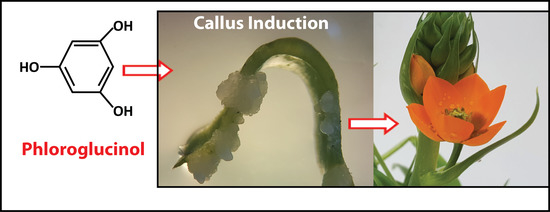
Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments
Abstract
1. Introduction
2. Results
2.1. Media Assessment and Hormones Baseline Establishment
2.2. Phloroglucinol as an Auxin-Like Hormone
2.3. Phloroglucinol and Callus Induction: Young versus Older Tissue Type
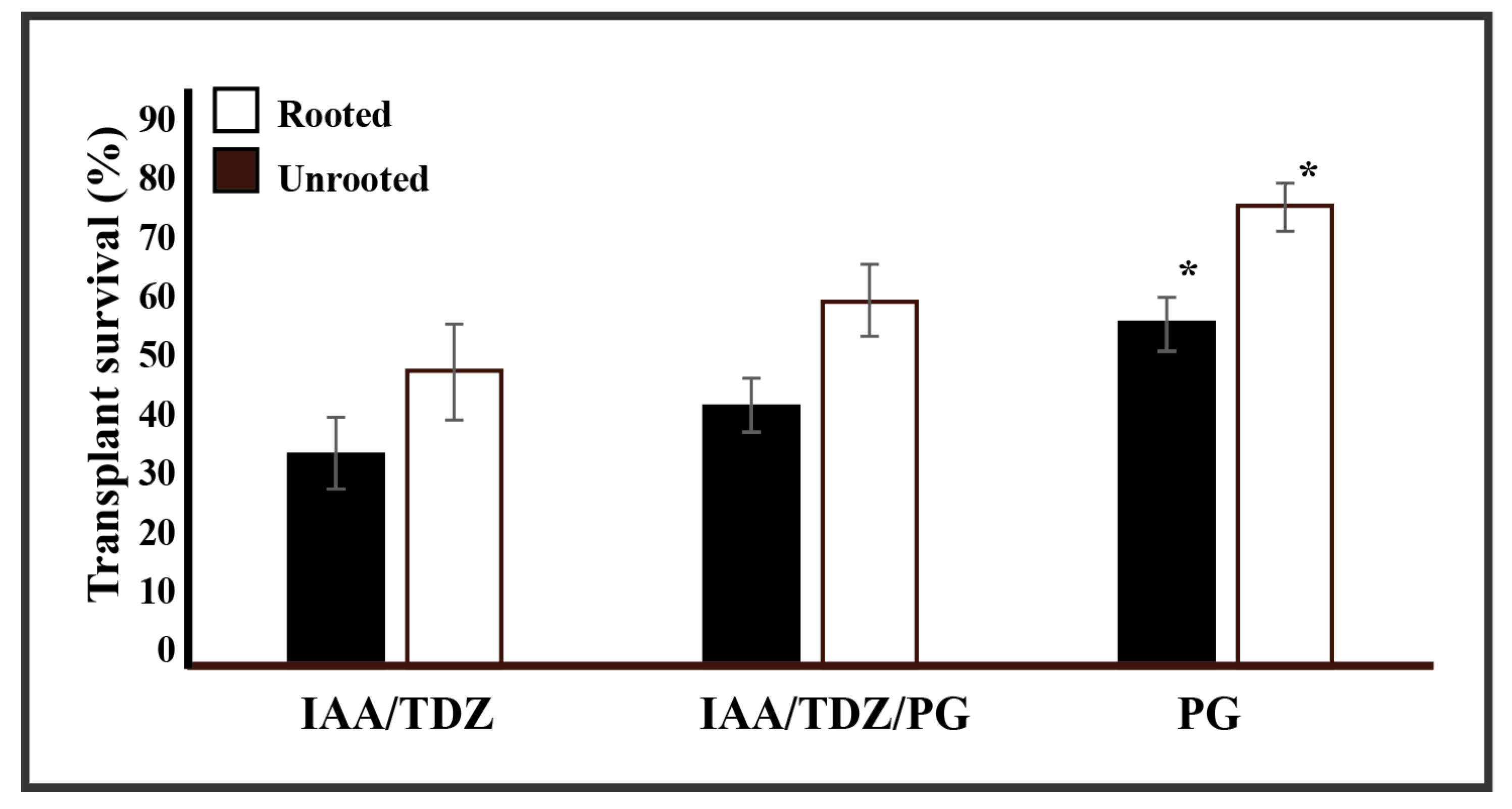
2.4. Plant Transplant Vigour and Phloroglucinol
2.5. Morphological Characteristics
3. Discussion
4. Materials and Methods
4.1. Original and Produced Plant Material
4.2. Tissue Culture
4.3. Phenotypical Characterization
4.4. DNA Extraction and RAPD Analysis
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Manning, J.C.; Martínez-Azorín, M.; Crespo, M.B. A revision of Ornithogalum subgenus Aspasia section Aspasia, the chincherinchees (Hyacinthaceae). Bothalia 2007, 37, 133–164. [Google Scholar] [CrossRef][Green Version]
- Reinten, E.Y.; Coetzee, J.H.; Van Wyk, B.E. The potential of South African indigenous plants for the international cut flower trade. S. Afr. J. Bot. 2011, 77, 934–946. [Google Scholar] [CrossRef]
- Niederwieser, J.G. Role of biotechnology in the development and production of Lachenalia and Ornithogalum cultivars in South Africa. S. Afr. J. Bot. 2004, 70, 47–51. [Google Scholar] [CrossRef]
- Griesbach, R.J.; Meyer, F.; Koopowitz, H. Creation of new flower colors in Ornithogalum via interspecific hybridization. J. Am. Soc. Hort. Sci. 1993, 118, 409–414. [Google Scholar] [CrossRef]
- Sharma, D.R.; Kaur, R.; Kumar, K. Embryo rescue in plants—A review. Euphytica 1996, 89, 325–337. [Google Scholar]
- Niederwieser, J.G.; Van De Venter, H.A.; Robbertse, P.J. Embryo rescue in Ornithogalum. HortScience 1990, 25, 565–566. [Google Scholar] [CrossRef]
- Ziv, M. Simple bioreactors for mass propagation of plants. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 79–93. [Google Scholar]
- Paek, K.Y.; Chakrabarty, D.; Hahn, E.J. Application of bioreactor systems for large scale production of horticultural and medicinal plants. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 95–116. [Google Scholar]
- Sugiyama, M. Historical review of research on plant cell dedifferentiation. J. Plant Res. 2015, 128, 349–359. [Google Scholar] [CrossRef]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef]
- Depuydt, S.; Hardtke, C.S. Hormone signalling crosstalk in plant growth regulation. Curr. Biol. 2011, 21, R365–R373. [Google Scholar] [CrossRef]
- McSteen, P.; Zhao, Y. Plant hormones and signaling: Common themes and new developments. Dev. Cell 2008, 14, 467–473. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Pospíšil, T. Structure and activity of strigolactones: New plant hormones with a rich future. Mol. Plant 2013, 6, 38–62. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Pospíšil, T.; Zeljković, S.Ć. Strigolactones: New plant hormones in action. Planta 2016, 243, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195. [Google Scholar] [CrossRef] [PubMed]
- Khripach, V.; Zhabinskii, V.; de Groot, A. Twenty years of brassinosteroids: Steroidal plant hormones warrant better crops for the XXI century. Ann. Bot. 2000, 86, 441–447. [Google Scholar] [CrossRef]
- Lindsey, K. Plant peptide hormones: The long and the short of it. Curr. Biol. 2001, 11, R741–R743. [Google Scholar] [CrossRef]
- Singh, I.P.; Sidana, J.; Bharate, S.B.; Foley, W.J. Phloroglucinol compounds of natural origin: Synthetic aspects. Nat. Prod. Rep. 2010, 27, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef] [PubMed]
- Bloor, S.J. Antiviral phloroglucinols from New Zealand kunzea species. J. Nat. Prod. 1992, 55, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, M.; Konoshima, T.; Fujitani, K.; Yoshida, S.; Nishimura, H.; Tokuda, H.; Nishino, H.; Iwashima, A.; Kozuka, M. Inhibitors of Skin-Tumor Promotion. VIII: Inhibitory Effects of Euglobals and Their Related Compounds on Epstein-Barr Virus Activation. Chem. Pharm. Bull. 1990, 38, 2737–2739. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, R.; Murata, M.; Homma, S.; Aida, K. Antibacterial compounds from Eucalyptus perriniana. Agric. Biol. Chem. 1990, 54, 231–232. [Google Scholar] [CrossRef]
- Shang, Z.-C.; Yang, M.-H.; Liu, R.-H.; Wang, X.-B.; Kong, L.-Y. New Formyl Phloroglucinol Meroterpenoids from the Leaves of Eucalyptus robusta. Sci. Rep. 2016, 6, 39815. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Q.; Shen, Z.B.; Chen, Y.F.; Wu, J.Y.; Yang, C.Y.; Liang, W.N.; Tang, C.P. Study on antifungal susceptibility of different extract of Dryopteris fragrans. J. Chin. Med. Mater. 2012, 35, 1981–1985. [Google Scholar]
- Mayer, A.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar]
- Barros, F.M.; Pippi, B.; Dresch, R.R.; Dauber, B.; Luciano, S.C.; Apel, M.A.; Fuentefria, A.M.; von Poser, G.L. Antifungal and antichemotactic activities and quantification of phenolic compounds in lipophilic extracts of Hypericum spp. native to South Brazil. Ind. Crops Prod. 2013, 44, 294–299. [Google Scholar] [CrossRef]
- do Rego, J.C.; Benkiki, N.; Chosson, E.; Kabouche, Z.; Seguin, E.; Costentin, J. Antidepressant-like effect of hyperfoliatin, a polyisoprenylated phloroglucinol derivative from Hypericum perfoliatum (Clusiaceae) is associated with an inhibition of neuronal monoamines uptake. Eur. J. Pharm. 2007, 569, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.C.; Viana, A.F.; Müller, L.G.; Nunes, J.M.; Stolz, E.D.; Do Rego, J.C.; Costentin, J.; von Poser, G.L.; Rates, S.M.; Uliginosin, B. A phloroglucinol derivative from Hypericum polyanthemum: A promising new molecular pattern for the development of antidepressant drugs. Behav. Brain Res. 2012, 228, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Bridi, H.; Beckenkamp, A.; Ccana-Ccapatinta, G.V.; de Loreto Bordignon, S.A.; Buffon, A.; von Poser, G.L. Characterization of Phloroglucinol-enriched Fractions of Brazilian Hypericum Species and Evaluation of Their Effect on Human Keratinocytes Proliferation. Phytother. Res. 2017, 31, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Deiana, M.; Atzeri, A.; Corona, G.; Incani, A.; Melis, M.P.; Appendino, G.; Dessì, M. Evaluation of the antioxidant and cytotoxic activity of arzanol, a prenylated α-pyrone–phloroglucinol etherodimer from Helichrysum italicum subsp. microphyllum. Chem. Biol. Interact. 2007, 165, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Yuvakkumar, R.; Vijayakumar, S.; Vaseeharan, B. Cytotoxicity of phloroglucinol engineered silver (Ag) nanoparticles against MCF-7 breast cancer cell lines. Mater. Chem. Phys. 2018, 220, 402–408. [Google Scholar] [CrossRef]
- Decosterd, L.A.; Stoeckli-Evans, H.; Chapuis, J.C.; Sordat, B.; Hostettmann, K. New Constituents with Growth-Inhibitory Activity against the Co-115 Human Colon Carcinoma Cell Line and A New Antifungal Phloroglucinol Derivative from Hypericum calycinum. Planta Med. 1989, 55, 629–630. [Google Scholar] [CrossRef]
- Taborga, L.; Espinoza, L.; Moller, A.; Carrasco, H.; Cuellar, M.; Villena, J. Antiproliferative effect and apoptotic activity of linear geranylphenol derivatives from phloroglucinol and orcinol. Chem. Biol. Interact. 2016, 247, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Cha, S.H.; Kim, K.N.; Lee, S.H.; Ahn, G.; Kang, D.H.; Oh, C.; Choi, Y.U.; Affan, A.; Kim, D.; et al. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl. Biochem. Biotechnol. 2012, 166, 1520–1532. [Google Scholar] [CrossRef]
- Hammatt, N. Promotion by phloroglucinol of adventitious root formation in micropropagated shoots of adult wild cherry (Prunus avium L.). Plant Growth Reg. 1994, 14, 127–132. [Google Scholar] [CrossRef]
- Thomas, V.; Mehta, A.R. Effect of phloroglucinol on shoot growth and initiation of roots in carob tree cultures grown in vitro. In Plant Cell Culture in Crop Improvement; Springer: Boston, MA, USA, 1983; pp. 451–457. [Google Scholar]
- Daud, N.; Faizal, A.; Geelen, D. Adventitious rooting of Jatropha curcas L. is stimulated by phloroglucinol and by red LED light. In Vitro Cell. Dev. Biol. Plant 2013, 49, 183–190. [Google Scholar] [CrossRef]
- Sharifian, S.; Vahdati, K.; Mirmasoumi, M.; Ghaem Maghami, S.A. Assessment of phloroglucinol effect on rooting of tissue cultured Persian walnut. In III International Symposium on Acclimatization and Establishment of Micropropagated Plants 812; Acta Horticulture: Faro, Portugal, 2007; pp. 189–196. [Google Scholar]
- Pérez, L.P.; Montesinos, Y.P.; Olmedo, J.G.; Rodriguez, R.B.; Sánchez, R.R.; Montenegro, O.N.; Escriba, R.C.R.; Daniels, D.; Gómez-Kosky, R. Effect of phloroglucinol on rooting and in vitro acclimatization of papaya (Carica papaya L. var. Maradol Roja). In Vitro Cell. Dev. Biol. Plant 2016, 52, 196–203. [Google Scholar] [CrossRef]
- Ross, S.; Castillo, A. Propagación masal de Vaccinium corymbosum en bioreactores. Agrociencia Urug. 2009, 13, 1–8. [Google Scholar]
- Vélez-Mora, D.P.; Armijos González, R.; Zimmermann, M.J. Enhancement of germination, hyperhydricity control and in vitro shoot formation of Vasconcellea stipulata Badillo. Rev. Colomb. Biotecnol. 2015, 17, 16–21. [Google Scholar] [CrossRef]
- Chandrika, R.; Shivakameshwari, M.N.; Saraswathi, K.T. Reduction of vitrification in in vitro shoot cultures of Eryngium foetidum l.-a potential aromatic and medicinal herb. Indian J. Plant Sci. 2015, 4, 2319–3824. [Google Scholar]
- da Silva, J.A.T.; Dobránszki, J.; Ross, S. Phloroglucinol in plant tissue culture. In Vitro Cell. Dev. Biol. Plant 2013, 49, 1–16. [Google Scholar] [CrossRef]
- Sarkar, D.; Naik, P.S. Phloroglucinol enhances growth and rate of axillary shoot proliferation in potato shoot tip cultures in vitro. Plant Cell Tissue Organ Cult. 2000, 60, 139–149. [Google Scholar] [CrossRef]
- Siwach, P.; Gill, A.R. Enhanced shoot multiplication in Ficus religiosa L. in the presence of adenine sulphate, glutamine and phloroglucinol. Physiol. Mol. Biol. Plants 2011, 17, 271. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.; Slovin, J.P.; Chen, C. A simplified method for differential staining of aborted and non-aborted pollen grains. Int. J. Plant Biol. 2010, 1, e13. [Google Scholar] [CrossRef]
- Pitman, N.C.; Jørgensen, P.M. Estimating the size of the world’s threatened flora. Science 2002, 298, 989. [Google Scholar] [CrossRef] [PubMed]
- Ziv, M.; Lilien-Kipnis, H. Bud cluster proliferation in bioreactor cultures of Ornithogalum dubium. Acta Hortic. 1997, 430, 307–310. [Google Scholar] [CrossRef]
- Cohen, A.; Lipsky, A.; Arazi, T.; Ion, A.; Stav, R.; Sandler-Ziv, D.; Pintea, C.; Barg, R.; Salts, Y.; Shabtai, S.; et al. Efficient genetic transformation of Lilium longiflorum and Ornithogalum dubium by particle acceleration followed by prolonged selection in liquid medium. Acta Hortic. 2004, 651, 131–140. [Google Scholar] [CrossRef]
- Cohen, A.; Lipsky, A.; Arazi, T.; Ion, A.; Stav, R.; Sandler-Ziv, D.; Fintea, C.; Gaba, V.; Gera, A. Particle bombardment-mediated transformation of Ornithogalum dubium for Ornithogalum mosaic virus resistance. Acta Hortic. 2005, 673, 183–190. [Google Scholar] [CrossRef]
- Lipsky, A.; Cohen, A.; Ion, A.; Yedidia, I. Genetic transformation of Ornithogalum via particle bombardment and generation of Pectobacterium carotovorum-resistant plants. Plant Sci. 2014, 228, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Tun, O.M.; Lipsky, A.; Knaan, T.L.; Kerem, Z.; Yedidia, I. The plant activator BTH promotes Ornithogalum dubium and O. thyrsoides differentiation and regeneration in vitro. Biol. Plant. 2013, 57, 41–48. [Google Scholar] [CrossRef]
- Watad, A.A.; Yun, D.J.; Matsumoto, T.; Niu, X.; Wu, Y.; Kononowicz, A.K.; Bressan, R.A.; Hasegawa, P.M. Microprojectile bombardment-mediated transformation of Lilium longiflorum. Plant Cell Rep. 1998, 17, 262–267. [Google Scholar] [CrossRef]
- Lipsky, A.; Joshi, J.R.; Carmi, N.; Yedidia, I. Expression levels of antimicrobial peptide tachyplesin I in transgenic Ornithogalum lines affect the resistance to Pectobacterium infection. J. Biotechnol. 2016, 238, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Van Emmenes, L.; Veale, A.; Cohen, A.; Arazi, T. Agrobacterium-mediated transformation of the bulbous flower Ornithogalum. Acta Hortic. 2008, 766, 477–484. [Google Scholar] [CrossRef]
- Smertenko, A.; Bozhkov, P.V. Somatic embryogenesis: Life and death processes during apical–basal patterning. J. Exp. Bot. 2014, 65, 1343–1360. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A. Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochem. Biophys. Acta (BBA) Gene Reg. Mech. 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Fehér, A. Callus, Dedifferentiation, Totipotency, Somatic Embryogenesis: What These Terms Mean in the Era of Molecular Plant Biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Gaj, M.D.; Zhang, S.; Harada, J.J.; Lemaux, P.G. Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 2005, 222, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Niu, Q.W.; Frugis, G.; Chua, N.H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002, 30, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhang, C.; Xu, J. Control of cell fate reprogramming towards de novo shoot organogenesis. Plant Cell Physiol. 2017, 59, 713–719. [Google Scholar] [CrossRef]
- Rensing, S.A.; Lang, D.; Schumann, E.; Reski, R.; Hohe, A. EST sequencing from embryogenic Cyclamen persicum cell cultures identifies a high proportion of transcripts homologous to plant genes involved in somatic embryogenesis. J. Plant Growth Regul. 2005, 24, 102–115. [Google Scholar] [CrossRef]
- Egertsdotter, U.; von Arnold, S. Development of somatic embryos in Norway spruce. J. Exp. Bot. 1998, 49, 155–162. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, X.; Zhu, L.; Tu, L.; Guo, X.; Nie, Y. Isolation and characterization of genes associated to cotton somatic embryogenesis by suppression subtractive hybridization and macroarray. Plant Mol. Biol. 2006, 60, 167–183. [Google Scholar] [CrossRef]
- Meslet-Cladière, L.; Delage, L.; Leroux, C.J.J.; Goulitquer, S.; Leblanc, C.; Creis, E.; Gall, E.A.; Stiger-Pouvreau, V.; Czjzek, M.; Potin, P. Structure/function analysis of a type III polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant Cell 2013, 25, 3089–3103. [Google Scholar] [CrossRef]
- Bertoni, G. A key step in phlorotannin biosynthesis revealed. Plant Cell 2013, 25, 2770. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mitra, A. Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol. Mol. Plant Pathol. 2007, 71, 201–209. [Google Scholar] [CrossRef]
- Underwood, W. The plant cell wall: A dynamic barrier against pathogen invasion. Front. Plant Sci. 2012, 3, 85. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rubio, R.; Centeno, M.L.; García-Angulo, P.; Álvarez, J.M.; Acebes, J.L.; Encina, A. The role of cell wall phenolics during the early remodelling of cellulose-deficient maize cells. Phytochemistry 2020, 170, 112219. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Igamberdiev, A.U.; Debnath, S.C. Thidiazuron-induced somatic embryogenesis and changes of antioxidant properties in tissue cultures of half-high blueberry plants. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jani, J.N.; Jha, S.K.; Nagar, D.S.; Chauhan, R.S.; Hegde, H. Phloroglucinol plays role in shoot bud induction and in vitro Tuberization in Tinospora cordifolia-a medicinal plant with multi-therapeutic application. Adv. Tech. Biol. Med. 2015, 8, 1–5. [Google Scholar]
- Golan, A.; Kerem, Z.; Tun, O.M.; Luzzatto, T.; Lipsky, A.; Yedidia, I. Combining flow cytometry and gfp reporter gene for quantitative evaluation of Pectopbacterium carotovorum ssp. carotovorum in Ornithogalum dubium plantlets. J. Appl. Microbiol. 2010, 108, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.M. Tissue culture-derived variation in crop improvement. Euphytica 2001, 118, 153–166. [Google Scholar] [CrossRef]
- Bradaï, F.; Pliego-Alfaro, F.; Sánchez-Romero, C. Somaclonal variation in olive (Olea europaea L.) plants regenerated via somatic embryogenesis: Influence of genotype and culture age on genetic stability. Sci. Hortic. 2019, 251, 260–266. [Google Scholar] [CrossRef]
- Landi, L.; Mezzetti, B. TDZ, auxin and genotype effects on leaf organogenesis in Fragaria. Plant Cell Rep. 2006, 25, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]



| MS Basal Salts | MS Plus Gamborg Vitamins | ||||
|---|---|---|---|---|---|
| Hormone | Concentration (mg/L) | Callus/Shoot Formation (%) | 1 Average Callus/Shoot Size (cm) ± SD | Callus/Shoot Formation (%) | 1,2 Average Callus/Shoot Size (cm) ± SD |
| None | N/A | 0 | 0 | 0 | 0 |
| IAA | 0.5 | 65.9 a | 0.88 ± 0.24 a | 64.6 a | 0.97 ± 0.17 a |
| 1 | 77 b | 1.31± 0.34 bc | 74.1 b | 1.19 ± 0.25 b | |
| 1.5 | 75.7 b | 1.14 ± 0.14 b | 74.3 b | 0.82 ± 0.33 a | |
| TDZ | 0.5 | 61.1 a | 0.92 ± 0.25 a | 55.9 a | 0.98 ± 0.25 a |
| 1 | 75.6 b | 1.50 ± 0.33 bcd | 71.1 b | 1.18 ± 0.41 b | |
| 1.5 | 78.7 bc | 1.01 ± 0.45 a | 77.2 bc | 0.95 ± 0.36 a | |
| IAA + TDZ | 0.5 | 82.1 b | 0.89 ± 0.25 a | 77 b | 0.98 ± 0.25 a |
| 1 | 90.6 bc | 1.63 ± 0.36 bce | 81 b | 1.62 ± 0.35 bc | |
| 1.5 | 92 bcd | 1.32 ± 0.17 bc | 86 bc | 1.34 ± 0.27 b | |
| BAP | 0.5 | 52.4 a | 0.76 ± 0.22 a | 55 a | 0.72 ± 0.31 a |
| 1 | 65 a | 1.28 ± 0.15 bc | 69.9 a | 1.33 ± 0.32 b | |
| 1.5 | 71.6 b | 1.09 ± 0.35 b | 72.9 b | 1.25 ± 0.45 b | |
| Phloroglucinol Concentration (mg/L) | Callus/Shoot Formation (%) | 1,2 Average Callus/Shoot Size (cm) ± SD |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 25.2 a | 0.83 ± 0.36 a |
| 2 | 33.3 b | 0.87 ± 0.52 a |
| 4 | 37.5 bc | 1.51 ± 0.47 bc |
| 10 | 32.1 b | 1.12 ± 0.49 b |
| 20 | 27.8 a | 1.08 ± 0.53 a |
| Phloroglucinol Concentration (mg/L) | Callus/Shoot Formation (%) | 1,2 Average Callus/Shoot Size (cm) ± SD |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 37.2 a | 0.90 ± 0.25 a |
| 2 | 44.5 b | 1.12 ± 0.36 b |
| 4 | 52.1 bc | 1.65 ± 0.51 bc |
| 10 | 37.8 a | 1.08 ± 0.44 a |
| 20 | 33.1 a | 1.12 ± 0.21 a |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petti, C. Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments. Plants 2020, 9, 929. https://doi.org/10.3390/plants9080929
Petti C. Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments. Plants. 2020; 9(8):929. https://doi.org/10.3390/plants9080929
Chicago/Turabian StylePetti, Carloalberto. 2020. "Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments" Plants 9, no. 8: 929. https://doi.org/10.3390/plants9080929
APA StylePetti, C. (2020). Phloroglucinol Mediated Plant Regeneration of Ornithogalum dubium as the Sole “Hormone-Like Supplement” in Plant Tissue Culture Long-Term Experiments. Plants, 9(8), 929. https://doi.org/10.3390/plants9080929