Turkish Propolis and Its Nano Form Can Ameliorate the Side Effects of Cisplatin, Which Is a Widely Used Drug in the Treatment of Cancer
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Body Weight Change and Feed Intake
2.2. Biochemical Parameters
2.3. Antioxidant Status in Liver and Kidney Tissues
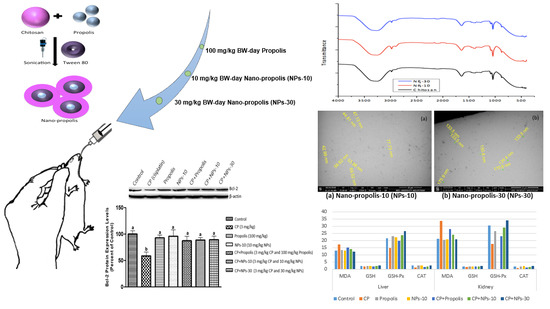
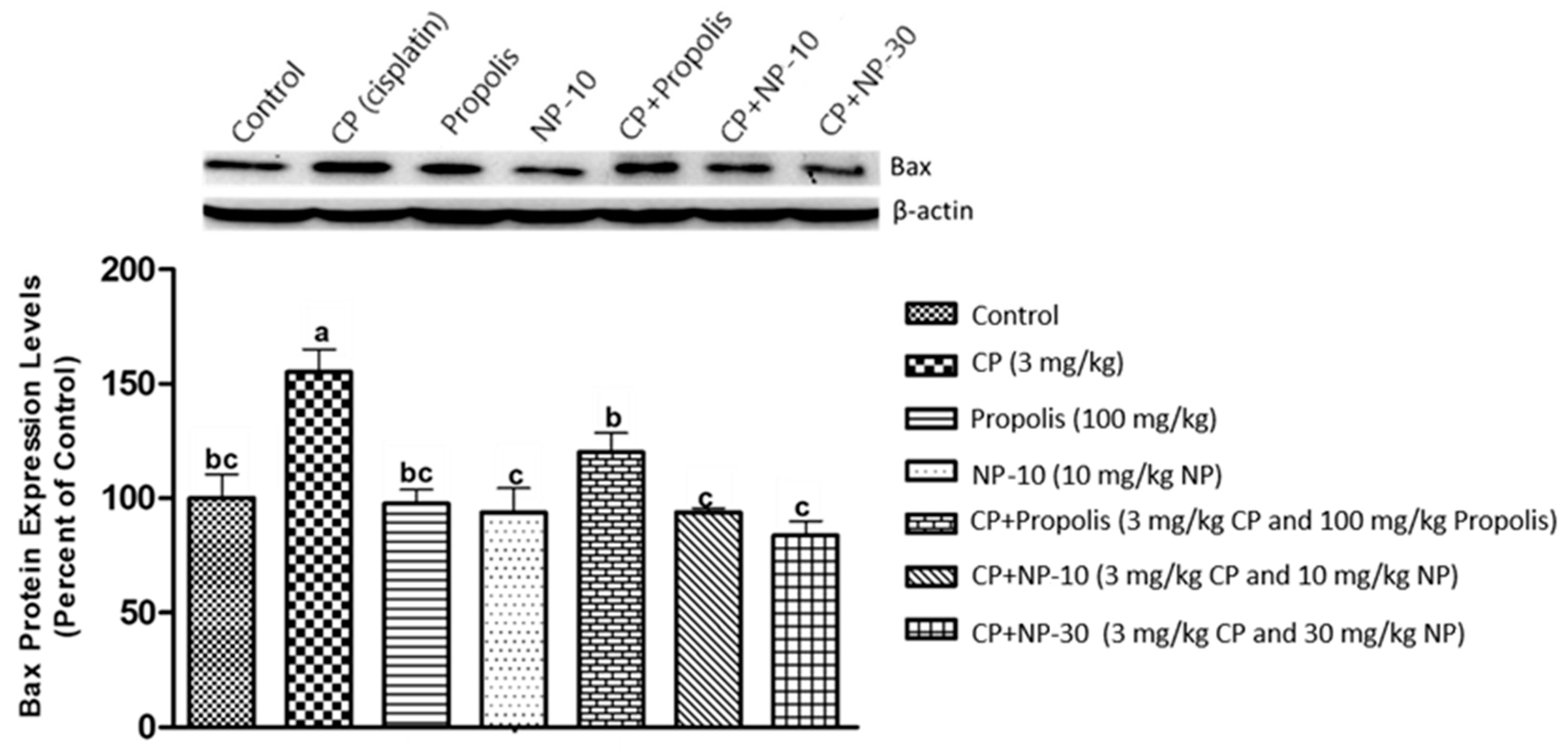
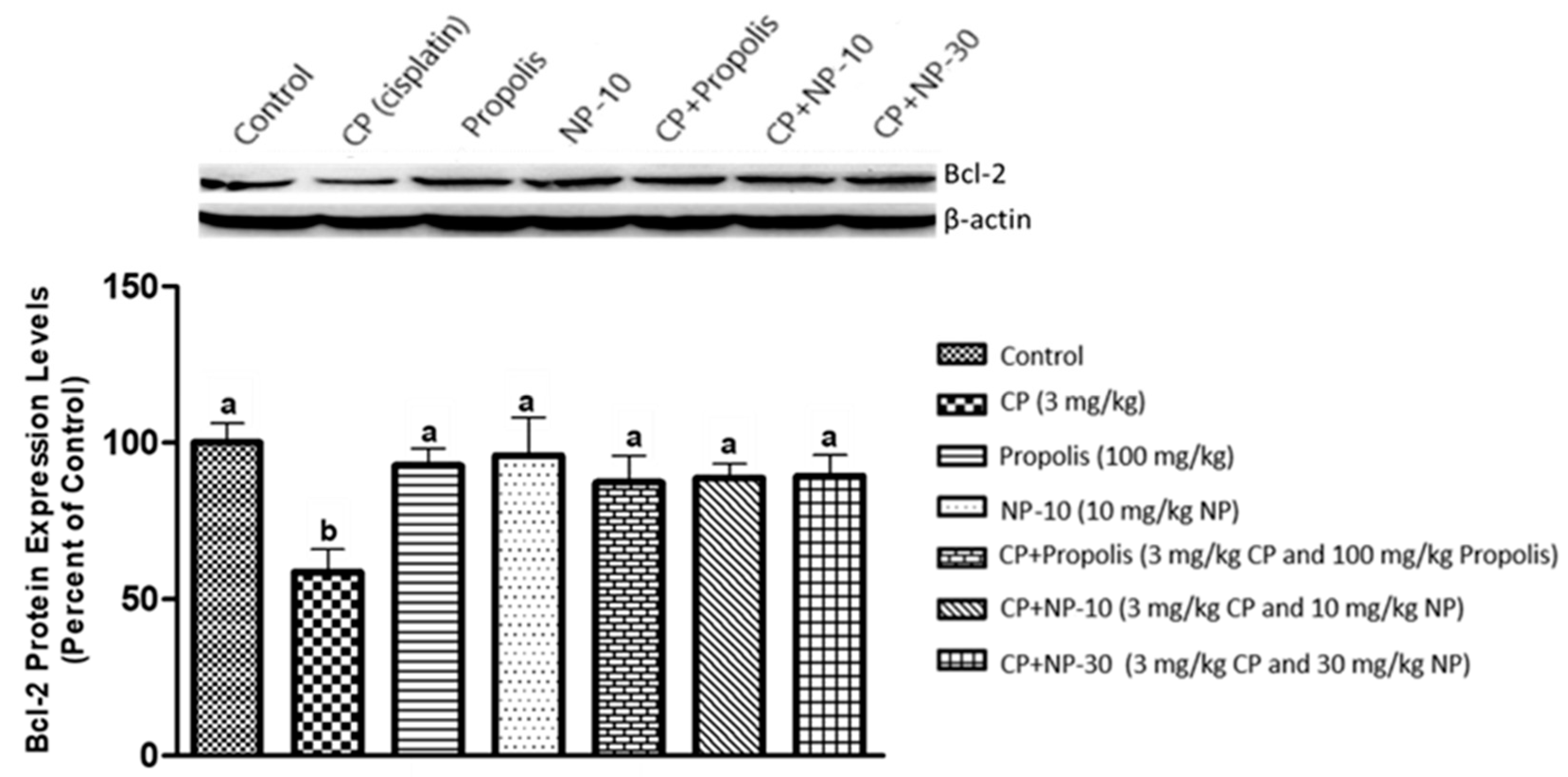
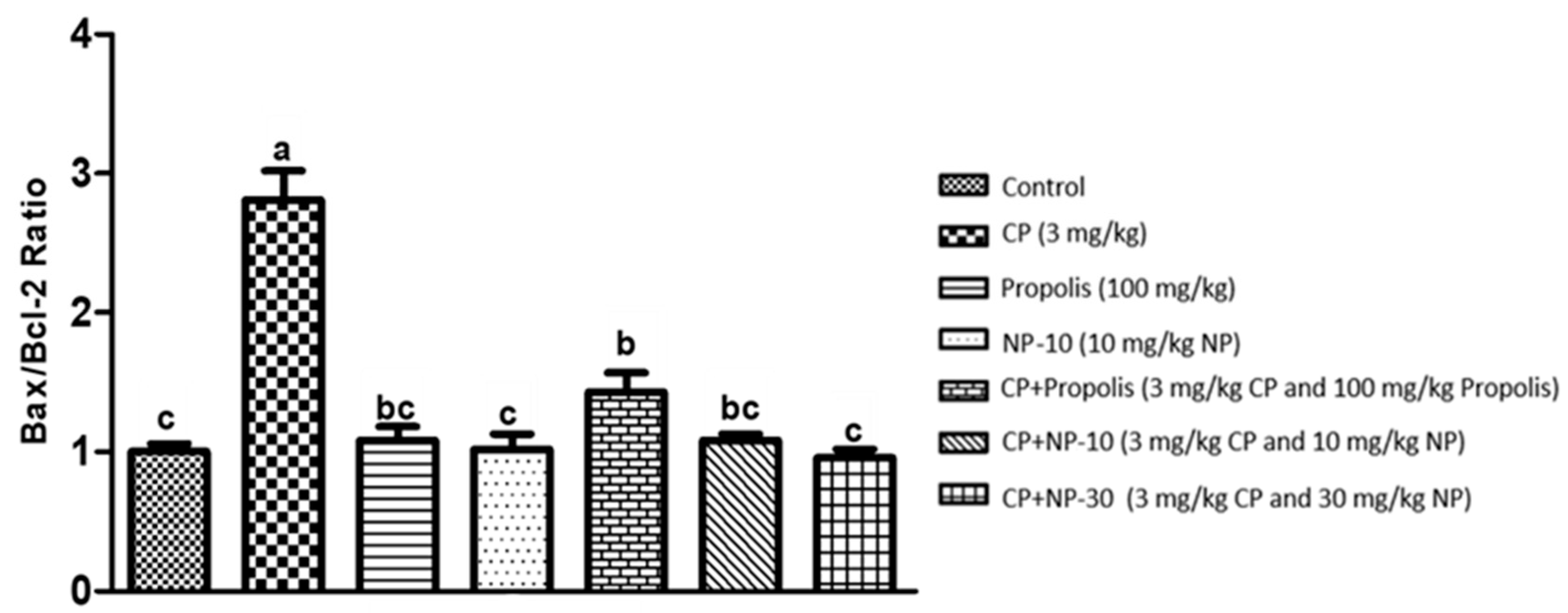
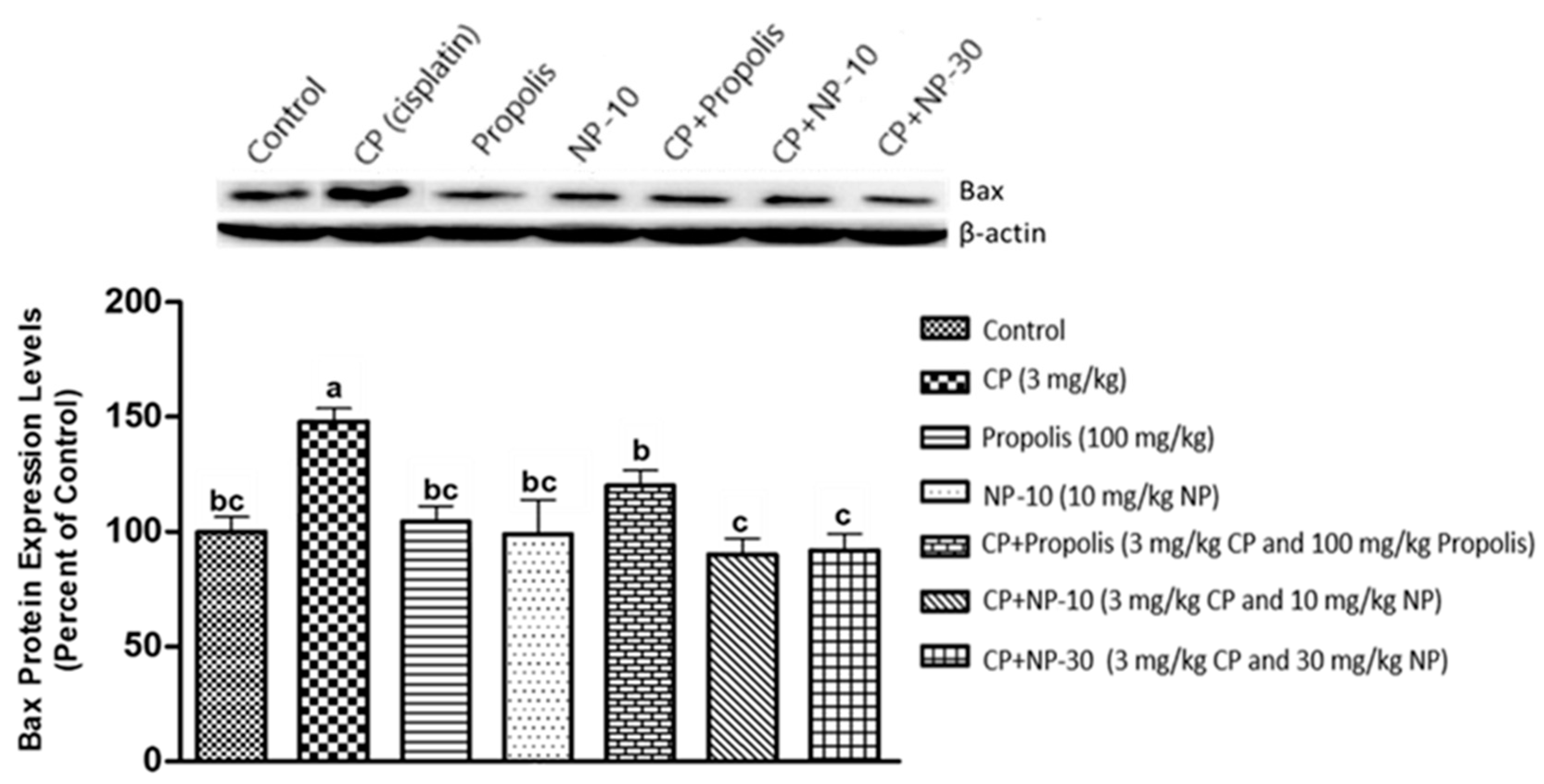
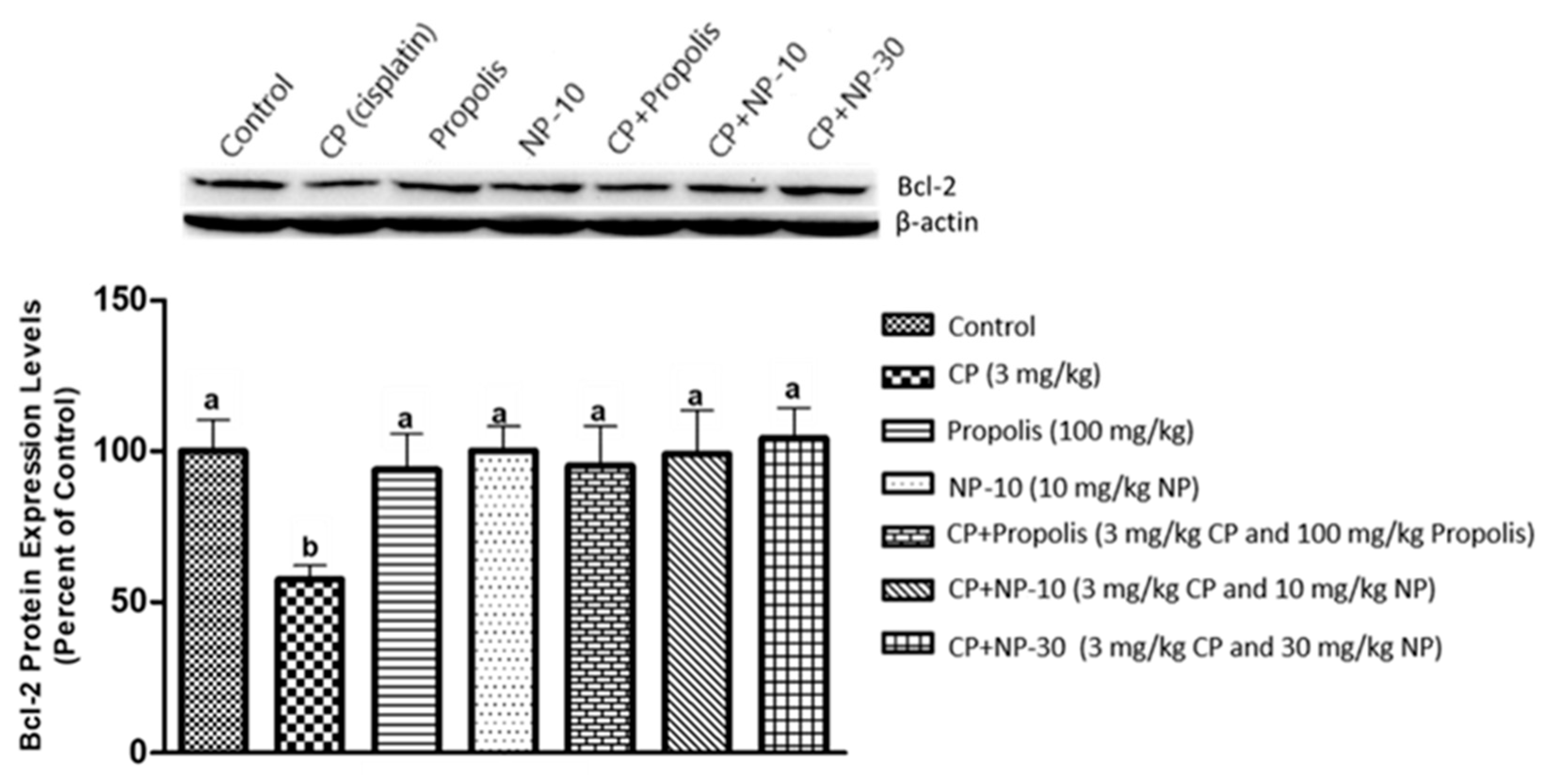
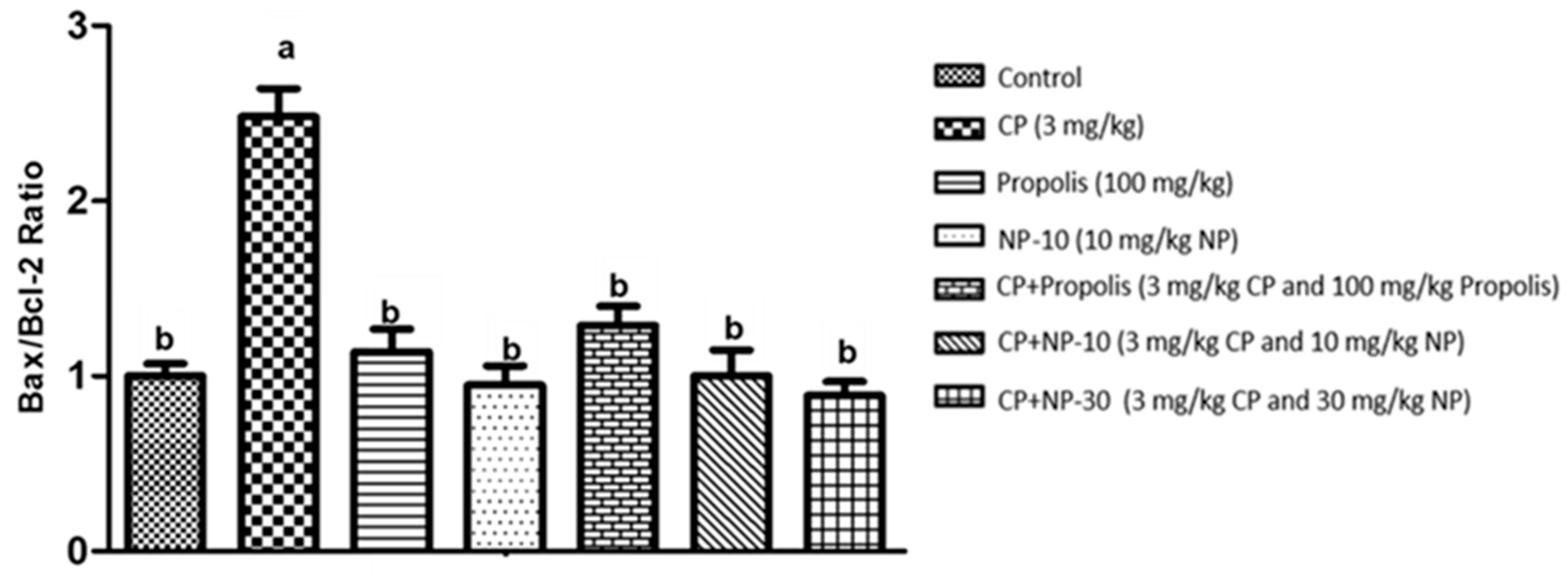
2.4. Bcl-2, Bax Protein Expression of Liver and Kidney Tissue
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Animal Housing and Experimental Design
4.3. Analysis of Propolis
4.3.1. Extraction of Propolis
4.3.2. The Analysis of Total Phenolic Content
4.3.3. Content of Total Flavonoid
4.3.4. Total Antioxidant Capacity (TAC) of Propolis
Total Antioxidant Capacity According to the DPPH Method
Total Antioxidant Capacity According to the CUPRAC Method
4.3.5. Major Individual Phenolic Profile of Propolis
4.4. Preparation of Nano-Propolis
4.4.1. Nano-Propolis-10 (NP-10)
4.4.2. Nano-Propolis-30 (NP-30)
4.4.3. Characterization Technique
4.5. Performance Parameters
4.6. Blood Pparameters
4.7. Oxidative Stress Analyses
4.7.1. Sample Collection and Homogenate Preparation
4.7.2. Determination of Malondialdehyde (MDA) Levels
4.7.3. Measurement of Catalase (CAT) Enzyme Level in Tissues
4.7.4. Glutathione (GSH) Levels in Tissues
4.7.5. The Glutathione Peroxidase (GSH-Px) Activity
4.8. Determination of Protein Expressions by Western Blotting Technique
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ates, S.; Olgun, N. Chemotherapy induced alopecia and quality of life. J. Hacet. Univ. Fac. Nurs. 2014, 1, 67–80. [Google Scholar]
- Sabuncuoglu, S.; Ozgunes, H. Cisplatin toxicity: Importance of oxidative stress and effect of antioxidants. J. Ist. Fac. Med. 2011, 74, 18–25. [Google Scholar]
- Kim, D.M.; Lee, G.D.; Aum, S.H.; Kim, H.J. Preparation of propolis nanofood and application to human cancer. Biol. Pharm. Bull. 2008, 31, 1704–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-de-Lucía, I.; Gonçalves, S.F.; Leganés, F.; Fernández-Piñas, F.; Rosal, R.; Loureiro, S. Combined toxicity of graphite-diamond nanoparticles and thiabendazole to Daphnia magna. Sci. Total Environ. 2019, 688, 1145–1154. [Google Scholar] [CrossRef]
- Rippner, D.A.; Lien, J.; Balla, H.; Guo, T.; Green, P.G.; Young, T.M.; Parikh, S.J. Surface modification induced cuprous oxide nanoparticle toxicity to duckweed at sub-toxic metal concentrations. Sci. Total Environ. 2020, 722, 137607. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Dos Santos, N.A.; Martins, N.M.; Curti, C.; Pires Bianchi Mde, L.; Dos Santos, A.C. Dimethylthiourea protects against mitochondrial oxidative damage induced by cisplatin in liver of rats. Chem. Biol. Interact 2007, 170, 177–186. [Google Scholar] [CrossRef]
- Iseri, S.; Ercan, F.; Gedik, N.; Yuksel, M.; Alican, I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology 2007, 230, 256–264. [Google Scholar] [CrossRef]
- Caro, A.A.; Cederbaum, A.I. Oxidative stress, toxicology, and pharmacology of CYP2E1. Ann. Rev. Pharmacol. Toxicol. 2004, 44, 27–42. [Google Scholar] [CrossRef]
- Cetin, R.; Devrim, E.; Kilicoglu, B.; Avci, A.; Candir, O.; Durak, I. Cisplatin impairs antioxidant system and causes oxidation in rat kidney tissues: Possible protective roles of natural antioxidant foods. J. Appl. Toxicol. 2006, 26, 42–46. [Google Scholar] [CrossRef]
- Kart, A.; Cigremis, Y.; Karaman, M.; Ozen, H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin induced hepatotoxicity in rabbit. Exp. Toxicol. Pathol. 2010, 62, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Hevia, D.; Patchva, S.; Park, B.; Koh, W.; Aggarwal, B.B. Upsides and downsides of reactive oxygen species for cancer: The roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid. Redox. Signal. 2012, 16, 1295–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Hampton, M.B.; Orrenius, S. Dual regulation of caspase activity by hydrogen peroxide: Implications for apoptosis. FEBS Lett. 1997, 414, 552–556. [Google Scholar] [CrossRef] [Green Version]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Fulda, S. Regulation of necroptosis signaling and cell death by reactive oxygen species. Biol. Chem. 2016, 397, 657–660. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- FSAI-Food Safety Authority of Ireland. Available online: https://www.fsai.ie/WorkArea/DownloadAsset.aspx?id=7858 (accessed on 25 June 2020).
- Troncarelli, M.Z.; Brandão, H.M.; Gern, J.C.; Guimarães, A.S.; Langoni, H. Nanotechnology and Antimicrobials in Veterinary Medicine. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education, 1st ed.; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; Volume 1, pp. 543–556. [Google Scholar]
- Afrouzan, H.; Amirinia, C.; Mirhadi, S.A.; Ebadollahi, A.; Vaseji, N.; Tahmasbi, G. Evaluation of antimicrobial activity of propolis and nanopropolis against Staphylococcus aureus and Candida albicans. Afr. J. Microbiol. Res. 2012, 6, 421–425. [Google Scholar]
- Brandão, H.M.; Gern, J.C.; Vicentini, N.M.; Pereira, M.M.; Andrade, P.V.D. Nanotecnologia: A próxima revolução na agropecuária. Rev. CFMV 2011, 17, 61–67. [Google Scholar]
- Madureira, E.H. Entrevista sobre desenvolvimento de novas formulações farmacêuticas para uso veterinário, incluindo emprego de micro e nanopartículas. Rev. CFMV Ano. XVII 2011, 53, 5–8. [Google Scholar]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; English, M.E.; Schaeffer, S.M.; Miles, C.A.; Zelenyuk, A.; Suski, K.; Flury, M. Release of micro-and nanoparticles from biodegradable plastic during in situ composting. Sci. Total Environ. 2019, 675, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Loher, S.; Schneider, O.D.; Maienfisch, T.; Bokorny, S.; Stark, W.J. Micro-organism-triggered release of silver nanoparticles from biodegradable oxide carriers allows preparation of self-sterilizing polymer surfaces. Small 2008, 4, 824–832. [Google Scholar] [CrossRef]
- Rizzi, V.; Lacalamita, D.; Gubitosa, J.; Fini, P.; Petrella, A.; Romita, R.; Agostiano, A.; Gabaldón, J.A.; Fortea Gorbe, M.I.; Gómez-Morte, T.; et al. Removal of tetracycline from polluted water by chitosan-olive pomace adsorbing films. Sci. Total Environ. 2019, 693, 133620. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Biodegradable polymers for biomedical additive manufacturing. Appl. Mater. Today 2020, 20, 100700. [Google Scholar] [CrossRef]
- Kasálková, N.S.; Slepička, P.; Bačáková, L.; Sajdl, P.; Švorčík, V. Biocompatibility of plasma nanostructured biopolymers. Nucl. Instrum. Meth. B 2013, 307, 642–646. [Google Scholar] [CrossRef]
- Seven, I.; Tatli Seven, P.; Gul Baykalir, B.; Parlak Ak, T.; Ozer Kaya, S.; Yaman, M. Bee glue (propolis) improves reproductive organs, sperm quality and histological changes and antioxidant parameters of testis tissues in rats exposed to excess copper. Andrologia 2020, 52, e13540. [Google Scholar] [CrossRef] [PubMed]
- Basista, K.; Filipek, B. Allergic potential of propolis—A literature review. Artic. Alerg. Astma Immunol. 2013, 18, 32–38. [Google Scholar]
- Basista-Sołtys, K. Allergy to propolis in beekeepers—A literature review. Occup. Med. Health Aff. 2013, 1, 1–3. [Google Scholar]
- Gamazo, C.; Gastaminza, G.; Ferrer, M.; Sanz, M.L.; Irache, J.M. Nanoparticle based-immunotherapy against allergy. Immunotherapy 2014, 6, 885–897. [Google Scholar] [CrossRef]
- Dragostin, I.; Dragostin, O.M.; Dragan, M.; Stan, C.D.; Zamfir, C.L. Drug hypersensitivity reduction using encapsulation method with chitosan-cetirizine derivatives. Rev. De Chimie 2018, 69, 3731–3735. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamazo, C.; D’Amelio, C.; Gastaminza, G.; Ferrer, M.; Irache, J.M. Adjuvants for allergy immunotherapeutics. Hum. Vaccines Immunother. 2017, 13, 2416–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza Rebouças, J.; Esparza, I.; Ferrer, M.; Sanz, M.L.; Irache, J.M.; Gamazo, C. Nanoparticulate adjuvants and delivery systems for allergen immunotherapy. J. Biomed. Biotechnol. 2012, 474605. [Google Scholar] [CrossRef] [PubMed]
- Sikora, P.; Cendrowski, K.; Horszczaruk, E.; Mijowska, E. The effects of Fe3O4 and Fe3O4/SiO2 nanoparticles on the mechanical properties of cement mortars exposed to elevated temperatures. Constr. Build. Mater. 2018, 182, 441–450. [Google Scholar] [CrossRef]
- Xu, G.; Wang, X.L.; Liu, G.Z. Facile solvothermal synthesis of abnormal growth of one-dimensional ZnO nanostructures by ring-opening reaction of polyvinylpyrrolidone. Appl. Surf. Sci. 2015, 329, 137–142. [Google Scholar] [CrossRef]
- Tunesi, M.M.; Kalwar, N.H.; Soomro, R.A.; Karakus, S.; Jawaid, S.; Abro, M.I. Tartaric acid assisted in-situ growth of CuO nanostructures over ITO substrate for the electrocatalytic detection of Sudan I. Mat. Sci. Semicon. Proc. 2018, 75, 296–300. [Google Scholar] [CrossRef]
- Pehlivan, A.O.; Karakuş, S.; Karapınar, I.S.; Özbay, A.E.Ö.; Yazgan, A.U.; Taşaltın, N.; Kilislioğlu, A. Effect of Novel Synthesized Nanoeggshell on the Properties of Cementitious Composites. J. Adv. Concr. Tech. 2020, 18, 294–306. [Google Scholar] [CrossRef]
- Prasetyo, R.; Hasan, A.E.Z.; Siregar, R. Application of nanoparticle technology Trigona spp propolis from Bogor as an antibacterial Escherichia coli in vitro. Ekol 2011, 11, 36–43. [Google Scholar]
- Hasan, A.E.Z.; Artika, I.M.; Fahri, V.R.; Sari, N. Application of nanoparticle technology for medicine preparate (Antibiotic base on natural product, Propolis Trigona spp.). Chem. Prog. 2012, 5, 1–7. [Google Scholar]
- Chung, N.K.; Cho, Y.C.; Ha, C.S.; Kim, H.S. Hypoglycemic effects of nano powder propolis on streptozotocin-induced diabetic rats. Korean J. Vet. Serv. 2010, 33, 199–206. [Google Scholar]
- Satyanarayan, G.B.; Snigdha, M.; Yun, M.; Zhongzhen, N.; Craig, A.W.; Leonard, P.R.; Vickram, R. Cisplatin up-regulates the adenosine A1 receptor in the rat kidney. Eur. J. Pharmacol. 2002, 442, 251–264. [Google Scholar]
- Gulec, M.; Yılmaz, H.R.; Iraz, M.; Aglamıs, S.; Sogut, S. The effects of ginkgo biloba extract on plasma glutathion peroxidase, superoxide dismutase, adenosine deaminase and nitric oxide levels in cisplatin-induced nephrotoxicity. Turkiye Klinikleri J. Med. Sci. 2004, 24, 585–591. [Google Scholar]
- Malik, N.M.; Liu, Y.L.; Cole, N.; Sanger, G.J.; Andrews, P.L.R. Differential effects of dexamethasone, ondansetron and a tachykinin NK1 receptor antagonist (GR205171) on cisplatin-induced changes in behaviour, food intake, pica and gastric function in rats. Eur. J. Pharmacol. 2007, 555, 164–173. [Google Scholar] [CrossRef]
- Seven, İ.; Aksu, T.; Tatlı Seven, P. The effects of propolis and vitamin C supplemented feed on performance, nutrient utilization and carcass characteristics in broilers exposed to lead. Livest. Sci. 2012, 148, 10–15. [Google Scholar] [CrossRef]
- Shalmany, S.K.; Shivazad, M. The effect of diet propolis supplementation on Ross broiler chicks performance. Int. J. Poult. Sci. 2006, 5, 84–88. [Google Scholar]
- Kasala, E.R.; Bodduluru, L.N.; Barua, C.C.; Madhana, R.M.; Dahiya, V.; Budhani, M.K.; Mallugari, R.R.; Maramreddy, S.R.; Gogoi, R. Chemopreventive effect of chrysin, a dietary flavone against benzo(a)pyrene induced lung carcinogenesis in Swiss albino mice. Pharmacol. Rep. 2016, 68, 310–318. [Google Scholar] [CrossRef]
- Cetin, A.; Arslanbas, U.; Saraymen, B.; Canoz, O.; Ozturk, A.; Sagdic, O. Effects of grape seed extract and origanum onites essential oil on cisplatin-ınduced hepatotoxicity in rats. UHOD 2011, 21, 133–140. [Google Scholar] [CrossRef]
- Tatli Seven, P.; Yilmaz, S.; Seven, I.; Tuna Kelestemur, G. Effects of propolis in animals exposed oxidative stress. In Oxidative Stress-Environmental Induction and Dietary Antioxidants, 1st ed.; Volodymyr, I.L., Ed.; InTech: Rijeka, Croatia, 2012; Volume 13, pp. 267–288. [Google Scholar]
- Katanic, J.; Matic, S.; Pferschy-Wenzig, E.M.; Kretschmer, N.; Boroja, T.; Mihailovic, V.; Stankovic, V.; Stankovic, N.; Mladenovic, M.; Stanic, S.; et al. Filipendula ulmaria extracts attenuate cisplatin-induced liver and kidney oxidative stress in rats: In vivo investigation and LC-MS analysis. Food Chem. Toxicol. 2017, 99, 86–102. [Google Scholar] [CrossRef]
- Tatli Seven, P.; Gul Baykalir, B.; Parlak Ak, T.; Seven, I.; Basak, N.; Yaman, M. The protective effects of propolis and flunixin meglumine on feed intake, antioxidant status and histological parameters in liver and kidney tissues against excess copper in rats. Ankara Univ. Vet. Fak. 2018, 65, 395–406. [Google Scholar]
- Tatli Seven, P.; Seven, I.; Gul Baykalir, B.; Iflazoglu Mutlu, S.; Salem, A.Z.M. Nanotechnology and nano-propolis in animal production and health: An overview. Ital. J. Anim. Sci. 2018, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Seven, I.; Aksu, T.; Seven, P.T. The effects of propolis on biochemical parameters and activity of antioxidant enzymes in broilers exposed to lead-induced oxidative stress. Asian-Aust. J. Anim. Sci. 2010, 23, 1482–1489. [Google Scholar] [CrossRef]
- Chan, G.C.F.; Cheung, K.W.; Sze, D.M.Y. The immunomodulatory and anticancer properties of propolis. Clin. Rev. Allergy Immunol. 2013, 44, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Tatli Seven, P.; Yilmaz, S.; Seven, I.; Cerci, I.H.; Azman, M.A.; Yilmaz, M. Effects of propolis on selected blood indicators and antioxidant enzyme activities in broilers under heat stress. Acta Vet. Brno. 2009, 78, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.J.; Lien, Y.H.; Yu, Z.R. Supercritical fluid extractive fractionation study of the antioxidant activities of propolis. Food Chem. 2004, 86, 237–243. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Hosnuter, M.; Gurel, A.; Babuccu, O.; Armutcu, F.; Kargi, E.; Isikdemir, A. The effect of CAPE on lipid peroxidation and nitric oxide levels in the plasma of rats following thermal injury. Burns 2004, 30, 121–125. [Google Scholar] [CrossRef]
- Boroja, T.; Katanic, J.; Rosic, G.; Selakovic, D.; Joksimovic, J.; Misic, D.; Stankovic, V.; Jovicic, N.; Mihailovic, V. Summer savory (Satureja hortensis L.) extract: Phytochemical profile and modulation of cisplatin-induced liver, renal and testicular toxicity. Food Chem. Toxicol. 2018, 118, 252–263. [Google Scholar] [CrossRef]
- Rezvanfar, M.A.; Rezvanfar, M.A.; Shahverdi, A.R.; Ahmadi, A.; Baeeri, M.; Mohammadirad, A.; Abdollahi, M. Protection of cisplatin-induced spermatotoxicity, DNA damage and chromatin abnormality by selenium nano-particles. Toxicol. Appl. Pharmacol. 2013, 266, 356–365. [Google Scholar] [CrossRef]
- Abd-Elrazek, A.M.; El-Dash, H.A.; Said, N.I. The role of propolis against paclitaxel-induced oligospermia, sperm abnormality, oxidative stress and DNA damage in testes of male rats. Andrologia 2020, 52, e13394. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. Life or death decions by the Bcl-2 family. Trends Biochem. Sci. 2001, 26, 61–66. [Google Scholar] [CrossRef]
- Coskun, G.; Ozgur, H. Apoptoz ve Nekrozun Moleküler Mekanizması. ARŞİV 2011, 20, 145–158. [Google Scholar]
- Cho, H.J.; Kim, J.K.; Kim, K.D.; Yoon, H.K.; Cho, M.Y.; Park, Y.P.; Jeon, J.H.; Lee, E.S.; Byun, S.S.; Lim, H.M.; et al. Upregulation of Bcl-2 is associated with cisplatin-resistance via inhibition of Bax translocation in human bladder cancer cells. Cancer Lett. 2006, 237, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Tomar, A.; Vasisth, S.; Khan, S.I.; Malik, S.; Nag, T.C.; Arya, D.S.; Bhatia, J. Galangin ameliorates cisplatin induced nephrotoxicity in vivo by modulation of oxidative stress, apoptosis and inflammation through interplay of MAPK signaling cascade. Phytomedicine 2017, 34, 154–161. [Google Scholar] [CrossRef]
- Oršolić, N.; Car, N.; Lisičić, D.; Benković, V.; Knežević, A.H.; Dikić, D.; Petrik, J. Synergism between propolis and hyperthermal intraperitoneal chemotherapy with cisplatin on ehrlich ascites tumor in mice. J. Pharm. Sci. 2013, 102, 4395–4405. [Google Scholar] [CrossRef]
- Salem, M.M.; Donia, T.; Abu-Khudir, R.; Ramadan, H.; Ali, E.M.M.; Mohamed, T.M. Propolis potentiates methotrexate anticancer mechanism and reduces its toxic effects. Nutr. Cancer 2020, 72, 460–480. [Google Scholar] [CrossRef]
- Zheng, X.N.; Wang, X.W.; Li, L.Y.; Xu, Z.W.; Huang, H.Y.; Zhao, J.S.; Zhang, D.; Yin, X.; Sheng, J.; Tang, J.T. Pu-erh tea powder preventive effects on cisplatin-induced liver oxidative damage in Wistar rats. Asian Pac. J. Cancer Prev. 2014, 15, 7389–7394. [Google Scholar] [CrossRef] [Green Version]
- Seven, İ.; Baykalir, B.G.; Tatli Seven, P.; Dagoglu, G. The ameliorative effects of propolis against cyclosporine-A induced hepatotoxicity and nephrotoxicity in rats. Kafkas Univ. Vet. Fak. 2014, 20, 641–648. [Google Scholar]
- Coneac, G.; Gafiţanu, E.; Hădărugă, D.I.; Hădărugă, N.G.; Pînzaru, I.A.; Bandur, G.; Urşica, L.; Păunescu, V.; Gruia, A. Flavonoid contents of propolis from the west side of Romania and correlation with the antioxidant activity. Chem. Bull. Politehnica Univ. (Timisoara) 2008, 53, 56–60. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Wahile, A.; Mukherjee, K.; Saha, B.P.; Mukherjee, P.K. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J. Ethnopharmacol. 2006, 104, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; Hall, R.; De Vos, C.H.R. Changes in antioxidants and metabolite profiles during production of tomato paste. J. Agric. Food Chem. 2008, 56, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Nori, M.P.; Favaro-Trindade, C.S.; De Alencar, S.M.; Thomazini, M.; De Camargo Balieiro, J.C.; Castillo, C.J.C. Microencapsulation of propolis extract by complex coacervation. LWT-Food Sci. Technol. 2011, 44, 429–435. [Google Scholar] [CrossRef]
- Dhamodharan, G.; Mirunalini, S. Dose response study of agaricus bisporus (white button mushroom) and its encapsulated chitosan nanoparticles against 7,12-dimethylbenz(A)anthracene induced mammary carcinogenesis in female sprague-dawley rats. Int. J. Pharm. Pharm. Sci. 2012, 4, 348–354. [Google Scholar]
- Ong, T.H.; Chitra, E.; Ramamurthy, S.; Siddalingam, R.P.; Yuen, K.H.; Ambu, S.P.; Davamani, F. Chitosan-propolis nanoparticle formulation demonstrates anti-bacterial activity against Enterococcus faecalis biofilms. PLoS ONE 2017, 12, 1–22. [Google Scholar]
- Hussein, U.; Hassan, N.; Elhalwagy, M.; Zaki, A.; Abubakr, H.; Nagulapalli Venkata, K.; Bishayee, A. Ginger and propolis exert neuroprotective effects against monosodium glutamate-induced neurotoxicity in rats. Molecules 2017, 22, 1928. [Google Scholar] [CrossRef] [Green Version]
- El-Guendouz, S.; Lyoussi, B.; Lourenço, J.P.; da Costa, A.M.R.; Miguel, M.G.; Dias, C.B.; Faleiro, M.L. Magnetite nanoparticles functionalized with propolis against methicillin resistant strains of Staphylococcus aureus. J. Taiwan Inst. Chem. Eng. 2019, 102, 25–33. [Google Scholar] [CrossRef]
- Zancanela, D.C.; Funari, C.S.; Herculano, R.D.; Mello, V.M.; Rodrigues, C.M.; Borges, F.A.; Guastaldi, A.C. Natural rubber latex membranes incorporated with three different types of propolis: Physical-chemistry and antimicrobial behaviours. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 576–582. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Hu, Y.; You, J.; Higashisaka, K.; Nagano, K.; Tsutsumi, Y.; Gao, J. Chitosan nanoparticles and their tween 80 modified counterparts disrupt the developmental profile of zebrafish embryos. Int. J. Pharm. 2016, 515, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Sukmawati, A.; Utami, W.; Yuliani, R.; Da’i, M.; Nafarin, A. Effect of tween 80 on nanoparticle preparation of modified chitosan for targeted delivery of combination doxorubicin and curcumin analogue. IOP Conf. Series Mater. Sci. Eng. 2018, 311, 12024. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randal, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef]
- Goth, L.A. Simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [Green Version]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Expressing Cloned Genes for Protein Production, Purification, and Analysis. In Molecular Cloning: A Laboratory Manual, 4th ed.; Green, M.R., Sambrook, J., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012; Volume 19, pp. 1599–1625. [Google Scholar]
- Bass, J.J.; Wilkinson, D.J.; Rankin, D.; Phillips, B.E.; Szewczyk, N.J.; Smith, K.; Atherton, P.J. An overview of technical considerations for Western blotting applications to physiological research. Scand. J. Med. Sci. Sports 2017, 27, 4–25. [Google Scholar] [CrossRef] [PubMed]




| Days | Control | CP | Propolis | NP-10 | CP + Propolis | CP + NP-10 | CP + NP-30 | p | |
|---|---|---|---|---|---|---|---|---|---|
| BW | IW | 246.60 ± 3.94 | 247.75 ± 3.18 | 247.63 ± 13.42 | 245.33 ± 3.96 | 247.00 ± 1.48 | 243.93 ± 3.88 | 247.00 ± 3.63 | NS |
| 7. | 273.50 ± 8.75 | 260.44 ± 2.17 | 275.63 ± 13.09 | 270.67 ± 1.86 | 264.60 ± 4.56 | 260.36 ± 3.60 | 261.25 ± 3.32 | NS | |
| 14. | 291.30 ± 3.52 a,b | 272.44 ± 3.63 c | 295.25 ± 3.40 a | 299.08 ± 2.96 a | 280.20 ± 9.29 b,c | 276.64 ± 2.97 c | 277.50 ± 4.32 b,c | ** | |
| 21. | 312.80 ± 1.69 a | 288.81 ± 2.38 b | 314.25 ± 13.21 a | 314.08 ± 4.67 a | 310.70 ± 10.26 a | 290.50 ± 3.90 b | 294.81 ± 6.42 a,b | ** | |
| BWC | 1–7 | 3.84 ± 1.10 | 1.81 ± 0.29 | 4.00 ± 0.50 | 3.62 ± 0.66 | 2.52 ± 0.68 | 2.35 ± 0.35 | 2.04 ± 0.50 | NS |
| 8–14 | 2.54 ± 0.98 | 1.72 ± 0.40 | 2.80 ± 1.41 | 4.06 ± 0.62 | 2.23 ± 0.97 | 2.33 ± 0.50 | 2.32 ± 0.52 | NS | |
| 15–21 | 3.07 ± 0.52 | 2.34 ± 0.54 | 2.72 ± 1.49 | 2.14 ± 0.66 | 4.36 ± 1.17 | 1.98 ± 0.47 | 2.48 ± 0.76 | NS | |
| 1–21 | 3.15 ± 0.25 a,b | 1.95 ± 0.21 c | 3.18 ± 0.31 a,b | 3.28 ± 0.34 a | 3.03 ± 0.47 a,b | 2.22 ± 0.21 b,c | 2.28 ± 0.30 b,c | ** | |
| FI | 1–7 | 19.87 ± 0.86 | 19.59 ± 0.86 | 20.76 ± 0.47 | 19.89 ± 0.67 | 19.64 ± 1.08 | 19.33 ± 1.40 | 20.18 ± 0.91 | NS |
| 8–14 | 22.80 ± 0.52 | 19.67 ± 1.23 | 19.30 ± 0.36 | 22.00 ± 1.00 | 19.52 ± 0.75 | 19.14 ± 0.89 | 20.89 ± 1.23 | NS | |
| 15–21 | 24.50 ± 0.49 a | 20.49 ± 0.32 c | 23.87 ± 0.11 a,b | 23.70 ± 1.11 a,b | 23.64 ± 1.35 a,b | 22.07 ± 0.46 b,c | 23.81 ± 0.75 a,b | ** | |
| 1–21 | 22.39 ± 0.13 a | 19.92 ± 0.39 c | 21.31 ± 0.24 a,b,c | 21.87 ± 0.71 a,b | 20.93 ± 0.63 a,b,c | 20.18 ± 0.50 b,c | 21.63 ± 0.78 a,b,c | * |
| Control | CP | Propolis | NP-10 | CP + Propolis | CP + NP-10 | CP + NP-30 | p | |
|---|---|---|---|---|---|---|---|---|
| GLU (mg/dL) | 119.00 ± 1.58 d | 155.75 ± 4.33 a | 121.00 ± 4.23 d | 120.75 ± 0.85 d | 142.40 ± 1.17 b | 140.75 ± 2.10 bc | 130.40 ± 6.73 c,d | *** |
| AST (U/L) | 224.60 ± 8.61 b | 298.80 ± 13.93 a | 223.67 ± 12.65 b | 222.60 ± 10.32 b | 242.40 ± 8.85 b | 238.83 ± 11.26 b | 234.14 ± 17.20 b | ** |
| ALT (U/L) | 88.50 ± 3.74 b | 103.40 ± 4.26 a | 87.00 ± 5.19 b | 89.00 ± 1.78 a,b | 99.20 ± 9.23 a,b | 97.43 ± 4.60 a,b | 93.88 ± 2.01 a,b | * |
| ALP (U/L) | 289.75 ± 4.84 c | 355.40 ± 8.15 a | 282.50 ± 2.32 c | 285.75 ± 2.06 c | 312.20 ± 4.05 b | 313.75 ± 4.59 b | 311.50 ± 7.16 b | *** |
| TP (g/dL) | 6.13 ± 0.05 a,b | 5.57 ± 0.10 c | 6.10 ± 0.12 a,b | 6.26 ± 0.07 a | 5.78 ± 0.14 b,c | 5.86 ± 0.18 a,b,c | 5.88 ± 0.13 a,b,c | * |
| Alb (g/dL) | 3.62 ± 0.06 a,b | 3.27 ± 0.09 c | 3.63 ± 0.06 a,b | 3.75 ± 0.06 a | 3.48 ± 0.03 b | 3.52 ± 0.04 b | 3.54 ± 0.04 b | *** |
| Cre (mg/dL) | 0.28 ± 0.01 b,c | 0.33 ± 0.01 a | 0.28 ± 0.02 b,c | 0.25 ± 0.02 c | 0.31 ± 0.01 a,b | 0.31 ± 0.02 a,b | 0.30 ± 0.01 a,b | * |
| BUN (mg/dL) | 52.80 ± 1.85 b,c | 65.00 ± 0.53 a | 49.75 ± 1.38 c,d | 47.25 ± 0.63 d | 56.40 ± 0.93 b | 54.33 ± 1.45 b | 54.00 ± 1.29 b | *** |
| Control | CP | Propolis | NP-10 | CP + Propolis | CP + NP-10 | CP + NP-30 | p | ||
|---|---|---|---|---|---|---|---|---|---|
| Liver | MDA | 13.10 ± 0.77 c | 17.32 ± 0.74 a | 13.28 ± 0.77 b,c | 12.97 ± 0.49 c | 15.10 ± 0.41 b | 14.07 ± 0.67 b,c | 12.30 ± 0.24 c | *** |
| GSH | 2.23 ± 0.07 b,c | 1.80 ± 0.06 d | 2.21 ± 0.08 b,c | 2.32 ± 0.08 a,b | 2.06 ± 0.07 c | 2.21 ± 0.08 b,c | 2.55 ± 0.08 a | *** | |
| GSH-Px | 21.44 ± 1.84 b | 14.60 ± 0.69 c | 22.97 ± 1.25 a,b | 22.25 ± 1.57 a,b | 19.79 ± 2.47 b | 23.56 ± 1.01 a,b | 26.42 ± 1.22 a | *** | |
| CAT | 2.80 ± 0.16 a | 1.13 ± 0.05 d | 2.68 ± 0.27 a | 2.80 ± 0.15 a | 1.63 ± 0.04 c | 2.01 ± 0.08 b,c | 2.52 ± 0.17 a,b | *** | |
| Kidney | MDA | 21.32 ± 0.97 c,d | 33.53 ± 1.07 a | 20.41 ± 0.73 d | 20.76 ± 1.34 c,d | 27.82 ± 2.10 b | 24.07 ± 0.89 c | 20.73 ± 0.63 c,d | *** |
| GSH | 1.93 ± 0.08 b | 1.56 ± 0.08 c | 1.90 ± 0.05 b | 1.94 ± 0.08 b | 1.95 ± 0.06 a,b | 1.99 ± 0.09 a,b | 2.21 ± 0.06 a | *** | |
| GSH-Px | 30.43 ± 1.66 a,b | 17.78 ± 1.34 d | 26.46 ± 1.36 b,c | 27.01 ± 2.64 b,c | 23.00 ± 1.45 c | 28.99 ± 1.29 a,b | 33.86 ± 1.62 a | *** | |
| CAT | 1.79 ± 0.17 b | 1.00 ± 0.12 d | 1.86 ± 0.20 b | 2.16 ± 0.12 a,b | 1.40 ± 0.12 c | 1.78 ± 0.06 b | 2.27 ± 0.07 a | *** | |
| Nutritional Composition | % | Nutritional Composition | % |
|---|---|---|---|
| Dry matter 1 | 92.5 | Ether extract 1 | 3.21 |
| Crude ash 1 | 6.48 | Ca 2 | 0.89 |
| Crude protein 1 | 24.00 | P 2 | 0.98 |
| Crude cellulose 1 | 6.15 | Metabolizable energy (kcal/kg) 2 | 2650 |
| Content | mg/g |
|---|---|
| Total phenolic content, GAE | 17.18 ± 0.45 |
| Total flavonoid content, QE | 42.28 ± 1.23 |
| Total antioxidant capacity—CUPRAC, TEAC | 143.16 ± 1.31 |
| Total antioxidant capacity—DPPH, TEAC | 20.09 ± 1.31 |
| Phenolics | mg/g | Phenolics | mg/g |
|---|---|---|---|
| Caffeic acid | 0.17 ± 0.00 | Pinostrobin | 2.93 ± 0.03 |
| Vanillin | 0.23 ± 0.00 | Pinocembrin | 1.22 ± 0.15 |
| Ferulic acid | 0.36 ± 0.00 | Chrysin | 2.94 ± 0.07 |
| t-cinnamic acid | 3.95 ± 0.00 | Galangin | 0.09 ± 0.01 |
| Pinobanksin | 0.58 ± 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatli Seven, P.; Seven, I.; Karakus, S.; Iflazoglu Mutlu, S.; Arkali, G.; Muge Sahin, Y.; Kilislioglu, A. Turkish Propolis and Its Nano Form Can Ameliorate the Side Effects of Cisplatin, Which Is a Widely Used Drug in the Treatment of Cancer. Plants 2020, 9, 1075. https://doi.org/10.3390/plants9091075
Tatli Seven P, Seven I, Karakus S, Iflazoglu Mutlu S, Arkali G, Muge Sahin Y, Kilislioglu A. Turkish Propolis and Its Nano Form Can Ameliorate the Side Effects of Cisplatin, Which Is a Widely Used Drug in the Treatment of Cancer. Plants. 2020; 9(9):1075. https://doi.org/10.3390/plants9091075
Chicago/Turabian StyleTatli Seven, Pinar, Ismail Seven, Selcan Karakus, Seda Iflazoglu Mutlu, Gozde Arkali, Yesim Muge Sahin, and Ayben Kilislioglu. 2020. "Turkish Propolis and Its Nano Form Can Ameliorate the Side Effects of Cisplatin, Which Is a Widely Used Drug in the Treatment of Cancer" Plants 9, no. 9: 1075. https://doi.org/10.3390/plants9091075
APA StyleTatli Seven, P., Seven, I., Karakus, S., Iflazoglu Mutlu, S., Arkali, G., Muge Sahin, Y., & Kilislioglu, A. (2020). Turkish Propolis and Its Nano Form Can Ameliorate the Side Effects of Cisplatin, Which Is a Widely Used Drug in the Treatment of Cancer. Plants, 9(9), 1075. https://doi.org/10.3390/plants9091075