Fruit Morphology, Citrulline, and Arginine Levels in Diverse Watermelon (Citrullus lanatus) Germplasm Collections
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Plant Material
2.2.1. Cultivation
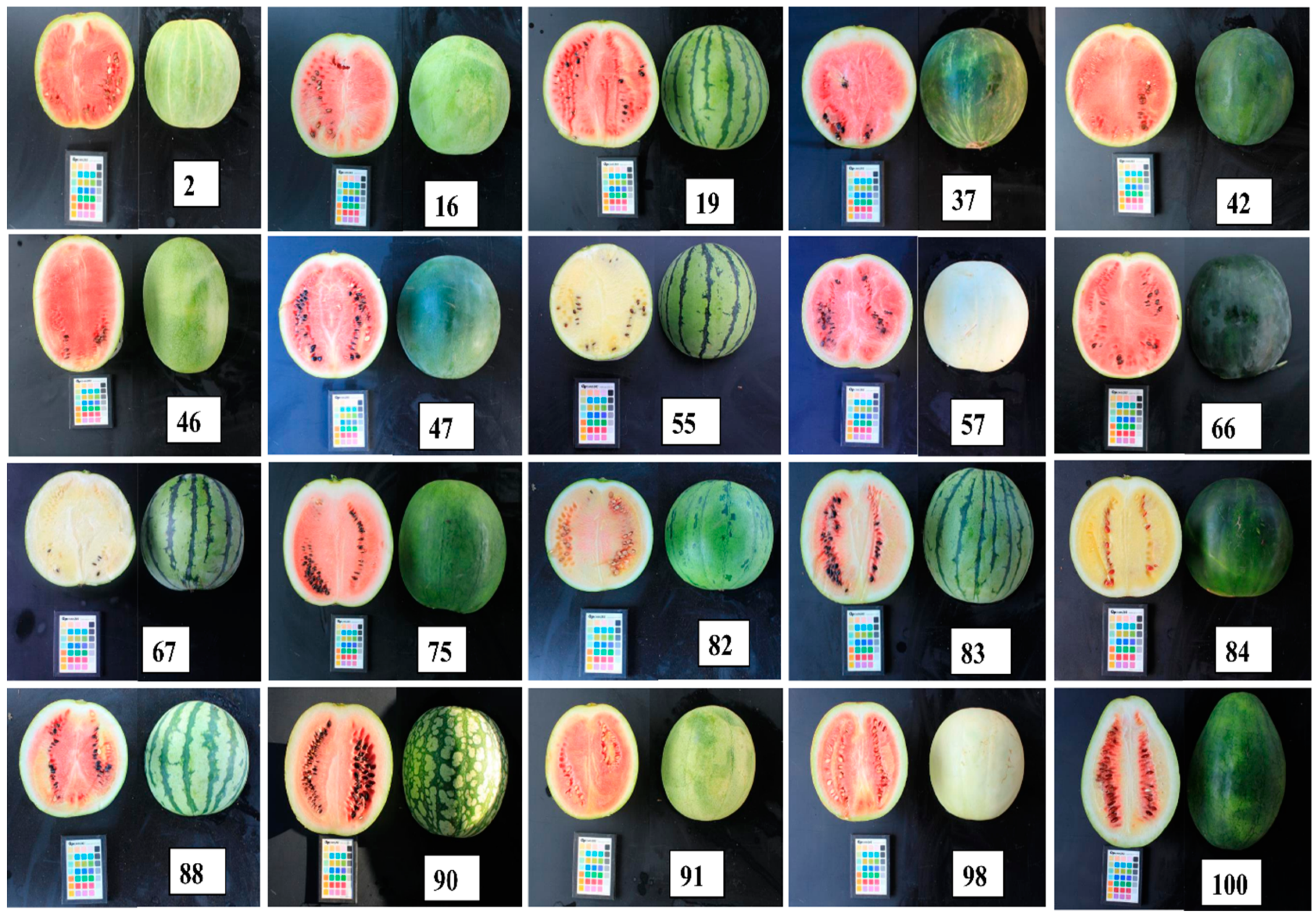
2.2.2. Sampling
2.2.3. Analysis of Morphological Properties
2.3. Extraction and Analysis of Citrulline and Arginine
2.4. Analysis of Citrulline Using Citrulline Assay Kit
2.5. Statistical Analysis
3. Results and Discussion
3.1. Fruit Morphological Characteristics
3.2. Analysis of Citrulline and Arginine Using HPLC and
3.2.1. Method Development and Validation
3.2.2. Determination of Citrulline and Arginine Content Using HPLC
3.2.3. Quantification of Citrulline Using Citrulline Assay Kit
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Park, Y.H.; Cho, S.K. Watermelon production and breeding in South Korea. Isr. J. Plant Sci. 2012, 60, 415–424. [Google Scholar]
- Wehner, T.C. Watermelon. In Vegetables I. Handbook of Plant Breeding; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; Volume 1, pp. 381–418. [Google Scholar]
- Perkins-Veazie, P.; Davis, A.; Collins, J.K. Watermelon: From dessert to functional food. Isr. J. Plant Sci. 2012, 60, 37–41. [Google Scholar]
- Liu, W.; King, S.R.; Zhao, S.; Cheng, Z.; Wan, X.; Yan, Z. Lycopene and citrulline contents in watermelon (Citrullus lanatus) fruit with different ploidy and changes during fruit development. Acta Hortic. 2010, 871, 543–550. [Google Scholar]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of citrulline in watermelon rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.R.; Fish, W.W.; Levi, A.; King, S.; Wehner, T.; Perkins-veazie, P. L-Citrulline levels in watermelon cultivars from three locations. Curcubit Genet. Coop. Rep. 2010, 39, 36–39. [Google Scholar]
- Akashi, K.; Mifune, Y.; Morita, K.; Ishitsuka, S.; Ishihara, T. Spatial accumulation pattern of citrulline and other nutrients in immature and mature watermelon fruits. J. Sci. Food Agric. 2016, 97, 479–487. [Google Scholar] [CrossRef]
- Hartman, J.L.; Wehner, T.C.; Ma, G.; Perkins-Veazie, P. Citrulline and arginine content of taxa of Cucurbitaceae. Horticulturae 2019, 5, 22. [Google Scholar] [CrossRef]
- Davis, A.R.; Webbber, C.L., III; Liu, W.; Perkins-veazie, P.; Levi, A.; King, S. Watermelon quality traits as affected by ploidy. HortScience 2013, 48, 1113–1118. [Google Scholar] [CrossRef]
- Akashi, K.; Miyake, C.; Yokota, A. Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 2001, 508, 438–442. [Google Scholar] [CrossRef]
- Fish, W.W. A reliable methodology for quantitative extraction of fruit and vegetable physiological amino acids and their subsequent analysis with commonly available HPLC systems. Food Nutr. Sci. 2012, 3, 863–871. [Google Scholar] [CrossRef]
- Mapelli, S.; Brambilla, I.; Bertani, A. Free amino acids in walnut kernels and young seedlings. Tree Physiol. 2001, 21, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Benazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Collins, J.K.; Perkins-Veazie, P.; Siddiq, M.; Dolan, K.D.; Kelly, K.A.; Heaps, C.L.; Meininger, C.J. Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in zucker diabetic fatty rats. J. Nutr. 2007, 137, 2680–2685. [Google Scholar] [CrossRef]
- Flynn, N.E.; Meininger, C.J.; Haynes, T.E.; Wu, G. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed. Pharmacother. 2002, 56, 427–438. [Google Scholar] [CrossRef]
- Young, M.; Beidler, J.; Hooshmand, S.; Figueroa, A.; Kern, M. Watermelon and L-arginine consumption improve serum lipid profile and reduce inflammation and oxidative stress by altering gene expression in rats fed an atherogenic diet. Nutr. Res. 2018, 58, 46–54. [Google Scholar]
- Clarkson, P.; Adams, M.R.; Powe, A.J.; Donald, A.E.; McCredie, R.; Robinson, J.; McCarthy, S.N.; Keech, A.; Celermajer, D.S.; Deanfield, J.E. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolemic young adults. J. Clin. Invest. 1996, 97, 1989–1994. [Google Scholar] [CrossRef]
- Chin-Dusting, J.P.; Alexander, C.T.; Arnold, P.J.; Hodgson, W.C.; Lux, A.S.; Jennings, G.L. Effects of in vivo and in vitro L-arginine supplementation on healthy human vessels. J. Cardiovasc. Pharmacol. 1996, 28, 158–166. [Google Scholar] [CrossRef]
- Tarazona-Diaz, M.P.; Alacid, F.; Mart, I.; Aguayo, E. Watermelon juice: Potential functional drink for sore muscle relief in athletes. J. Agric. Food Chem. 2013, 61, 7522–7528. [Google Scholar] [CrossRef]
- Szamosi, C.; Solmaz, I.; Sari, N.; Barsony, C. Morphological characterization of Hungarian and Turkish watermelon (Citrullus lanatus (Thunb.) Matsum. et Nakai) genetic resources. Genet. Resour. Crop Evol. 2009, 56, 1091–1105. [Google Scholar] [CrossRef]
- Solmaz, I.; Sari, N. Characterization of watermelon (Citrullus lanatus) accessions collected from Turkey for morphological traits. Genet. Resour. Crop Evol. 2009, 56, 173–188. [Google Scholar] [CrossRef]
- Huh, Y.C.; Choi, H.S.; Solmaz, I.; Sari, N.; Kim, S. Morphological characterization of Korean and Turkish watermelon germplasm. Korean J. Agric. Sci. 2014, 41, 309–314. [Google Scholar] [CrossRef]
- Davis, A.R.; Webber, C.L.; Fish, W.W.; Wehner, T.C.; King, S.; Perkins-veazie, P. L-Citrulline levels in watermelon cultigens tested in two environments. HortScience 2011, 46, 1572–1575. [Google Scholar] [CrossRef]
- International Union for the Protection of New Varieties of Plants (UPOV) Watermelon, TG/142/5. Guidelines for the Conduct of Tests for Distinctness, uniformity and stabability. Available online: http://www.upov.int/edocs/tgdocs/en/tg142.pdf (accessed on 1 April 2020).
- Sari, N.; Solmaz, I.; Yetisir, H.; Unlu, H. Watermelon genetic resources in Turkey and their characteristics. Acta Hortic. 2007, 731, 433–438. [Google Scholar] [CrossRef]
- Wehner, T.C.; Naegele, R.P.; Perkins-Veazie, P. Heritablity and genetic variance components associated with citrulline, arginine, and lycopene conetent in diverse watermelon cultigens. HortScience 2017, 52, 936–940. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Murthy, K.N.C.; Patil, B.S. Rapid HPLC-UV method for quantification of L-citrulline in watermelon and its potential role on smooth muscle relaxation markers. Food Chem. 2011, 127, 240–248. [Google Scholar] [CrossRef]
- Ridwan, R.; Razak, H.R.A.; Adenan, M.I.; Saad, W.M.M. Development of isocratic RP-HPLC method for separation and quantification of L-citrulline and L-arginine in watermelons. Int. J. Anal. Chem. 2018, 2018, 4798530. [Google Scholar] [CrossRef]
- Borges, E.M. Silica, hybrid silica, hydride silica and non-silica stationary phases for liquid chromatography. J. Chromatogr. Sci. 2015, 53, 580–597. [Google Scholar] [CrossRef]
- Soteriou, G.A.; Siomos, A.S.; Gerasopoulos, D.; Rouphael, Y.; Georgiadou, S.; Kyriacou, M.C. Biochemical and histological contributions to textural changes in watermelon fruit modulated by grafting. Food Chem. 2017, 237, 133–140. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Sun, H.; Salse, J.; Lucas, W.J.; Zhang, H.; Zheng, Y.; Mao, L.; Ren, Y.; Wang, Z.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 2013, 45, 51–60. [Google Scholar] [CrossRef]
- Fish, W.W. The expression of citrulline and other members of the arginine metabolic family in developing watermelon fruit. Int. J. Agric. Innov. Res. 2014, 2, 1473–2319. [Google Scholar]
- Song, Q.; Joshi, M.; Dipiazza, J.; Joshi, V. Functional relevance of citrulline in the vegetative tissues of watermelon during abiotic stresses. Front. Plant Sci. 2020, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Dasgan, H.Y.; Kusvuran, S.; Abak, K.; Leport, L.; Larher, F.; Bouchereau, A. The relationship between citrulline accumulation and salt tolerance during the vegetative growth of melon. Plant Soil Environ. 2009, 55, 51–57. [Google Scholar] [CrossRef]
- Hartman, J.L.; Perkins-veazie, P.; Wehner, T.C. Citrulline and arginine are moderately heritable in two red-fleshed watermelon populations. HortScience 2019, 54, 200–205. [Google Scholar] [CrossRef]
- Park, C.J.; Shaughnessy, M.P.; Aremenia, S.J.; Cowles, R.A. Serum citrulline levels exhibit circadian variation and fluctuations in relation to food intake in mice. Gastrocenterol. Res. 2019, 12, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Yang, K.; Mao, J.Y.; Shen, W.F.; Lu, L.; Wu, Q.H.; Wang, Y.P.; Wu, L.P.; Zhang, R.Y. Cyanate-impaired angiogenesis: Association with poor coronary collateral growth in patients with stable angina and chronic total occlusion. J. Am. Heart Assoc. 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Muñoz-cruz, S.; Gomez-garcía, A.; Matadamas-martínez, F.; Alvarado-torres, J.A.; Meza-Cervantez, P.; Arriaga-Pizano, L.; Yepez-Mulia, L. Giardia lamblia: Identification of molecules that contribute to direct mast cell activation. Parasitol. Res. 2018, 117, 2555–2567. [Google Scholar] [CrossRef]
- Inatomi, H.; Sasaki, T.; Suyama, Y.; Inukai, F. Meiji Daigaku, Nogakubu Kenkyu Hokoku. Bull. Chem. Soc. Jpn. 2007, 80, 2413–2417. [Google Scholar]
- Kameya, M.; Asano, Y. Enzyme and microbial technology rapid enzymatic assays for L-citrulline and L-arginine based on the platform of pyrophosphate detection. Enzyme Microb. Technol. 2014, 57, 36–41. [Google Scholar] [CrossRef]
- Knipp, M.; Vasak, M. A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal. Biochem. 2000, 286, 257–264. [Google Scholar] [CrossRef]
- Sugawara, K.; Yoshizawa, Y.; Tzeng, S.; Epstein, W.L.; Fukuyama, K. Colorimetric determination of citrulline residues in proteins. Anal. Biochem. 1998, 265, 92–96. [Google Scholar] [CrossRef]




| No | Trait | Description | Class | n | % | Min/Max/Ave * |
|---|---|---|---|---|---|---|
| 1 | Fruit shape in longitudinal section | Circular | 1 | 63 | 60.6 | - |
| Broad elliptic | 2 | 30 | 28.8 | - | ||
| Medium elliptic | 3 | 9 | 8.7 | - | ||
| Narrow elliptic | 4 | 2 | 1.9 | - | ||
| 2 | Ground color of skin | White | 1 | 6 | 5.7 | - |
| Yellow | 2 | 0 | 0.0 | - | ||
| Green | 3 | 99 | 94.3 | - | ||
| 3 | Intensity of green color of skin | Very light | 1 | 2 | 1.9 | - |
| Very light to light | 2 | 5 | 4.8 | - | ||
| Light | 3 | 3 | 2.9 | - | ||
| Light to medium | 4 | 6 | 5.7 | - | ||
| Medium | 5 | 18 | 17.1 | - | ||
| Medium to dark | 6 | 21 | 20.0 | - | ||
| Dark | 7 | 27 | 25.7 | - | ||
| Dark to very dark | 8 | 13 | 12.4 | - | ||
| Very dark | 9 | 10 | 9.5 | - | ||
| 4 | Fruit shape of apical part | Truncate | 1 | 52 | 49.5 | - |
| Little rounded | 2 | 43 | 41.0 | - | ||
| Rounded | 3 | 9 | 8.6 | - | ||
| Little acute | 4 | 1 | 1.0 | - | ||
| Acute | 5 | 0 | 0.0 | - | ||
| 5 | Grooving distribution of fruit | Absent | 1 | 85 | 81.0 | - |
| At basal half | 2 | 1 | 1.0 | - | ||
| At apical half | 3 | 0 | 0.0 | - | ||
| On whole fruit | 4 | 19 | 18.1 | - | ||
| 6 | Conspicuousness of stripes | Inconspicuous | 0 | 35 | 33.3 | - |
| Conspicuous | 1 | 70 | 66.7 | - | ||
| 7 | Main color of flesh | White | 1 | 0 | 0.0 | - |
| Yellow | 2 | 5 | 4.8 | - | ||
| Orange | 3 | 4 | 3.8 | - | ||
| Pink | 4 | 30 | 28.6 | - | ||
| Pink/red | 5 | 37 | 35.2 | - | ||
| Red | 6 | 29 | 27.6 | - | ||
| 8 | Size of pistil scar (mm) | 2.0/32.6/14.1 | ||||
| 9 | Width of stripes (mm) | 0.0/29.8/8.4 | ||||
| 10 | Weight of fruit (kg) | 3.2/10.4/5.2 | ||||
| 11 | Length of fruit (cm) | 17.0/48.4/23.3 | ||||
| 12 | Width of fruit(cm) | 17.3/23.2/20.7 | ||||
| 13 | Thickness of outer layer of the pericarp (mm) | 4.6/22.6/10.6 | ||||
| 14 | Soluble solids content (Brix) | 5.1/12.3/8.5 | ||||
| Trait | SPS | WS | WF | LF | WIF | TP | SSC | Citrulline | Arginine | Citrulline # |
|---|---|---|---|---|---|---|---|---|---|---|
| WS | 0.020 | - | ||||||||
| WF | −0.362 ** | 0.144 | - | |||||||
| LF | −0.662 ** | 0.052 | 0.737 ** | - | ||||||
| WIF | 0.351 ** | 0.156 | 0.439 ** | -0.204 * | - | |||||
| TP | 0.094 | 0.197 * | 0.174 | 0.191 | 0.146 | - | ||||
| SSC | −0.013 | 0.142 | −0.026 | −0.094 | 0.046 | −0.419 ** | - | |||
| Citrulline | 0.191 | 0.014 | −0.235 * | −0.203 * | −0.161 | −0.083 | −0.375 ** | - | ||
| Arginine | 0.043 | −0.289 ** | 0.072 | 0.084 | -0.052 | 0.120 | −0.375 ** | 0.283 ** | - | |
| Citrulline # | 0.116 | 0.004 | -0.223 * | −0.150 | −0.218 * | −0.041 | −0.351 ** | 0.919 ** | 0.318 ** | - |
| Citrulline + Arginine | 0.172 | −0.103 | −0.166 | −0.133 | −0.152 | −0.021 | −0.452 | 0.926 | 0.624 | 0.874 |
| S/No | Origin | IT NO/ Variety Name | Citrulline (HPLC) | Arginine (HPLC) | Citrulline (CAK) |
|---|---|---|---|---|---|
| 1 | KOR | IT104713 | 22.85 ± 1.67 i-s | 12.37 ± 0.48 c-m | 26.39 ± 0.69 e-r |
| 2 | TUR | IT119709 | 44.30 ± 0.49 ab | 9.51 ± 0.27 e-q | 39.54 ± 0.54 a-c |
| 3 | ITA | IT119743 | 30.03 ± 0.06 c-r | 8.51 ± 0.16 f-r | 28.46 ± 0.70 c-q |
| 4 | TWN | IT120006 | 28.52 ± 2.01 c-r | 9.48 ± 0.26 e-q | 25.23 ± 0.35 f-r |
| 5 | TWN | IT120008 | 27.16 ± 0.48 d-s | 11.64 ± 0.18 c-n | 27.29 ± 0.30 e-q |
| 6 | Unknown | IT138188 | 22.26 ± 0.39 i-s | 8.37 ± 0.18 f-r | 23.46 ± 0.45 h-r |
| 7 | CHN | IT160392 | 26.04 ± 0.59 e-s | 7.62 ± 0.32 h-s | 29.42 ± 0.24 c-q |
| 8 | Unknown | IT174803 | 28.62 ± 0.51 c-r | 6.34 ± 0.09 m-s | 26.75 ± 0.20 e-r |
| 9 | Unknown | IT185447 | 20.39 ± 0.42 l-s | 6.61 ± 0.36 m-s | 21.76 ± 0.15 j-r |
| 10 | Unknown | IT185456 | 18.10 ± 0.17 p-t | 4.39 ± 0.34 p-s | 19.72 ± 0.20 o-r |
| 11 | USA | IT190057 | 21.92 ± 0.63 j-s | 12.18 ± 0.12 c-m | 22.57 ± 0.25 h-r |
| 12 | USA | IT190058 | 6.90 ± 0.12 t | 1.76 ± 0.19 s | 6.54 ± 0.28 s |
| 13 | UKR | IT190059 | 25.22 ± 0.53 g-s | 14.39 ± 0.33 c-g | 23.64 ± 0.30 h-r |
| 14 | TKM | IT190077 | 21.52 ± 0.26 j-s | 11.54 ± 0.31 c-n | 20.19 ± 0.11 n-r |
| 15 | KGZ | IT190084 | 29.17 ± 1.30 c-r | 9.59 ± 0.3 e-q | 24.99 ± 0.59 g-r |
| 16 | TJK | IT190110 | 33.45 ± 0.98 b-n | 13.50 ± 0.43 c-j | 32.51 ± 0.16 a-j |
| 17 | TJK | IT190116 | 23.51 ± 0.17 i-s | 10.74 ± 0.18 d-p | 23.50 ± 0.51 h-r |
| 18 | KAZ | IT190123 | 25.96 ± 0.43 e-s | 7.66 ± 0.22 h-s | 28.22 ± 0.06 d-q |
| 19 | TKM | IT190135 | 33.46 ± 1.29 b-n | 12.39 ± 0.18 c-m | 31.98 ± 0.72 b-l |
| 20 | BRA | IT190141 | 26.63 ± 0.73 e-s | 6.36 ± 0.1 m-s | 27.45 ± 0.20 d-q |
| 21 | RUS | IT190146 | 23.54 ± 0.66 i-s | 6.47 ± 0.19 l-s | 25.10 ± 0.69 g-r |
| 22 | RUS | IT190148 | 19.31 ± 0.51o-s | 8.47 ± 0.08 f-r | 21.51 ± 0.47 j-r |
| 23 | Unknown | IT190151 | 26.54 ± 0.76 e-s | 11.81 ± 0.14 c-n | 26.24 ± 0.39 e-r |
| 24 | RUS | IT199769 | 31.47 ± 0.24 b-p | 11.96 ± 0.04 c-n | 28.79 ± 0.50 c-q |
| 25 | RUS | IT199772 | 29.22 ± 0.58 c-r | 8.37 ± 0.18 f-r | 27.45 ± 0.32 d-q |
| 26 | RUS | IT199773 | 20.57 ± 0.57 l-s | 10.07 ± 0.19 d-q | 20.84 ± 0.10 l-r |
| 27 | RUS | IT199788 | 33.00 ± 0.38 b-o | 6.76 ± 0.18 k-s | 31.23 ± 0.51 c-n |
| 28 | RUS | IT199796 | 24.75 ± 0.75 g-s | 8.56 ± 0.2 f-r | 26.89 ± 0.45 e-r |
| 29 | UKR | IT199805 | 20.76 ± 0.41 k-s | 6.52 ± 0.31 l-s | 22.56 ± 0.38 h-r |
| 30 | UKR | IT199806 | 21.80 ± 0.80 j-s | 8.53 ± 0.33 f-r | 26.55 ± 0.36 e-r |
| 31 | KAZ | IT199814 | 25.83 ± 0.43 f-s | 8.89 ± 0.18 e-r | 27.30 ± 0.17e-q |
| 32 | UZB | IT199823 | 24.65 ± 0.48 g-s | 8.34 ± 0.05 f-r | 26.50 ± 0.32 e-r |
| 33 | NPL | IT200493 | 28.08 ± 0.15 c-r | 7.53 ± 0.24 h-s | 25.58 ± 0.52 e-r |
| 34 | PHL | IT201722 | 29.63 ± 0.51 c-r | 9.50 ± 0.36 e-q | 28.51 ± 0.39 c-q |
| 35 | UZB | IT202998 | 19.65 ± 0.20 n-s | 8.61 ± 0.12 f-r | 21.76 ± 0.48 j-r |
| 36 | RUS | IT203017 | 26.38 ± 0.30 e-s | 10.14 ± 0.12 d-q | 25.79 ± 0.08 e-r |
| 37 | RUS | IT203019 | 34.73 ± 0.62 b-k | 11.61 ± 0.41 c-n | 32.04 ± 0.63 b-l |
| 38 | KAZ | IT203029 | 24.01 ± 0.52 i-s | 9.07 ± 0.05 e-q | 33.81 ± 0.08 a-h |
| 39 | UZB | IT203034 | 26.09 ± 1.55 e-s | 8.87 ± 0.03 e-r | 26.55 ± 0.91 e-r |
| 40 | RUS | IT203037 | 30.32 ± 0.49 c-q | 10.24 ± 0.11 d-q | 27.28 ± 0.20 e-q |
| 41 | UZB | IT203049 | 31.44 ± 2.27 b-p | 7.36 ± 0.37 i-s | 31.32 ± 0.29 c-n |
| 42 | UZB | IT203067 | 34.11 ± 2.50 b-l | 10.78 ± 0.11 d-p | 35.26 ± 0.26 a-g |
| 43 | AZE | IT203072 | 31.82 ± 3.32 b-p | 9.60 ± 0.07 e-q | 32.48 ± 0.38 a-k |
| 44 | Unknown | IT203627 | 32.79 ± 0.45 b-o | 11.40 ± 0.35 c-n | 29.34 ± 0.19 c-q |
| 45 | MNG | IT204167 | 25.98 ± 1.18 e-s | 11.53 ± 0.11 c-n | 25.81 ± 0.58 e-r |
| 46 | Unknown | IT208441 | 30.10 ± 1.30 c-r | 15.28 ± 0.3bcde | 30.39 ± 0.51 c-p |
| 47 | UZB | IT213903 | 33.22 ± 1.88 b-o | 16.50 ± 0.18abcd | 31.01 ± 0.24 c-o |
| 48 | Unknown | IT216860 | 21.20 ± 2.83 j-s | 9.13 ± 0.36 e-q | 21.57 ± 0.24 j-r |
| 49 | Unknown | IT251845 | 33.38 ± 1.62 b-n | 9.36 ± 0.48 e-q | 32.59 ± 0.39 a-j |
| 50 | Unknown | IT251849 | 34.91 ± 1.48 b-j | 8.66 ± 0.31 f-r | 31.23 ± 0.52 c-n |
| 51 | Unknown | IT251851 | 23.08 ± 0.93 i-s | 7.38 ± 0.14 i-s | 23.48 ± 0.34 h-r |
| 52 | RUS | IT251860 | 35.95 ± 2.25 b-i | 7.52 ± 0.12 h-s | 31.60 ± 0.36 b-m |
| 53 | USA | IT271064 | 20.89 ± 1.09 k-s | 7.34 ± 0.18 j-s | 20.54 ± 0.09 m-r |
| 54 | CHN | IT294452 | 24.85 ± 1.61 g-s | 5.58 ± 0.36 n-s | 23.46 ± 0.56 h-r |
| 55 | JPN | IT302244 | 39.30 ± 0.84 b-f | 9.12 ± 0.22 e-q | 36.74 ± 0.54 a-e |
| 56 | UZB | IT305108 | 26.65 ± 1.63 e-s | 13.98 ± 0.05 c-h | 24.53 ± 0.19 g-r |
| 57 | RUS | IT321060 | 32.52 ± 1.86 b-o | 12.97 ± 0.15 c-l | 33.53 ± 0.39 a-i |
| 58 | UZB | IT321075 | 24.79 ± 1.53 g-s | 8.52 ± 0.10 f-r | 22.61 ± 0.49 h-r |
| 59 | Unknown | IT32839 | 23.09 ± 0.45 i-s | 4.70 ± 0.21 o-s | 21.11 ± 0.62 k-r |
| 60 | URY | IT119741 | 16.94 ± 0.42 q-t | 6.37 ± 0.23 m-s | 22.47 ± 0.71 h-r |
| 61 | RUS | IT199776 | 31.57 ± 2.04 b-p | 9.80 ± 0.03 e-q | 31.09 ± 0.37 c-o |
| 62 | RUS | IT199783 | 27.11 ± 3.47 d-s | 14.90 ± 0.08 c-f | 28.15 ± 0.32 d-q |
| 63 | UKR | IT199804 | 14.00 ± 0.22 st | 9.69 ± 0.19 e-q | 15.75 ± 0.20 r |
| 64 | USA | IT199834 | 25.91 ± 0.98 f-s | 13.93 ± 0.26 c-i | 23.2 ± 0.52 h-r |
| 65 | CHN | 803617 | 26.82 ± 0.70 d-s | 8.42 ± 0.18 f-r | 26.13 ± 0.54 e-r |
| 66 | RUS | 805656 | 28.20 ± 0.60 c-r | 17.48 ± 0.21 a-c | 26.35 ± 0.34 e-r |
| 67 | JPN | 807364 | 41.38 ± 2.12 a-c | 6.63 ± 0.39 l-s | 35.81 ± 1.00 a-g |
| 68 | KOR | 906976 | 22.90 ± 0.90 i-s | 9.94 ± 0.02 e-q | 21.04 ± 0.44 l-r |
| 69 | Unknown | 908581 | 24.79 ± 0.93 g-s | 8.48 ± 0.11 f-r | 22.75 ± 0.58 h-r |
| 70 | UZB | 908835 | 22.02 ± 1.38 i-s | 13.41 ± 0.11 c-j | 20.42 ± 0.29 m-r |
| 71 | Unknown | K004668 | 26.78 ± 1.18 d-s | 12.13 ± 0.08 c-n | 25.69 ± 0.40 e-r |
| 72 | USA | K012424 | 28.67 ± 1.14 c-r | 11.00 ± 0.29 d-o | 28.54 ± 0.26 c-q |
| 73 | KOR | K038117 | 24.05 ± 0.67 i-s | 8.44 ± 0.12 f-r | 21.33 ± 0.46 j-r |
| 74 | IND | K192260 | 29.26 ± 1.62 c-r | 11.74 ± 0.14 c-n | 29.85 ± 0.36 c-q |
| 75 | IND | K192264 | 38.19 ± 0.67 b-h | 20.69 ± 0.23 ab | 42.06 ± 0.29 ab |
| 76 | TUR | K192296 | 19.91 ± 0.54 m-s | 9.70 ± 0.15 e-q | 20.97 ± 0.31 l-r |
| 77 | TUR | K192319 | 26.98 ± 0.64 d-s | 7.56 ± 0.21 h-s | 25.94 ± 0.52 e-r |
| 78 | TUR | K192321 | 30.68 ± 0.70 c-q | 8.45 ± 0.35 f-r | 30.89 ± 0.74 c-p |
| 79 | TUR | K192324 | 25.02 ± 0.68 g-s | 11.37 ± 0.23c-n | 27.83 ± 0.82 d-q |
| 80 | TUR | K192338 | 22.53 ± 0.54 i-s | 12.33 ± 0.15 c-m | 23.69 ± 0.62 h-r |
| 81 | TUR | K192352 | 26.08 ± 0.38 e-s | 7.87 ± 0.19 g-s | 25.40 ± 0.25 f-r |
| 82 | TUR | K192365 | 52.06 ± 0.59 a | 8.46 ± 0.20 f-r | 42.79 ± 0.48 a |
| 83 | TUR | K192370 | 40.53 ± 0.51 b-d | 12.25 ± 0.33 c-m | 36.51 ± 0.46 a-f |
| 84 | TUR | K192373 | 32.36 ± 0.34 b-o | 13.26 ± 0.30 c-k | 29.78 ± 0.29 c-q |
| 85 | TUR | K192378 | 28.84 ± 0.30 c-r | 11.10 ± 0.07 c-o | 24.97 ± 0.66 g-r |
| 86 | TUR | K192379 | 24.51 ± 1.04 h-s | 10.45 ± 0.15 d-p | 22.08 ± 0.23 j-r |
| 87 | TUR | K192381 | 28.33 ± 0.47 c-r | 10.43 ± 0.18 d-p | 26.02 ± 0.18 e-r |
| 88 | TUR | K192386 | 38.42 ± 0.86 b-g | 10.00 ± 0.18 e-q | 35.47 ± 0.30 a-g |
| 89 | TUR | K192390 | 33.35 ± 0.58 b-n | 8.57 ± 0.20 f-r | 30.07 ± 0.61 c-p |
| 90 | TUR | K192394 | 39.76 ± 0.87 b-e | 7.84 ± 0.13 g-s | 38.56 ± 0.28 a-d |
| 91 | TUR | K192397 | 33.05 ± 0.09 b-o | 11.82 ± 0.04 c-n | 29.02 ± 0.09 c-q |
| 92 | TUR | K192403 | 26.51 ± 0.84 e-s | 12.39 ± 0.36 c-m | 25.60 ± 0.52 e-r |
| 93 | TUR | K192432 | 19.80 ± 0.53 n-s | 7.54 ± 0.24 h-s | 22.67 ± 0.45 h-r |
| 94 | TUR | K192444 | 25.04 ± 0.54 g-s | 9.24 ± 0.06 e-q | 25.64 ± 0.27 e-r |
| 95 | TUR | K192446 | 33.79 ± 0.36 b-m | 6.50 ± 0.35 l-s | 30.68 ± 0.36 c-p |
| 96 | TUR | K192467 | 33.03 ± 0.42 b-o | 7.56 ± 0.33 h-s | 31.21 ± 0.60 c-n |
| 97 | TUR | K192469 | 25.41 ± 0.45 g-s | 8.89 ± 0.22 e-r | 25.78 ± 0.22 e-r |
| 98 | IRQ | K192471 | 31.14 ± 0.46 b-p | 13.81 ± 0.26 c-j | 28.95 ± 0.94 c-q |
| 99 | TUR | K192502 | 16.19 ± 0.61 r-t | 3.82 ± 0.25 q-s | 18.57 ± 0.38 qr |
| 100 | TUR | K192504 | 26.91 ± 0.34 d-s | 21.25 ± 0.11 a | 28.26 ± 0.51 d-q |
| 101 | KOR | Speedggul | 22.07 ± 0.78 i-s | 8.50 ± 0.19 f-r | 25.23 ± 0.40 f-r |
| 102 | KOR | Sambokggul | 25.70 ± 0.23 f-s | 8.44 ± 0.15 f-r | 29.67 ± 0.22 c-q |
| 103 | KOR | Seo Tae Ja | 24.58 ± 0.86 g-s | 9.39 ± 0.31 e-q | 26.53 ± 0.33 e-r |
| 104 | KOR | Uriggul | 23.88 ± 0.47 i-s | 10.61 ± 0.35 d-p | 28.85 ± 0.52 c-q |
| 105 | KOR | Newkkokkoma | 19.50 ± 0.43 n-s | 2.54 ± 0.03 rs | 19.62 ± 0.34 p-r |
| 106 | KOR | Lycofreshi | 18.33 ± 0.31 p-t | 9.39 ± 0.13 e-q | 22.35 ± 0.35 i-r |
| 107 | KOR | Norangsambokggul | 23.85 ± 0.95 i-s | 11.46 ± 0.2 c-n | 30.13 ± 0.56 c-p |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assefa, A.D.; Hur, O.-S.; Ro, N.-Y.; Lee, J.-E.; Hwang, A.-J.; Kim, B.-S.; Rhee, J.-H.; Yi, J.-Y.; Kim, J.-H.; Lee, H.-S.; et al. Fruit Morphology, Citrulline, and Arginine Levels in Diverse Watermelon (Citrullus lanatus) Germplasm Collections. Plants 2020, 9, 1054. https://doi.org/10.3390/plants9091054
Assefa AD, Hur O-S, Ro N-Y, Lee J-E, Hwang A-J, Kim B-S, Rhee J-H, Yi J-Y, Kim J-H, Lee H-S, et al. Fruit Morphology, Citrulline, and Arginine Levels in Diverse Watermelon (Citrullus lanatus) Germplasm Collections. Plants. 2020; 9(9):1054. https://doi.org/10.3390/plants9091054
Chicago/Turabian StyleAssefa, Awraris Derbie, On-Sook Hur, Na-Young Ro, Jae-Eun Lee, Ae-Jin Hwang, Bich-Saem Kim, Ju-Hee Rhee, Jung-Yoon Yi, Ji-Hyun Kim, Ho-Sun Lee, and et al. 2020. "Fruit Morphology, Citrulline, and Arginine Levels in Diverse Watermelon (Citrullus lanatus) Germplasm Collections" Plants 9, no. 9: 1054. https://doi.org/10.3390/plants9091054
APA StyleAssefa, A. D., Hur, O.-S., Ro, N.-Y., Lee, J.-E., Hwang, A.-J., Kim, B.-S., Rhee, J.-H., Yi, J.-Y., Kim, J.-H., Lee, H.-S., Sung, J.-S., Kim, M.-K., & Noh, J.-J. (2020). Fruit Morphology, Citrulline, and Arginine Levels in Diverse Watermelon (Citrullus lanatus) Germplasm Collections. Plants, 9(9), 1054. https://doi.org/10.3390/plants9091054





