The Cypriot Indigenous Grapevine Germplasm Is a Multi-Clonal Varietal Mixture
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Extraction Protocol
2.3. Microsatellite Genotyping
2.4. Genetic Relationships and Analysis of Population Structure
3. Results
3.1. Genetic Affiliations across the Cypriot Germplasm
3.2. Population Structure of the Main Cypriot Grapevine Varieties
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, K.; Wittwer, G.U.K. and Global Wine Markets by 2025, and Implications of Brexit. J. Wine Econ. 2017, 12, 221–251. [Google Scholar] [CrossRef]
- Wen, J. Vitaceae. In The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin, Germany, 2007; Volume 9, pp. 466–478. [Google Scholar]
- Chen, I.; Manchester, S.R. Seed morphology of modern and fossil Ampelocissus (Vitaceae) and implications for phytogeography. Am. J. Bot. 2007, 94, 1534–1553. [Google Scholar] [CrossRef] [PubMed]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of domesticated Plants in Southwest Asia, Europe, and The Mediterranean Basin; Oxford University Press on Demand: Oxford, UK, 2012; ISBN 0199549060. [Google Scholar]
- Murphy, C. Grapes: Origins and Development BT. In Encyclopedia of Global Archaeology; Smith, C., Ed.; Springer: New York, NY, USA, 2014; pp. 3107–3111. ISBN 978-1-4419-0465-2. [Google Scholar]
- Cunha, J.; Ibáñez, J.; Teixeira-Santos, M.; Brazão, J.; Fevereiro, P.; Martínez-Zapater, J.M.; Eiras-Dias, J.E. Genetic Relationships Among Portuguese Cultivated and Wild Vitis vinifera L. Germplasm. Front. Plant Sci. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519. [Google Scholar] [CrossRef]
- McGovern, P.E. Ancient Wine: The Search for the Origins of Viniculture. Econ. Bot. 2004, 58, 488. [Google Scholar]
- Riaz, S.; De Lorenzis, G.; Velasco, D.; Koehmstedt, A.; Maghradze, D.; Bobokashvili, Z.; Musayev, M.; Zdunic, G.; Laucou, V.; Andrew Walker, M.; et al. Genetic diversity analysis of cultivated and wild grapevine (Vitis vinifera L.) accessions around the Mediterranean basin and Central Asia. BMC Plant Biol. 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Arroyo-García, R.; Ruiz-García, L.; Bolling, L.; Ocete, R.; López, M.A.; Arnold, C.; Ergul, A.; Söylemezoǧlu, G.; Uzun, H.I.; Cabello, F.; et al. Multiple origins of cultivated grapevine (Vitis vinifera L. ssp. sativa) based on chloroplast DNA polymorphisms. Mol. Ecol. 2006, 15, 3707–3714. [Google Scholar] [CrossRef]
- Imazio, S.; Labra, M.; Grassi, F.; Scienza, A.; Failla, O. Chloroplast microsatellites to investigate the origin of grapevine. Genet. Resour. Crop Evol. 2006, 53, 1003–1011. [Google Scholar] [CrossRef]
- Myles, S.; Boyko, A.R.; Owens, C.L.; Brown, P.J.; Grassi, F.; Aradhya, M.K.; Prins, B.; Reynolds, A.; Chia, J.M.; Ware, D.; et al. Genetic structure and domestication history of the grape. Proc. Natl. Acad. Sci. USA 2011, 108, 3530–3535. [Google Scholar] [CrossRef]
- Drori, E.; Rahimi, O.; Marrano, A.; Henig, Y.; Brauner, H.; Salmon-Divon, M.; Netzer, Y.; Prazzoli, M.L.; Stanevsky, M.; Failla, O.; et al. Collection and characterization of grapevine genetic resources (Vitis vinifera) in the Holy Land, towards the renewal of ancient winemaking practices. Sci. Rep. 2017, 7, 44463. [Google Scholar] [CrossRef]
- Riaz, S.; Pap, D.; Uretsky, J.; Laucou, V.; Boursiquot, J.M.; Kocsis, L.; Andrew Walker, M. Genetic diversity and parentage analysis of grape rootstocks. Theor. Appl. Genet. 2019, 132, 1847–1860. [Google Scholar] [CrossRef] [PubMed]
- Olmo, H.P. Grapes. Evol. Crop Plants 1976, 114, 294–298. [Google Scholar]
- Emanuelli, F.; Lorenzi, S.; Grzeskowiak, L.; Catalano, V.; Stefanini, M.; Troggio, M.; Myles, S.; Martinez-Zapater, J.M.; Zyprian, E.; Moreira, F.M.; et al. Genetic diversity and population structure assessed by SSR and SNP markers in a large germplasm collection of grape. BMC Plant Biol. 2013, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Vrontis, D.; Thrassou, A. The renaissance of Commandaria: A strategic branding prescriptive analysis Demetris Vrontis * and Alkis Thrassou. J. Glob. Bus. Adv. 2011, 4, 302–316. [Google Scholar] [CrossRef]
- Johnson, H.; Robinson, J. The World Atlas of Wine.; Mitchell Beazley: London, UK, 2001. [Google Scholar]
- EFSA Panel on Plant Health (PLH). Scientific Opinion on the risk to plant health posed by Daktulosphaira vitifoliae (Fitch) in the EU territory, with the identification and evaluation of risk reduction options. EFSA J. 2014, 12, 3678. [Google Scholar] [CrossRef]
- Galet, P. Vines Wines Cyprus. 4000 Years Tradit; Food and Agriculture Organization of the United Nations: Roma, Italy, 1993; pp. 61–73. [Google Scholar]
- Hvarleva, T.; Hadjinicoli, A.; Atanassov, I.; Atanassov, A.; Ioannou, N. Genotyping Vitis vinifera L. cultivars of Cyprus by microsatellite analysis. Vitis J. Grapevine Res. 2005, 44, 93–97. [Google Scholar]
- Ioannou-Papayianni, E.; Kokkinofta, R.I.; Theocharis, C.R. Authenticity of cypriot sweet wine commandaria using FT-IR and chemometrics. J. Food Sci. 2011, 76, 420–427. [Google Scholar] [CrossRef]
- Constantinou, S.; Gómez-Caravaca, A.M.; Goulas, V.; Segura-Carretero, A.; Koundouras, S.; Manganaris, G.A. The impact of postharvest dehydration methods on qualitative attributes and chemical composition of ‘Xynisteri’ grape (Vitis vinifera) must. Postharvest Biol. Technol. 2018, 135, 114–122. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Antoniou, O.; Tzortzakis, N. Climate change due to heat and drought stress can alter the physiology of maratheftiko local cyprian grapevine variety. J. Water Clim. Chang. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Anesi, A.; Stocchero, M.; Dal Santo, S.; Commisso, M.; Zenoni, S.; Ceoldo, S.; Tornielli, G.B.; Siebert, T.E.; Herderich, M.; Pezzotti, M.; et al. Towards a scientific interpretation of the terroir concept: Plasticity of the grape berry metabolome. BMC Plant Biol. 2015, 15, 1–17. [Google Scholar] [CrossRef]
- Inglis, P.W.; Marilia de Castro, R.P.; Resende, L.V.; Grattapaglia, D. Fast and inexpensive protocols for consistent extraction of high quality DNA and RNA from challenging plant and fungal samples for high-throughput SNP genotyping and sequencing applications. PLoS ONE 2018, 13, e0206085. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, R.; Sudharma, K.N.; Kecke, S.; Marx, G.; Eibach, R.; Maghradze, D.; Maul, E. The Vitis International Variety Catalogue (VIVC): New design and more information. Bull. L′OIV. 2009, 82, 45–55. [Google Scholar]
- Schuelke, M. An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 2000, 18, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Matschiner, M.; Salzburger, W. TANDEM: Integrating automated allele binning into genetics and genomics workflows. Bioinformatics 2009, 25, 1982–1983. [Google Scholar] [CrossRef]
- Peakall, R.O.D.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Brooks, J.C.; Grünwald, N.J. Novel R tools for analysis of genome-wide population genetic data with emphasis on clonality. Front. Genet. 2015, 6, 208. [Google Scholar] [CrossRef]
- Clark, L.V.; Jasieniuk, M. Polysat: An R package for polyploid microsatellite analysis. Mol. Ecol. Resour. 2011, 11, 562–566. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, 1–4. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar]
- Laucou, V.; Launay, A.; Bacilieri, R.; Lacombe, T.; Adam-Blondon, A.-F.; Bérard, A.; Chauveau, A.; De Andrés, M.T.; Hausmann, L.; Ibáñez, J.; et al. Extended diversity analysis of cultivated grapevine Vitis vinifera with 10K genome-wide SNPs. PLoS ONE 2018, 13, e0192540. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, T.; Boursiquot, J.-M.; Laucou, V.; Di Vecchi-Staraz, M.; Péros, J.-P.; This, P. Large-scale parentage analysis in an extended set of grapevine cultivars (Vitis vinifera L.). Theor. Appl. Genet. 2013, 126, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Laucou, V.; Lacombe, T.; Dechesne, F.; Siret, R.; Bruno, J.-P.; Dessup, M.; Dessup, T.; Ortigosa, P.; Parra, P.; Roux, C.; et al. High throughput analysis of grape genetic diversity as a tool for germplasm collection management. Theor. Appl. Genet. 2011, 122, 1233–1245. [Google Scholar] [CrossRef]
- Pelsy, F. Molecular and cellular mechanisms of diversity within grapevine varieties. Heredity 2010, 104, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Aradhya, M.K.; Dangl, G.S.; Prins, B.H.; Boursiquot, J.-M.; Walker, M.A.; Meredith, C.P.; Simon, C.J. Genetic structure and differentiation in cultivated grape, Vitis vinifera L. Genet. Res. 2003, 81, 179–192. [Google Scholar] [CrossRef] [PubMed]
- This, P.; Jung, A.; Boccacci, P.; Borrego, J.; Botta, R.; Costantini, L.; Crespan, M.; Dangl, G.S.; Eisenheld, C.; Ferreira-Monteiro, F.; et al. Development of a standard set of microsatellite reference alleles for identification of grape cultivars. Theor. Appl. Genet. 2004, 109, 1448–1458. [Google Scholar] [CrossRef]
- Pelsy, F. Untranslated leader region polymorphism of Tvv1, a retrotransposon family, is a novel marker useful for analyzing genetic diversity and relatedness in the genus Vitis. Theor. Appl. Genet. 2007, 116, 15–27. [Google Scholar] [CrossRef]
- González Techera, A.; Jubany, S.; Ponce De León, I.; Boido, E.; Dellacassa, E.; Carrau, F.M.; Hinrichsen, P.; Gaggero, C. Molecular diversity within clones of cv. Tannat (Vitis vinifera). Vitis J. Grapevine Res. 2004, 43, 179–185. [Google Scholar]
- Pelsy, F.; Hocquigny, S.; Moncada, X.; Barbeau, G.; Forget, D.; Hinrichsen, P.; Merdinoglu, D. An extensive study of the genetic diversity within seven French wine grape variety collections. Theor. Appl. Genet. 2010, 120, 1219–1231. [Google Scholar] [CrossRef]
- Ibáñez, J.; Vélez, M.D.; Teresa de Andrés, M.; Borrego, J. Molecular markers for establishing distinctness in vegetatively propagated crops: A case study in grapevine. Theor. Appl. Genet. 2009, 119, 1213–1222. [Google Scholar] [CrossRef]
- Muzzalupo, I.; Vendramin, G.G.; Chiappetta, A. Genetic biodiversity of Italian olives (Olea europaea) germplasm analyzed by SSR markers. Sci. World J. 2014, 2014, 296590. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, G.; Spadotto, A.; Jurman, I.; Gaspero, G.D.; Crespan, M.; Meneghetti, S.; Frare, E.; Vignani, R.; Cresti, M.; Morgante, M.; et al. The SSR-based molecular profile of 1005 grapevine (Vitis vinifera L.) accessions uncovers new synonymy and parentages, and reveals a large admixture amongst varieties of different geographic origin. Theor. Appl. Genet. 2010, 121, 1569–1585. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.; Labra, M.; Imazio, S.; Spada, A.; Sgorbati, S.; Scienza, A.; Sala, F. Evidence of a secondary grapevine domestication centre detected by SSR analysis. Theor. Appl. Genet. 2003, 107, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- De Michele, R.; La Bella, F.; Gristina, A.S.; Fontana, I.; Pacifico, D.; Garfi, G.; Motisi, M.; Crucitti, D.; Abbate, L.; Carimi, F. Phylogenetic relationship among wild and cultivated Grapevine in Sicily: A hotspot in the Middle of the Mediterranean Basin. Front. Plant Sci. 2019, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, G.; Májer, J.; Varga, P.; SzÖke, B. Analysis of clones of Pinots grown in Hungary by SSR markers. Sci. Hortic. 2011, 129, 32–37. [Google Scholar] [CrossRef]
- Franks, T.; Botta, R.; Thomas, M.R.; Franks, J. Chimerism in grapevines: Implications for cultivar identity, ancestry and genetic improvement. Theor. Appl. Genet. 2002, 104, 192–199. [Google Scholar] [CrossRef]
- Zarouri, B.; Vargas, A.M.; Gaforio, L.; Aller, M.; de Andrés, M.T.; Cabezas, J.A. Whole-genome genotyping of grape using a panel of microsatellite multiplex PCRs. Tree Genet. Genomes 2015, 11, 17. [Google Scholar] [CrossRef]
- Sutton, J.T.; Robertson, B.C.; Jamieson, I.G. Dye shift: A neglected source of genotyping error in molecular ecology. Mol. Ecol. Resour. 2011, 11, 514–520. [Google Scholar] [CrossRef]
- Copper, A.W.; Johnson, T.E.; Danner, L.; Bastian, S.E.P.; Collins, C. Preliminary sensory and chemical profiling of Cypriot wines made from indigenous grape varieties Xynisteri, Maratheftiko and Giannoudhi and acceptability to Australian consumers. Oeno One 2019, 53, 229–248. [Google Scholar] [CrossRef]
- Anestiadou, K.; Nikoloudakis, N.; Hagidimitriou, M.; Katsiotis, A. Monumental olive trees of Cyprus contributed to the establishment of the contemporary olive germplasm. PLoS ONE 2017, 12, e0187697. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive response of a native mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef]
- Azri, W.; Cosette, P.; Guillou, C.; Rabhi, M.; Nasr, Z.; Mliki, A. Physiological and proteomic responses to drought stress in leaves of two wild grapevines (Vitis sylvestris): A comparative study. Plant Growth Regul. 2020, 91, 37–52. [Google Scholar] [CrossRef]
- Duan, D.; Halter, D.; Baltenweck, R.; Tisch, C.; Tröster, V.; Kortekamp, A.; Hugueney, P.; Nick, P. Genetic diversity of stilbene metabolism in Vitis sylvestris. J. Exp. Bot. 2015, 66, 3243–3257. [Google Scholar] [CrossRef] [PubMed]
- Toffolatti, S.L.; Maddalena, G.; Salomoni, D.; Maghradze, D.; Bianco, P.A.; Failla, O. Evidence of resistance to the downy mildew agent Plasmopara viticola in the Georgian Vitis vinifera germplasm. Vitis J. Grapevine Res. 2016, 55, 121–128. [Google Scholar] [CrossRef]
- Revilla, E.; Carrasco, D.; Benito, A.; Arroyo-García, R. Anthocyanin composition of several wild grape accessions. Am. J. Enol. Vitic. 2010, 61, 536–543. [Google Scholar] [CrossRef]
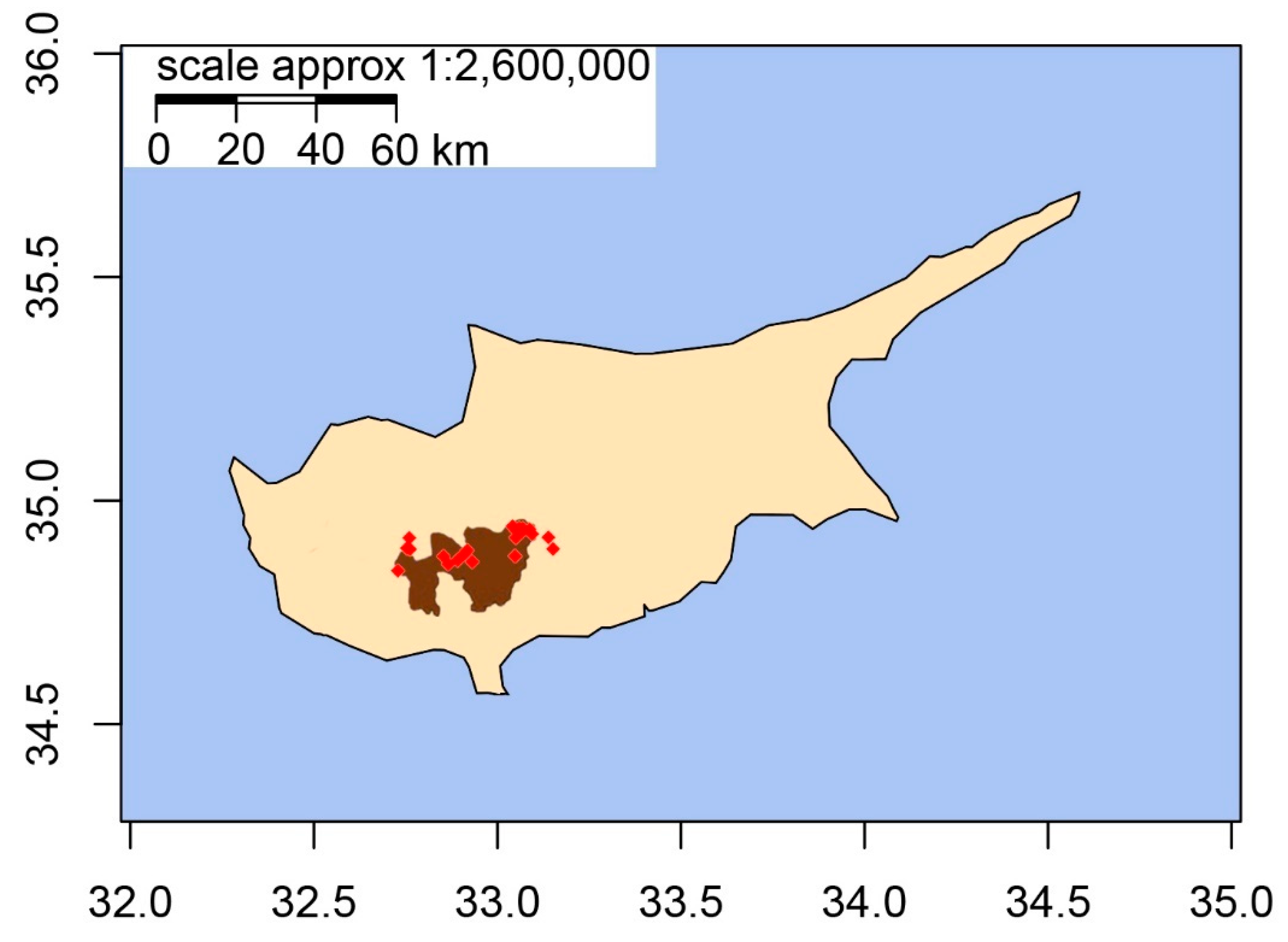
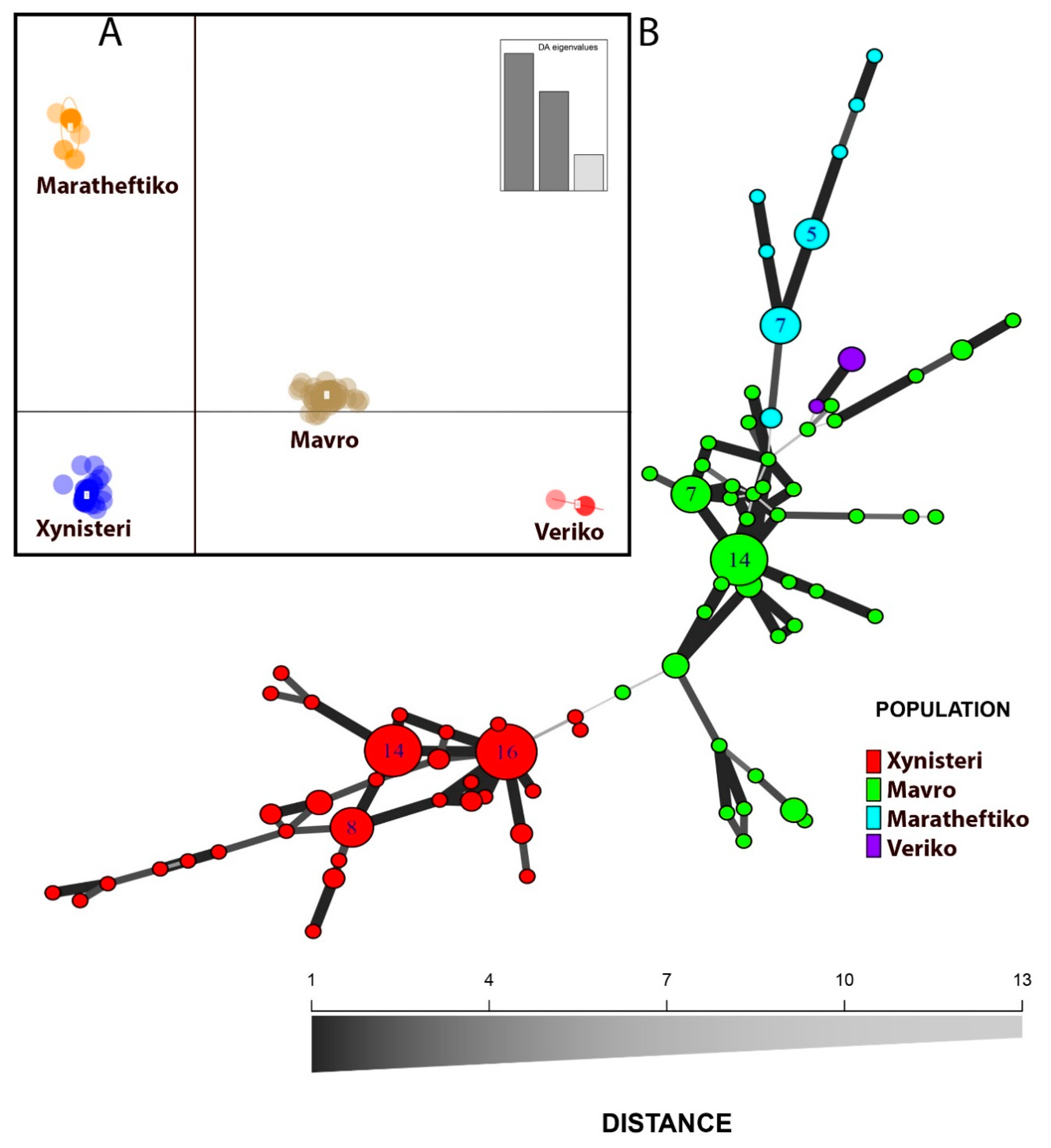
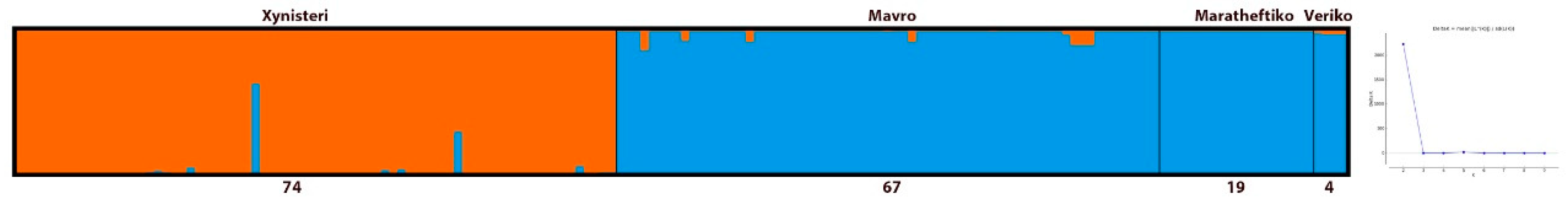
| Pop | MLG (N) | eMLG | H | Hexp | Ia * | rbarD * | Ho | He | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Xynisteri | 32 (74) | 10 | 3.466 | 0.489 | 1.03 | 0.118 | 0.700 | 0.430 |
| 2 | Mavro | 41 (67) | 10 | 3.714 | 0.667 | 4.141 | 0.427 | 0.923 | 0.626 |
| 3 | Maratheftiko | 8 (19) | 8 | 2.079 | 0.567 | −0.395 | −0.148 | 0.981 | 0.526 |
| 4 | Veriko | 2 (4) | 2 | 0.693 | 0.652 | NA | NA | 0.932 | 0.474 |
| 5 | Total | 83 (164) | 10 | 4.419 | 0.732 | 4.143 | 0.42 | 0.884 | 0.514 |
| Locus | Alleles | 1-D | Hexp | Evenness |
|---|---|---|---|---|
| VVMD27 | 7 | 0.75 | 0.75 | 0.8 |
| VrZAG79 | 13 | 0.82 | 0.82 | 0.7 |
| VrZAG67 | 12 | 0.71 | 0.71 | 0.64 |
| VrZAG62 | 8 | 0.81 | 0.81 | 0.89 |
| VrZAG112 | 6 | 0.5 | 0.5 | 0.52 |
| VVS2 | 7 | 0.68 | 0.68 | 0.68 |
| VVMD5 | 8 | 0.79 | 0.8 | 0.86 |
| VVMD7 | 8 | 0.75 | 0.75 | 0.75 |
| VVMD28 | 16 | 0.83 | 0.83 | 0.68 |
| VVMD25 | 6 | 0.58 | 0.58 | 0.65 |
| VVMD32 | 11 | 0.8 | 0.8 | 0.8 |
| mean | 9.27 | 0.73 | 0.73 | 0.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoriou, A.; Tsaniklidis, G.; Hagidimitriou, M.; Nikoloudakis, N. The Cypriot Indigenous Grapevine Germplasm Is a Multi-Clonal Varietal Mixture. Plants 2020, 9, 1034. https://doi.org/10.3390/plants9081034
Grigoriou A, Tsaniklidis G, Hagidimitriou M, Nikoloudakis N. The Cypriot Indigenous Grapevine Germplasm Is a Multi-Clonal Varietal Mixture. Plants. 2020; 9(8):1034. https://doi.org/10.3390/plants9081034
Chicago/Turabian StyleGrigoriou, Apostolis, Georgios Tsaniklidis, Marianna Hagidimitriou, and Nikolaos Nikoloudakis. 2020. "The Cypriot Indigenous Grapevine Germplasm Is a Multi-Clonal Varietal Mixture" Plants 9, no. 8: 1034. https://doi.org/10.3390/plants9081034
APA StyleGrigoriou, A., Tsaniklidis, G., Hagidimitriou, M., & Nikoloudakis, N. (2020). The Cypriot Indigenous Grapevine Germplasm Is a Multi-Clonal Varietal Mixture. Plants, 9(8), 1034. https://doi.org/10.3390/plants9081034






