 17, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Singh, A.; Srivastava, M.; Gupta, M.; Pandey, R. Augmentation of systemic resistance and secondary metabolites by chitinolytic microbes in Withania somnifera against Meloidogyne incognita. Biocontrol. Sci. Technol. 2016, 26, 1626–1642. [Google Scholar] [CrossRef]
- Adam, M.; Heuer, H.; Hallmann, J. Bacterial antagonists of fungal pathogens also control root-knot nematodes by induced systemic resistance of tomato plants. PLoS ONE 2014, 9, e90402. [Google Scholar] [CrossRef] [Green Version]
- Elhady, A.; Adss, S.; Hallmann, J.; Heuer, H. Rhizosphere microbiomes modulated by pre-crops assisted plants in defense against plant-parasitic nematodes. Front. Microbiol. 2018, 9, 1133. [Google Scholar] [CrossRef] [Green Version]
- Goellner, M.; Wang, X.; Davis, E.L. Endo-β-1, 4-glucanase expression in compatible plant–nematode interactions. Plant Cell 2001, 13, 2241–2255. [Google Scholar]
- Williamson, V.M.; Hussey, R.S. Nematode pathogenesis and resistance in plants. Plant Cell 1996, 8, 1735. [Google Scholar]
- Abad, P.; Favery, B.; Rosso, M.N.; Castagnone-Sereno, P. Root-knot nematode parasitism and host response: Molecular basis of a sophisticated interaction. Mol. Plant Pathol. 2003, 4, 217–224. [Google Scholar] [CrossRef]
- Celik, I.; Sogut, M.A.; Ozkaynak, E.; Doganlar, S.; Frary, A. Physical mapping of NBS-coding resistance genes to the Me-gene cluster on chromosome P9 reveals markers tightly linked to the N gene for root-knot nematode resistance in pepper. Mol. Breed. 2016, 36, 137. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Le Dang, Q.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Cha, B.; Luu, N.H.; Kim, J.-C. Nematicidal activities of 4-quinolone alkaloids isolated from the aerial part of Triumfetta grandidens against Meloidogyne incognita. J. Agric. Food Chem. 2014, 63, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, P.R.; Miller, A.J.; Dennis, P.G. Do root exudates exert more influence on rhizosphere bacterial community structure than other rhizodeposits? Mol. Microb. Ecol. Rhizosphere 2013, 1, 229–242. [Google Scholar]
- Hallmann, J. Plant Interactions with Endophytic Bacteria; CABI Publishing: New York, NY, USA, 2001. [Google Scholar]
- Sharma, M.; Jasrotia, S.; Ohri, P.; Manhas, R.K. Nematicidal potential of Streptomyces antibioticus strain M7 against Meloidogyne incognita. AMB Express 2019, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Sarwat, M.; Sharma, S. Reactive oxygen species, antioxidants and signaling in plants. J. Plant Biol. 2008, 51, 167–173. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, W.; Jin, Z.; Shen, Z. Response of antioxidative enzymes in cucumber chloroplasts to cadmium toxicity. J. Plant Nutr. 2003, 26, 1779–1788. [Google Scholar] [CrossRef]
- Kimura, S.; Sinha, N. Tomato (Solanum lycopersicum): A model fruit-bearing crop. Cold Spring Harb. Protoc. 2008, 2008, pdb. emo105. [Google Scholar] [CrossRef]
- de la Torre-González, A.; Navarro-León, E.; Albacete, A.; Blasco, B.; Ruiz, J.M. Study of phytohormone profile and oxidative metabolism as key process to identification of salinity response in tomato commercial genotypes. J. Plant Physiol. 2017, 216, 164–173. [Google Scholar] [CrossRef]
- Darekar, K.; Mhase, N. Assessment of yield losses due to root-knot nematode Meloidogyne incognita race 3 in tomato, brinjal and bittergourd. Int. Nematol. Netw. Newsl. (USA) 1988, 5, 7–9. [Google Scholar]
- Williamson, V.M.; Roberts, P.A.; Perry, R. Mechanisms and genetics of resistance. In Root-Knot Nematodes; CAB International: Wallingford, IA, USA, 2009; Volume 301. [Google Scholar]
- Weller, D.M. Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol. 1988, 26, 379–407. [Google Scholar] [CrossRef]
- Wang, C.; Hu, H.-J.; Li, X.; Wang, Y.-F.; Tang, Y.-Y.; Chen, S.-L.; Yan, S.-Z. Effects of varying environmental factors on the biological control of Meloidogyne incognita in tomato by Bacillus cereus strain BCM2. Biocontrol. Sci. Technol. 2018, 28, 359–376. [Google Scholar] [CrossRef]
- Zhou, L.; Yuen, G.; Wang, Y.; Wei, L.; Ji, G. Evaluation of bacterial biological control agents for control of root-knot nematode disease on tomato. Crop Prot. 2016, 84, 8–13. [Google Scholar] [CrossRef]
- Sayre, R.; Starr, M. Bacterial diseases and antagonisms of nematodes. In Nematode Pathology; Poinar, G.O., Jansson, H.B., Eds.; CRC Press: Boca Raton, FL, USA, 1988; Volume 1, pp. 69–101. [Google Scholar]
- Crawford, J.M.; Clardy, J. Bacterial symbionts and natural products. Chem. Commun. 2011, 47, 7559–7566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuthbertson, A.; Blackburn, L.; Northing, P.; Luo, W.; Cannon, R.; Walters, K. Chemical compatibility testing of the entomopathogenic fungus Lecanicillium muscarium to control Bemisia tabaci in glasshouse environment. Int. J. Environ. Sci. Technol. 2010, 7, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Larralde-Corona, C.P.; Santiago-Mena, M.; Sifuentes-Rincon, A.M.; Rodríguez-Luna, I.; Rodriguez-Perez, M.A.; Shirai, K.; Narvaez-Zapata, J.A. Biocontrol potential and polyphasic characterization of novel native Trichoderma strains against Macrophomina phaseolina isolated from sorghum and common bean. Appl. Microbiol. Biotechnol. 2008, 80, 167. [Google Scholar] [CrossRef]
- Monga, D. Effect of carbon and nitrogen sources on spore germination, bio-mass production and antifungal metabolites by species of Trichoderma and Gliocladium. Indian Phytopathol. 2012, 54, 435–437. [Google Scholar]
- Sharma, P.; Pandey, R. Biological control of root-knot nematode; Meloidogyne incognita in the medicinal plant; Withania somnifera and the effect of biocontrol agents on plant growth. Afr. J. Agric. Res. 2009, 4, 564–567. [Google Scholar]
- Anjos, É.C.T.d.; Cavalcante, U.M.T.; Gonçalves, D.M.C.; Pedrosa, E.M.R.; Santos, V.F.d.; Maia, L.C. Interactions between an arbuscular mycorrhizal fungus (Scutellospora heterogama) and the root-knot nematode (Meloidogyne incognita) on sweet passion fruit (Passiflora alata). Braz. Arch. Biol. Technol. 2010, 53, 801–809. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yang, G.-D.; Shu, H.-R.; Yang, Y.-T.; Ye, B.-X.; Nishida, I.; Zheng, C.-C. Colonization by the arbuscular mycorrhizal fungus Glomus versiforme induces a defense response against the root-knot nematode Meloidogyne incognita in the grapevine (Vitis amurensis Rupr.), which includes transcriptional activation of the class III chitinase gene VCH3. Plant Cell Physiol. 2006, 47, 154–163. [Google Scholar]
- Vos, C.; Claerhout, S.; Mkandawire, R.; Panis, B.; De Waele, D.; Elsen, A. Arbuscular mycorrhizal fungi reduce root-knot nematode penetration through altered root exudation of their host. Plant Soil 2012, 354, 335–345. [Google Scholar] [CrossRef]
- Goyal, D.; Pandey, J.; Prakash, O. Role of plant growth-promoting Rhizobacteria (PGPR) in degradation of xenobiotic compounds and Allelochemicals. In Advances in PGPR Research; CAB International: Wallingford, IA, USA, 2017; Volume 330. [Google Scholar]
- Gupta, G.; Snehi, S.K.; Singh, V. Role of PGPR in biofilm formations and its importance in plant health. Biofilms Plant Soil Health 2017, 27, 27–40. [Google Scholar]
- Gupta, R.; Singh, A.; Ajayakumar, P.; Pandey, R. Microbial interference mitigates Meloidogyne incognita mediated oxidative stress and augments bacoside content in Bacopa monnieri L. Microbiol. Res. 2017, 199, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Jasrotia, S.; Ohri, P.; Manhas, R.K. Evaluation of in vitro and in vivo nematicidal potential of a multifunctional streptomycete, Streptomyces hydrogenans strain DH16 against Meloidogyne incognita. Microbiol. Res. 2016, 192, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, U.; Inam-ul-Haq, M.; Saeed, M.; Altaf, A.; Azam, F.; Hayat, S. A brief review on plant growth promoting Rhizobacteria (PGPR): A key role in plant growth promotion. Plant Prot. 2018, 2, 77–82. [Google Scholar]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant Growth Promoting Rhizobacteria (PGPR) for Sustainable Agriculture: Perspectives and Challenges. In PGPR Amelioration in Sustainable Agriculture; Woodhead Publishing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–157. [Google Scholar]
- Sidhu, H.S. Potential of plant growth-promoting rhizobacteria in the management of nematodes: A review. J. Entomol. Zool. Stud. 2018, 6, 1536–1545. [Google Scholar]
- Verma, R.K.; Sachan, M.; Vishwakarma, K.; Upadhyay, N.; Mishra, R.K.; Tripathi, D.K.; Sharma, S. Role of PGPR in sustainable agriculture: Molecular approach toward disease suppression and growth promotion. In Role of Rhizospheric Microbes in Soil; Springer: Singapore, 2018; pp. 259–290. [Google Scholar]
- Siddiqui, Z.A. PGPR: Prospective biocontrol agents of plant pathogens. In PGPR: Biocontrol and Biofertilization; Springer: Singapore, 2005; pp. 111–142. [Google Scholar]
- Borah, B.; Ahmed, R.; Hussain, M.; Phukon, P.; Wann, S.B.; Sarmah, D.K.; Bhau, B.S. Suppression of root-knot disease in Pogostemon cablin caused by Meloidogyne incognita in a rhizobacteria mediated activation of phenylpropanoid pathway. Biol. Control 2018, 119, 43–50. [Google Scholar] [CrossRef]
- Zhai, Y.; Shao, Z.; Cai, M.; Zheng, L.; Li, G.; Huang, D.; Cheng, W.; Thomashow, L.S.; Weller, D.M.; Yu, Z. Multiple modes of nematode control by volatiles of Pseudomonas putida 1A00316 from Antarctic soil against Meloidogyne incognita. Front. Microbiol. 2018, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Sahebani, N.; Hadavi, N. Induction of H2O2 and related enzymes in tomato roots infected with root knot nematode (M. javanica) by several chemical and microbial elicitors. Biocontrol. Sci. Technol. 2009, 19, 301–313. [Google Scholar] [CrossRef]
- Mostafanezhad, H.; Sahebani, N.; Nourinejhad Zarghani, S. Control of root-knot nematode (Meloidogyne javanica) with combination of Arthrobotrys oligospora and salicylic acid and study of some plant defense responses. Biocontrol. Sci. Technol. 2014, 24, 203–215. [Google Scholar] [CrossRef]
- Čepulytė, R.; Danquah, W.B.; Bruening, G.; Williamson, V.M. Potent attractant for root-knot nematodes in exudates from seedling root tips of two host species. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesan, M.; Gaur, H.; Datta, S. Effect of root-knot nematode, Meloidogyne graminicola on the uptake of macronutrients and arsenic and plant growth of rice. Vegetos 2013, 26, 112–120. [Google Scholar] [CrossRef]
- Tiwari, S.; Pandey, S.; Chauhan, P.S.; Pandey, R. Biocontrol agents in co-inoculation manages root knot nematode [Meloidogyne incognita (Kofoid & White) Chitwood] and enhances essential oil content in Ocimum basilicum L. Ind. Crop. Prod. 2017, 97, 292–301. [Google Scholar]
- Shane, M.W.; Lambers, H. Systemic suppression of cluster-root formation and net P-uptake rates in Grevillea crithmifolia at elevated P supply: A proteacean with resistance for developing symptoms of ‘P toxicity’. J. Exp. Bot. 2005, 57, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, G.; Knight, J.; Vance, C.; Allan, D. Acid phosphatase activity in phosphorus-deficient white lupin roots. Plant Cell Environ. 1999, 22, 801–810. [Google Scholar] [CrossRef]
- Khajuria, A.; Ohri, P. Exogenously applied putrescine improves the physiological responses of tomato plant during nematode pathogenesis. Sci. Hortic. 2018, 230, 35–42. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Kohli, S.K.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Role of plant growth promoting Bacteria (PGPRs) as biocontrol agents of Meloidogyne incognita through improved plant defense of Lycopersicon esculentum. Plant Soil 2019, 436, 325–345. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Sharma, A.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Al-Huqail, A.A.; Siddiqui, M.H.; Marraiki, N.; Ahmad, P. Evaluation of the role of Rhizobacteria in controlling root knot nematode (RKN) infection in Lycopersicon esculentum plants by modulation in the secondary metabolite profiles. AoB PLANTS 2019, 11, 1–14. [Google Scholar] [CrossRef]
- Chubachi, K.; Furukawa, M.; Fukuda, S.; Matsumura, S.; Yanagisawa, T.; Itagawa, H.; Shimizu, T.; Nakagawa, A. Suppressive effects of antinematodal Streptomyces spp. on root-knot nematodes of cucumbers caused by Meloidogyne incognita. Biocontrol. Sci. 2002, 7, 25–29. [Google Scholar] [CrossRef]
- Vasil’Eva, I.; Vanyushkin, S.; Zinov’Eva, S.; Udalova, Z.V.; Bolychevtseva, Y.V.; Paseshnichenko, V. Photosynthetic pigments of tomato plants under conditions of biotic stress and effects of furostanol glycosides. Appl. Biochem. Microbiol. 2003, 39, 606–612. [Google Scholar] [CrossRef]
- Yang, C.-M.; Yang, M.-M.; Hsu, J.-M.; Jane, W.-N. Herbivorous insect causes deficiency of pigment-protein complexes in an oval-pointed cecidomyiid gall of Machilus thunbergii leaf. Bot. Bull. Acad. Sin. 2003, 44, 315–321. [Google Scholar]
- Jiang, C.-H.; Xie, P.; Li, K.; Xie, Y.-S.; Chen, L.-J.; Wang, J.-S.; Xu, Q.; Guo, J.-H. Evaluation of root-knot nematode disease control and plant growth promotion potential of biofertilizer Ning shield on Trichosanthes kirilowii in the field. Braz. J. Microbiol. 2018, 49, 232–239. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.N.; Pintado, M.; Sarmento, B.; Stamford, N.; Vasconcelos, M.W. A biofertilizer with diazotrophic bacteria and a filamentous fungus increases Pinus pinaster tolerance to the pinewood nematode (Bursaphelenchus xylophilus). Biol. Control 2019, 132, 72–80. [Google Scholar] [CrossRef]
- Korayem, A.; El-Bassiouny, H.; El-Monem, A.A.A.; Mohamed, M. Physiological and biochemical changes in different sugar beet genotypes infected with root-knot nematode. Acta Physiol. Plant. 2012, 34, 1847–1861. [Google Scholar] [CrossRef]
- Sharma, I.P.; Sharma, A. Physiological and biochemical changes in tomato cultivar PT-3 with dual inoculation of mycorrhiza and PGPR against root-knot nematode. Symbiosis 2017, 71, 175–183. [Google Scholar] [CrossRef]
- Kuźniak, E.; Skłodowska, M. Ascorbate, glutathione and related enzymes in chloroplasts of tomato leaves infected by Botrytis cinerea. Plant Sci. 2001, 160, 723–731. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Nikoo, F.S.; Sahebani, N.; Aminian, H.; Mokhtarnejad, L.; Ghaderi, R. Induction of systemic resistance and defense-related enzymes in tomato plants using Pseudomonas fluorescens CHAO and salicylic acid against root-knot nematode Meloidogyne javanica. J. Plant Prot. Res. 2014, 54, 383–389. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Li, Y.-L.; Lai, H.-X.; Guo, Q.; Xue, Q.-H. Effects of two strains of Streptomyces on root-zone microbes and nematodes for biocontrol of root-knot nematode disease in tomato. Appl. Soil Ecol. 2017, 112, 34–41. [Google Scholar] [CrossRef]
- Jimenez, A.; Hernandez, J.A.; del Río, L.A.; Sevilla, F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997, 114, 275–284. [Google Scholar] [CrossRef] [Green Version]
- Kesba, H.H.; El-Beltagi, H.S. Biochemical changes in grape rootstocks resulted from humic acid treatments in relation to nematode infection. Asian Pac. J. Trop. Biomed. 2012, 2, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Afifi, A.; Al-Sayed, A.; Mahfoud, N.; Farahat, A. Enzymatic and non-enzymatic oxidants and antioxidants involved in defense mechanisms against root-knot, reniform and citrus nematodes in their hosts. Egypt. J. Agron. 2014, 13, 172–188. [Google Scholar] [CrossRef] [Green Version]
- Korayem, A.; Mohamed, M.; El-Ashry, S. Yield and oil quality of sunflower infected with the root-knot nematode, Meloidogyne arenaria. Int. J. Chem. Tech. Res. 2016, 3, 207–2014. [Google Scholar]
- Mollavali, M.; Bolandnazar, S.A.; Schwarz, D.; Rohn, S.; Riehle, P.; Zaare Nahandi, F. Flavonol glucoside and antioxidant enzyme biosynthesis affected by mycorrhizal fungi in various cultivars of onion (Allium cepa L.). J. Agric. Food Chem. 2016, 64, 71–77. [Google Scholar] [CrossRef]
- Ray, S.; Singh, V.; Singh, S.; Sarma, B.K.; Singh, H.B. Biochemical and histochemical analyses revealing endophytic Alcaligenes faecalis mediated suppression of oxidative stress in Abelmoschus esculentus challenged with Sclerotium rolfsii. Plant Physiol. Biochem. 2016, 109, 430–441. [Google Scholar] [CrossRef]
- Nayak, D. Effects of nematode infection on contents of phenolic substances as influenced by root-knot nematode, Meloidogyne incognita in susceptible and resistant brinjal cultivars. Agric. Sci. Dig. Res. J. 2015, 35, 163–164. [Google Scholar] [CrossRef]
- Mahajan, R.; Kaur, D.; Bajaj, K. Nematicidal activity of phenolic compounds against Meloidogyne incognita. Nematol. Mediterr. 1992, 20, 217–219. [Google Scholar]
- Malik, M.S.; Dhindsa, K.S.; Pal, V.; Verma, K.; Sangwan, N.K.; Bhatti, D. Nematicidal efficacy of substituted phenols, phenoxyacetic acid esters and hydrazides: A structure-activity relationship study. Nematologica 1989, 35, 366–370. [Google Scholar] [CrossRef]
- Patel, V.S.; Shukla, Y.; Dhruve, J. Influence of root knot nematode (Meloidogyne spp.) on phenolic acid profile in root of tomato (Solanum lycopersicum L.). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 840–848. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef]
- Gupta, R.; Pandey, R. Microbial interference ameliorates essential oil yield and diminishes root-knot infestation in sweet basil under field conditions. Biocontrol. Sci. Technol. 2015, 25, 1165–1179. [Google Scholar] [CrossRef]
- Hussey, R.; Barker, K. A comparison of nematodes of collecting inocula for Meloidogyne spp., including a new technique Pl. Dis. Rep. 1973, 61, 328–331. [Google Scholar]
- Kaur, T.; Manhas, R.K. Antifungal, insecticidal, and plant growth promoting potential of Streptomyces hydrogenans DH16. J. Basic Microbiol. 2014, 54, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maclachlan, S.; Zalik, S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot. 1963, 41, 1053–1062. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; pp. 673–680. [Google Scholar]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Esterbauer, H.; Schwarzl, E.; Hayn, M. A rapid assay for catechol oxidase and laccase using 2-nitro-5-thiobenzoic acid. Anal. Biochem. 1977, 77, 486–494. [Google Scholar] [CrossRef]
- Pütter, J. Peroxidases. In Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; pp. 685–690. [Google Scholar]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-Transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Martinek, R.G. Method for the determination of vitamin E (total tocopherols) in serum. Clin. Chem. 1964, 10, 1078–1086. [Google Scholar] [CrossRef]
- Lamaison, J.; Carnet, A. Contents of main flavonoids flowers Crataegeus monogyna Jacq and Crataegeus laevigata (Poiret DC) at different developmental stages. Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar]
- Mancinelli, A.L. Photoregulation of anthocyanin synthesis: VIII. Effect of light pretreatments. Plant Physiol. 1984, 75, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.J.; May, M.J.; Fricker, M. Quantitative in vivo measurement of glutathione in Arabidopsis cells. Plant J. 2001, 27, 67–78. [Google Scholar] [CrossRef] [Green Version]

| Treatments | Parameters (Mean ± S.E.) | |||||
|---|---|---|---|---|---|---|
| Germination % | Root Length (cm) | Shoot Length (cm) | Root Weight (g) | Shoot Weight (g) | No. of Galls | |
| C | 70.67 ± 1.453 a | 8.827 ± 0.361 bcd | 7.337 ± 0.130 a | 0.150 ± 0.012 ab | 0.5600 ± 0.021 a | - |
| NI | 69.33 ± 1.202 a | 6.897 ± 0.529 a | 6.943 ± 0.135 a | 0.127 ± 0.008 a | 0.533 ± 0.015 a | 8 ± 0.333 a |
| S | 87 ± 1.527 b | 9.130 ± 0.460 cd | 7.9167 ± 0.202 ab | 0.170 ± 0.006 b | 0.693 ± 0.009 b | - |
| S + NI | 85.67 ± 1.202 b | 7.173 ± 0.061 ab | 8.620 ± 0.399 b | 0.1633 ± 0.003 ab | 0.690 ± 0.023 b | 6 ± 1.202 a |
| E | 86.67 ± 1.333 b | 9.150 ± 0.105 d | 7.456 ± 0.059 a | 0.170 ± 0.100 b | 0.690 ± 0.006 b | - |
| E + NI | 84 ± 1.732 b | 7.287 ± 0.525 abc | 7.157 ± 0.206 a | 0.153 ± 0.008 ab | 0.580 ± 0.012 a | 7 ± 0.575 a |
| F-value | 33.747 ** | 7.440 ** | 7.903 ** | 3.733 * | 23.431 ** | 52.235 (ns) |
| Treatments | Parameters (Mean ± S.E.) | |||||
|---|---|---|---|---|---|---|
| Catalase (CAT) (U/mg Protein) | Superoxide Dismutase (SOD) (U/mg Protein) | Ascorbate Peroxidise (APOX) (U/mg Protein) | Polyphenol Oxidise (PPO) (U/mg Protein) | Guaiacol Peroxidise (GuPOX) (U/mg Protein) | Glutathione-S-Transferase (GST) (U/mg Protein) | |
| C | 0.1249 ± 0.0195 a | 0.0030 ± 0.00028 a | 0.0651 ± 0.00451 a | 0.0119 ± 0.00040 a | 0.0534 ± 0.00266 a | 0.0471 ± 0.00375 a |
| NI | 0.1353 ± 0.0175 a | 0.0039 ± 0.00028 abc | 0.0886 ± 0.00426 b | 0.0138 ± 0.00048 a | 0.0827 ± 0.00632 b | 0.0659 ± 0.01176 ab |
| S | 0.1487 ± 0.0106 a | 0.0043 ± 0.00017 bc | 0.0809 ± 0.00330 ab | 0.0130 ± 0.00024 a | 0.0715 ± 0.00576 ab | 0.0591 ± 0.00452 ab |
| S + NI | 0.1407 ± 0.0133 a | 0.0045 ± 0.00032 c | 0.0918 ± 0.00070 b | 0.0140 ± 0.00033 a | 0.0954 ± 0.00796 b | 0.0791 ± 0.00279 b |
| E | 0.1357 ± 0.0042 a | 0.0032 ± 0.00010 ab | 0.0777 ± 0.00509 ab | 0.0128 ± 0.00083 a | 0.699 ± 0.00829 ab | 0.0503 ± 0.00321 ab |
| E + NI | 0.138 ± 0.00493 a | 0.0037 ± 0.00015 abc | 0.0908 ± 0.00474 b | 0.0139 ± 0.00016 a | 0.0950 ± 0.00111 b | 0.0753 ± 0.00890 ab |
| F-value | 0.355 (ns) | 6.632 ** | 6.372 ** | 3.174 (ns) | 7.452 ** | 3.761 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Khanna, K.; Manhas, R.K.; Bhardwaj, R.; Ohri, P.; Alkahtani, J.; Alwahibi, M.S.; Ahmad, P. Insights into the Role of Streptomyces hydrogenans as the Plant Growth Promoter, Photosynthetic Pigment Enhancer and Biocontrol Agent against Meloidogyne incognita in Solanum lycopersicum Seedlings. Plants 2020, 9, 1109. https://doi.org/10.3390/plants9091109
Sharma N, Khanna K, Manhas RK, Bhardwaj R, Ohri P, Alkahtani J, Alwahibi MS, Ahmad P. Insights into the Role of Streptomyces hydrogenans as the Plant Growth Promoter, Photosynthetic Pigment Enhancer and Biocontrol Agent against Meloidogyne incognita in Solanum lycopersicum Seedlings. Plants. 2020; 9(9):1109. https://doi.org/10.3390/plants9091109
Chicago/Turabian StyleSharma, Nandni, Kanika Khanna, Rajesh Kumari Manhas, Renu Bhardwaj, Puja Ohri, Jawaher Alkahtani, Mona S. Alwahibi, and Parvaiz Ahmad. 2020. "Insights into the Role of Streptomyces hydrogenans as the Plant Growth Promoter, Photosynthetic Pigment Enhancer and Biocontrol Agent against Meloidogyne incognita in Solanum lycopersicum Seedlings" Plants 9, no. 9: 1109. https://doi.org/10.3390/plants9091109
APA StyleSharma, N., Khanna, K., Manhas, R. K., Bhardwaj, R., Ohri, P., Alkahtani, J., Alwahibi, M. S., & Ahmad, P. (2020). Insights into the Role of Streptomyces hydrogenans as the Plant Growth Promoter, Photosynthetic Pigment Enhancer and Biocontrol Agent against Meloidogyne incognita in Solanum lycopersicum Seedlings. Plants, 9(9), 1109. https://doi.org/10.3390/plants9091109






