Abstract
Plant heat shock factors (Hsfs) play crucial roles in various environmental stress responses. Eggplant (Solanum melongena L.) is an agronomically important and thermophilic vegetable grown worldwide. Although the functions of Hsfs under environmental stress conditions have been characterized in the model plant Arabidopsis thaliana and tomato, their roles in responding to various stresses remain unclear in eggplant. Therefore, we characterized the eggplant SmeHsf family and surveyed expression profiles mediated by the SmeHsfs under various stress conditions. Here, using reported Hsfs from other species as queries to search SmeHsfs in the eggplant genome and confirming the typical conserved domains, we identified 20 SmeHsf genes. The SmeHsfs were further classified into 14 subgroups on the basis of their structure. Additionally, quantitative real-time PCR revealed that SmeHsfs responded to four stresses—cold, heat, salinity and drought—which indicated that SmeHsfs play crucial roles in improving tolerance to various abiotic stresses. The expression pattern of SmeHsfA6b exhibited the most immediate response to the various environmental stresses, except drought. The genome-wide identification and abiotic stress-responsive expression pattern analysis provide clues for further analysis of the roles and regulatory mechanism of SmeHsfs under environmental stresses.
1. Introduction
Plants have developed various defense mechanisms that are responsive to different environmental stresses, such as drought, cold, salinity, and heat [1]. Transcription factors, like AP2/ERF, HSP90, WRKY, MYB, NAC, LOX, bZip, and heat shock (Hsfs) [2,3,4,5,6,7,8], are activated and regulate multiple genes and signaling pathways that enable plant adaptation to unfavorable conditions. Among them, Hsfs are involved in many aspects of protein homeostasis under stress conditions [9] and are especially involved in responding to high-temperature stress [10]. In addition to stress responses, Hsfs also play important roles in developmental processes in animals and plants [11].
Although the sequences and sizes of Hsf genes vary, the basic structures and promoter recognition modes are considerably conserved in higher eukaryotes [12]. Almost all the Hsfs have a highly conserved DNA-binding domain (DBD), located close to the N-terminus and containing an antiparallel four-stranded β-sheet and a three-helical bundle, which are required for specific binding with heat stress promoter elements [13,14,15]. The oligomerization domain (HR-A/B region), separated from the DBD domain by a flexible linker of a variable length, contributes to the trimerization of Hsfs by forming a coiled-coil structure [16]. Additionally, three other conserved structures—a nuclear localization signal (NLS), nuclear export signal (NES) and activator motif (AHA)—are present. Some Hsfs also contain a repression domain at the C-terminus [17]. On the basis of structural characteristics and phylogenetic comparisons, plant Hsf genes can be further divided into A, B, and C classes [12,18], which contain insertions of 21, 0, and 7 amino acid residues, respectively, between the HR-A and HR-B regions [12,18]. In addition, the amino acid length from the DBD to HR-A/B differs among the three classes [12]. The AHA is present in class A, but absent in classes B and C [17]. Class A Hsfs are involved in transcriptional activation and responses to environmental stresses [19], while class B Hsfs function as transcriptional coactivators with class A Hsfs or as gene expression repressors [9,20]. At present, there are few studies on class C; only several studies show that class C Hsfs can be induced by a variety of stresses [21,22].
Hsfs are engaged in responses to abiotic stresses conditions. For example, Arabidopsis thaliana HSFA1s and HSFA2 participate in responses to various abiotic stresses, such as salinity, osmotic pressure, oxidation, and anoxia [23,24,25], while HSFA1b and HSFA3 are involved in drought-stress responses [17,26]. Tomato HsfA1a plays a critical role in the development of thermotolerance and cannot be replaced by other tomato Hsfs [27]; HsfA1b and HsfA1e are likely responding to stress in specific tissues, while HsfA1c functions as a co-regulator in mild heat stress response. Tomato HsfA2 can increase plant heat tolerance by accumulating to high levels [28] and is also involved in protecting maturing and germinating pollen under heat-stress conditions [29]. In addition, wheat HsfA4a is involved in cadmium tolerance [19]. Chrysanthemum HSFA4 confers salinity tolerance as a consequence of Na+/K+ ion and reactive oxygen species homeostasis [30]. HSFA2 and A6 from wheat, HSF3, -18, -24, -32, -37, and -40 from cotton and HSF-06, -10, -14, -20, and -21 from maize may be involved in responding to heat stress [22,31,32]. Owing to their essential modulatory functions in plants, Hsf gene family members have been studied in several agronomically important plants, such as rice (Oryza sativa), maize (Zea mays), apple (Malus domestica), poplar (Populus trichocarpa), and cabbage (Brassica oleracea) [31,33,34,35,36]. However, the Hsf gene family in eggplant (Solanum melongena L.) has not been systematically studied.
Eggplant is an economically important vegetable cultivated worldwide. The optimal season for eggplant growth is autumn, when the temperature ranges from 22 to 30 °C [37]. During year-round production in protected cultivation, eggplant encounters various environmental stresses, including heat, cold, drought, and salinity. Here, we performed a genome-wide study to comprehensively analyze the eggplant Hsf gene family. We identified 20 SmeHsf genes and determined protein properties, phylogenetic relationships, gene structures, and conserved protein domains. We also investigated the expression changes of Hsf genes in plants subjected to different abiotic stresses. Our study provides a foundation for further SmeHsfs functional investigations and could help better understand the environmental stress-response-related molecular mechanisms of Hsf genes in eggplant.
2. Results
2.1. Identification, Classification, and Characterization of the Hsf Gene Family in Eggplant
A total of 20 Hsf genes were identified in the eggplant genome (Table S1). This is less than in pepper (25), tomato (26), potato (25), and cultivated tobacco (65). Subsequently, the 20 SmeHsf genes were classified into three subgroups—A, B, and C—according to the HEATSTER websites [38]. Most of the SmeHsfs, 14 out of 20, were classified into subgroup A, and these SmeHsfs were further classified into seven subgroups (A1, A3, A4, A5, A6, A8, and A9). Class B had five members from four subgroups (B1, B2, B3, and B4). Subgroups A1, A4, A6, A9, and B2 contained more than one member (Table 1).

Table 1.
Physicochemical characteristics and classification of Hsf genes in eggplant.
The physical and chemical properties of the SmeHsf proteins were analyzed and some differences were observed. The lengths of SmeHsf proteins varied from 213 to 496 amino acids, and the molecular weights ranged from 24.62 to 55.07 kDa. Among the 20 SmeHsf proteins, SmeHsfB3a had the shortest length and lowest molecular weight. Additionally, the predicted aromaticity ranged from 0.05 to 0.11, and the isoelectric point ranged from 4.60 to 9.44 (Table 1). The instability index, which provides an estimate of the stability of a protein in a test tube, indicated that all the SmeHsf proteins are unstable (scores greater than 40), except SmeHsfB1, which had an instability index of 30.35. The grand average of hydropathy values of the SmeHsfs was less than 0, suggesting that they are hydrophilic. These differences mostly resulted from variations in the non-conserved regions’ amino acid sequences.
2.2. Conserved Domains and Structural Analysis of SmeHsfs
The functional domains of the Hsfs have been studied in some model plants [11]. Detailed information regarding the conserved domains, such as DBD, HR-A/B, NLS, NES, and AHA, are presented in Table 2. As the core functional domain of the Hsfs, the DBD was composed of approximately 90 amino acids and existed in all the predicted SmeHsf proteins. In addition, another conserved domain, HR-A/B, was also present in all the SmeHsfs. Based on the number of amino acid residues inserted into the HR-A/B regions, the 20 SmeHsfs were divided into three major classes. Class A and class C Hsfs contained 21 and 7 amino acid residues between the A and B regions, respectively (Figure 1). This classification confirmed the results of HEATSTER website. Most of the SmeHsf proteins (13 out of 20) included an NLS domain, and the NES domain was detected in seven SmeHsfs. The AHA domain was detected in the A4, A5, A6, and A9 subgroups; however, it was not found in class B or C.

Table 2.
The function domains and their position in SmeHsfs.

Figure 1.
Multiple sequence alignment of the HR-A/B regions (OD) of SmeHsfs.
To further analyze the motifs and structural variations of SmeHsfs, we constructed a separate phylogenetic tree containing only SmeHsf proteins, and then, compared motif compositions and exon/intron organizations (Figure 2A). Generally, most of the closely related members had similar motif compositions and exon/intron organizations and lengths. The Multiple Em for Motif Elicitation (MEME) web server was used to search for motifs in the SmeHsf proteins. There were 15 potential motifs distributed throughout the Hsf protein sequences (Figure 2B). Motifs 1 and 2, or Motifs 2 and 8, which corresponded to the DBD domain, were found in all the SmeHsfs. Motif 4 was also identified in all the SmeHsfs and corresponded to the HR-A/B region. Different subgroups had similar motifs and contained their own unique motifs. Motif 3 was found in class A and C members, while Motif 12 was only found in class B members.
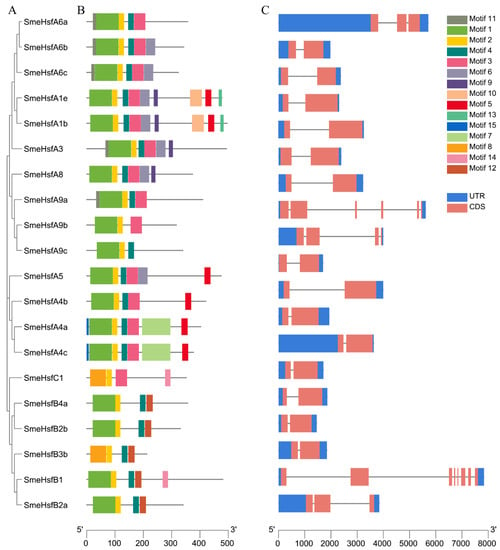
Figure 2.
Phylogenetic, motif and structural analyses of SmeHsfs. (A) Phylogenetic tree of SmeHsf proteins. (B) Schematic representation of the motif compositions of SmeHsfs. (C) Exon/intron structures of SmeHsf genes.
The exon/intron structures exhibited a highly conserved organization in 14 out of 20 SmeHsfs possessing strictly two exons, which was similar to the structures of Hsf genes in other plants [39,40]. In addition, we identified two genes possessing three exons (SmeHsfA6a and SmeHsfB2a), one gene containing four exons (SmeHsfA9b), and two genes (SmeHsfA9a and SmeHsfB1) having more than six exons (Figure 2C).
2.3. Phylogenetic Analysis of SmeHsfs
To study the evolutionary characteristics of SmeHsf proteins, we selected three other well-studied and representative plant species, including one related species (Solanum lycopersicum), a monocot (O. sativa), and a eudicot (A. thaliana). The full-length amino acid sequences of Hsf proteins in eggplant and these three species were used to construct a phylogenetic tree (Figure 3 and Table S2). The SmeHsfs in the same subgroup were classified together, which indicated that the SmeHsfs in the same subgroup not only have similar domain structures, and also, have similar sequences. The phylogenetic analysis also showed that the number of Hsf genes in different subclasses varied among land plants. For example, eggplant has no subclass A2 members, while rice has no subclass A9 and B3 members. Besides, the Hsf genes only varied slightly in different subclasses between eggplant and tomato, indicating an even distribution within the family Solanaceae.
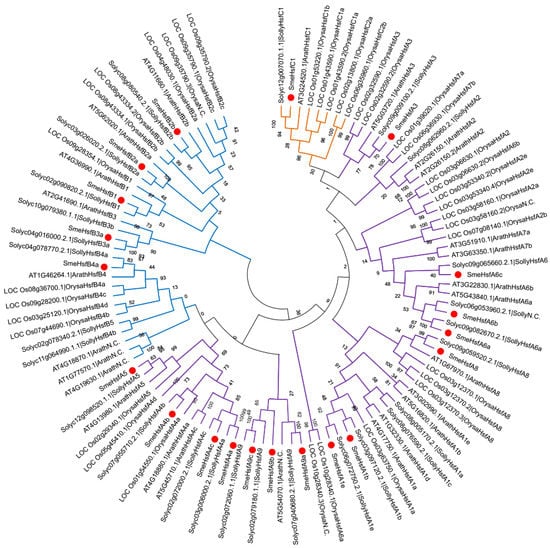
Figure 3.
The phylogenetic tree of the Hsf genes from four plant species. Individual species are distinguished by different gene code prefixes. The prefixes Arath, Orysa, Solyc, and Sme indicate that these genes are from A. thaliana, rice, tomato, and eggplant, respectively. Red circles indicate eggplant genes. Additionally, purple, blue, and yellow branches indicate classes A, B, and C, respectively.
2.4. Putative Regulatory cis-Elements of the SmeHsf Promoters
To further explore the potential regulatory mechanisms of SmeHsfs during stress responses, we used the PlantCARE database [41] to detect the cis-elements in the promoters (Table S3). In total, 88 cis-elements were identified, with 55 having known functions. The most commonly known function was responsiveness to light (26 out of 55), followed by other regulatory functions (14 out of 55), and responsiveness to hormones (9 out of 55). In addition, four abiotic stress-response elements—LTRs, MBSs, TC-rich repeats, and WUN motifs—were identified. The SmeHsfs, except for SmeHsfA4c, SmeHsfB2b, SmeHsfB3a, and SmeHsfC1, possessed at least one stress-response-related cis-element (Figure 4). In total, six SmeHsfs had one or more LTR, suggesting a potential cold-stress response under low temperature conditions. Additionally, MBSs, TC-rich repeats, and WUN motifs were found in 9, 2, and 10 SmeHsfs, respectively. The cis-element analysis indicated that SmeHsf genes could respond to different abiotic stresses.
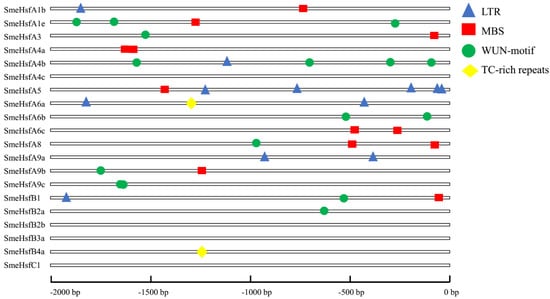
Figure 4.
The position of abiotic stress-response cis-elements on SmeHsf promoters.
2.5. qRT-PCR Analysis of SmeHsf Responses to Different Abiotic Stresses
The expression levels of Hsf genes are affected by heat and other abiotic stresses in plants [42]. In this study, we analyzed the expression levels of SmeHsfs under different stress conditions, including cold, heat, salinity, and drought, to determine the stress-responsive candidates (Figure 5). The expression level of SmeHsfA6b dramatically increased 43-, 54-, and 8-fold under cold, salinity, and heat treatments, respectively, indicating its function in increasing plant adaptability to these abiotic stresses. In total, 14, 10, 9, and 8 SmeHsfs showed significant differential expression levels under cold, heat, salinity, and drought treatments, respectively. Thus, the functions of these stress-induced SmeHsfs should be analyzed in further studies. Overall, the average ranges of expression level changes of these SmeHsfs under cold conditions were greater than those identified under other stress conditions. Under cold-stress conditions, the expression levels of SmeHsfC1 and SmeHsfA1b increased more than 10-fold, while those of SmeHsfA3, SmeHsfA4c, and SmeHsfB3a increased 3–5-fold. In addition, the expression levels of SmeHsfA5 and SmeHsfA6a were upregulated approximately 3–4-fold in response to a heat treatment.
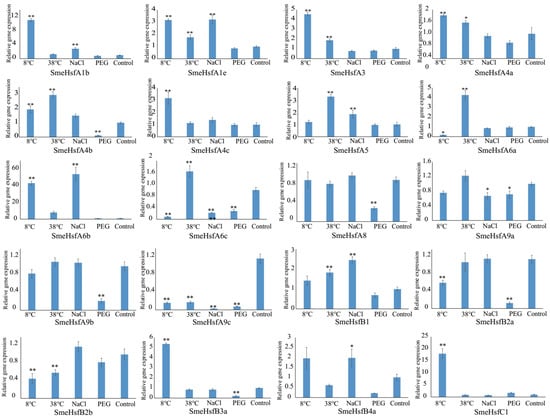
Figure 5.
qRT-PCR analysis of SmeHsf genes under various abiotic stress conditions. The expression level of the control CK treatment was normalized as 1.0. The results are shown as means ± SDs of three independent experiments. The significant differences at p ≤ 0.05 and p ≤ 0.01 are represented by one and two asterisks, respectively.
3. Discussion
Eggplant is an important vegetable belonging to the Solanaceae family, which encompasses crops like tobacco, tomato, potato, and pepper. The Hsfs act as terminal components of signal networks that participate in various abiotic stress responses [43]. Hsfs can regulate the expression of molecular chaperones, such as heat shock proteins, which are involved in heat-stress responses [44] and regulate the signaling networks of stress-related phytohormones, such as salicylic and abscisic acids [32,45]. However, a comprehensive characterization of the Hsf gene family in eggplant is lacking.
In this study, we identified 20 SmeHsf genes. In Solanaceae species, the genome sizes and gene numbers of eggplant (833.1 Mb and 42,035 coding genes, respectively), potato (844 Mb and 35,119 coding genes), and tomato (950 Mb and 34,727 coding genes) are similar [46,47,48,49]; however, eggplant has the lowest number of Hsf genes. Notably, the genome size of pepper (3.48 Gb) was approximately fourfold larger than those of these three species, but the coding genes (34,899) and Hsf gene numbers did not vary significantly [49]. However, cultivated tobacco, which has an almost fivefold larger genome size (4.41–4.57 Gb) than that of eggplant and has a high number of coding genes (85,439 coding genes) [50], has more than twice the number of Hsf genes than eggplant. Thus, the number of Hsf genes is not correlated with the genome size, but is proportionally related to the total number of coding genes. Consequently, because pepper is a diploid species and contains a large number of repetitive sequences [49], it has less Hsf genes compared with the tetraploid cultivated tobacco, which has undergone an allopolyploidization event.
An unrooted phylogenetic tree was constructed using previously reported Hsfs and SmeHsfs. The SmeHsfs in the same subgroups clustered together, corresponding to other Hsf genes, which confirmed the SmeHsf classification. Class A was the predominant class in both monocots and dicots. Like the Hsfs in other plants, all 20 SmeHsfs contained conserved DBD and HR-A/B domains, which are essential for their transcriptional functions. Although the overall gene structure of SmeHsfs in the A, B, and C classes were similar, the different groups contained characteristic domains.
Expression profile changes of Hsf genes that occur under various abiotic stresses have been extensively analyzed in different plants [40,51,52,53]. Investigating the expression changes of SmeHsfs under different stresses provides clues to their functions. SmeHsfs responded to the four stresses including heat, cold, salinity, and drought, which indicated that SmeHsfs increase tolerance levels to various abiotic stresses. Up to the present, many researchers found that class A and B Hsfs are involved in responding to environmental stresses, and few focused on the class C. In our study, we found that all class A and B SmeHsfs up- or downregulated under at least one stress, which indicated their functions in responding to environmental stresses. Interestingly, the expression level change of SmeHsfC1 was only less than that of SmeHsfA6b under low temperature treatment, which indicated the class C member also plays a role in responding to low temperature stress in eggplant.
Hsf genes are expected to always respond to heat stress [54]. However, more SmeHsfs showed significant differential expression levels and greater ranges in expression changes under cold conditions than under other stress conditions, which might be because eggplant is a warm-weather plant that is more sensitive to low temperature [55]. More SmeHsfs showed significant differential expression levels under cold- and heat-stress conditions than under saline and drought conditions, which might be because leaf tissue was detected in this study and the leaves being the first organs to perceive heat and cold stresses, while the roots are the first organs to sense drought and salinity stresses [40]. AtHsfA6a and AtHsfA6b participate in abscisic acid-mediated thermotolerance and drought tolerance [32]; however, the wheat HsfA6, which is the most inducible wheat Hsf gene, is only responsive to the oxidative stress-signaling pathway [40]. In our study, SmeHsfA6b was also the most inducible SmeHsf gene, being upregulated by cold, heat and salinity treatments in the leaves, but not in response to the drought stress, which indicated that homologous Hsf genes have different functions in different plants. Moreover, in tomato, HsfA1 plays a leading role in the heat-shock reaction and combines with HsfA2 to form a complex that increases plant heat tolerance [28]. However, HsfA2 was not identified in eggplant. Thus, the expression analysis indicated that SmeHsfs respond in unique manners to various environmental stresses, and the responses of these SmeHsfs are different in both magnitude and sensitivity to the above stresses.
4. Materials and Methods
4.1. Identification and Characterization of Hsfs in Eggplant
The eggplant genome (version SME_r2.5.1) and annotation data were downloaded from the Sol Genomics Network database [48]. Hmmsearch methods and BLAST searches were combined to identify Hsf genes in eggplant. Briefly, 325 Hsf gene sequences from A. thaliana (25), Capsicum annuum (25), Carica papaya (18), Glycine max (81), M. domestica (47), Nicotiana tabacum (65), O. sativa (36), and Solanum lycopersicum (26) were downloaded from PlantTFBD [56]. The downloaded Hsf proteins from different species were used as queries to search for all the possible Hsf protein sequences in the eggplant proteome file with an E-value of le-10 and identity of 60% as the thresholds. Then, Hmmsearch software was used to search for the Hsf domain (PF00447), which was downloaded from the Pfam database 32.0 [57], in the set of BLAST-identified proteins. The Hsf proteins were filtered with an E-value cutoff of 1 × 10−5 and at least a 60% coverage of the Pfam Hsf domain from the raw screening proteins. Furthermore, all the candidate Hsf protein sequences were analyzed to detect the DBD and coiled-coil structures using the SMART [58] and MARCOIL programs [59]. Those protein sequences, containing both a DBD and coiled-coil structure, were regarded as credible Hsf proteins. Moreover, the HEATSTER website [38] was used to confirm the 20 SmeHsf genes and classified them into subgroups. Finally, the Biopython module [60] was used to predict the molecular weight, isoelectric point, and other physical and chemical properties of the SmeHsf proteins. All the SmeHsf genes were renamed on the basis of their classifications and their phylogenetic relationships to S. lycopersicum and other species.
4.2. Gene Structure, Domain and Motif Analyses
Gene structural information was obtained from GFF3 files and visualized using TBtools software [61]. All the full-length amino acid sequences of the SmeHsfs were used to search for conserved motifs using the MEME tool [62]. The MEME parameters were set as follows: the maximum number to be found was set to 15 and the motif window length was set 8 to 100 bp. Additionally, the conserved NLS and NES domains were predicted using cNLS Mapper software [63] and NetNES 1.1 server software, respectively. The AHA domain was identified using the conserved motif FWxxF/L, F/I/L [64].
4.3. Phylogenetic Analysis and Classification of SmeHsf Genes
The amino acid sequences of SmeHsf proteins identified in this study and other Hsfs from A. thaliana, O. sativa, and S. lycopersicum downloaded from the HEATSTER website [38] were used in the phylogenetic analysis. The complete amino acid sequences of Hsf proteins and HR-A/B domain were aligned using the MUSCLE program [65]. Subsequently, the MEGA-X program was used to construct an unrooted maximum likelihood phylogenetic tree with the Jones–Taylor–Thornton model. Additionally, a bootstrap test was replicated 500 times and a partial deletion with a site coverage cutoff of 70% was used for gap treatment.
4.4. cis-Element Analysis of SmeHsf Promoters
The upstream 2000 bps of SmeHsf genes were abstracted as the promoter sequences from the eggplant genome file. Then, the PlantCARE database was used to determine the cis-regulatory elements present in each gene’s promoter [41]. Besides, the upstream 2000 bps of random selected 500 eggplant genes were also used to determine the cis-regulatory elements using PlantCARE and compared with SmeHsf.
4.5. Plant Materials and Stress Treatments
The seeds of eggplant inbred line ‘E22’ were grown in plastic pots on the horticultural farm of the Zhejiang Academy of Agriculture Science (Hangzhou, China). At the four true-leaf stage, seedlings were moved to a growth chamber set at 16 h day (28 °C)/8 h night (24 °C) and used for experiments. For drought- and salt-stress treatments, seedlings were subjected to 100 mL of 30% PEG6000 and 300 mM NaCl, respectively, for 48 h, and for heat- and cold-stress treatments, seedlings were subjected to 38 °C and 8 °C, respectively, for 24 h. Plants were cultured under normal conditions for the control. The new leaves of five seedlings were collected as biological replicates, and each treatment had three replicates. The freshly collected samples were immediately frozen in liquid nitrogen stored at −80 °C for RNA isolation.
4.6. RNA Extraction and qRT-PCR Analysis
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to independently extract total RNAs of all the samples, and genomic DNA contamination was removed using DNase I. Then, RNA concentrations were measured using a NanoDrop2000 microvolume spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA), and the RNA integrity was checked by 1.5% agarose gel electrophoresis. PrimeScript RTase (TaKaRa Biotechnology, Dalian, China) was used for first-strand cDNA synthesis following the manufacturer’s instructions. The primers for qRT-PCR reactions were designed using Primer Premier 5.0, and the SmEF1a gene was used as a stable reference gene [66]. The qRT-PCR reactions were performed on a TIB8600 machine using AceQ® qPCR SYBR® Green Master Mix kits (Vazyme Biotechnology, Nanjing, China) with the following settings: 95 °C for 5 min; followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. The relative expression levels of SmeHsf genes were calculated using the 2−ΔΔCt method [67]. The analysis included three biological replicates for each sample. All the primer sequences are listed in Table S4.
4.7. Statistical Analyses
The statistical analysis was carried out by calculating the average values and standard errors for the three replicates. SPSS software version 16.0 was used to determine the significant differences between controls and stress treatments using a one-way ANOVA procedure and post hoc analysis. A p value ≤ 0.05 indicates a significant difference and is represented by an asterisk (*); a p value ≤ 0.01 indicates a very significant difference and is represented by two asterisks (**).
5. Conclusions
In the present study, 20 full-length SmeHsf genes were identified in the eggplant genome. These SmeHsfs were comprehensively characterized using a systematic approach comprising analyses of sequence characteristics, phylogeny, classifications, gene structures, and motif compositions. Moreover, a qRT-PCR analysis of SmeHsf expression levels in response to various abiotic stresses indicated that SmeHsfs not only play crucial roles in heat tolerance, but also increase the tolerance levels to various abiotic stresses. This comprehensive analysis provides candidate genes for future functional analyses under stress conditions and also lays the foundation for investigating molecular mechanisms of abiotic stress tolerance in plants.
Supplementary Materials
Supplementary Materials can be found at https://www.mdpi.com/2223-7747/9/7/915/s1. Table S1: The alignment information and scaffold position of the SmeHsfs, Table S2: The protein sequences of the SmeHsfs, Table S3: cis-Element analysis of SmeHsf promoters, Table S4: qRT-PCR primers for the SmeHsf genes.
Author Contributions
Conceptualization, C.B.; methodology, J.W.; software, J.W.; validation, J.W., C.B.; formal analysis, J.W.; investigation, T.H.; resources, T.H.; data curation, W.W.; writing—original draft preparation, J.W.; writing—review and editing, J.W.; visualization, Q.W.; supervision, C.B., T.H.; project administration, H.H.; funding acquisition, C.B.; H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant from the New Variety Breeding Project of the Major Science Technology Projects of Zhejiang (2016C02051-2), Zhejiang Science and Technology Program (2018C02057) and the Natural Science Foundation of Zhejiang (LQ18C150004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Wu, C. Heat shock transcription factors: Structure and regulation. Annu. Rev. Cell. Dev. Biol. 1995, 11, 441–469. [Google Scholar] [CrossRef] [PubMed]
- Brash, A.R. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 1999, 274, 23679–23682. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2002, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Trends Plant Sci. 2010, 15, 247–258. [Google Scholar] [CrossRef]
- Puranik, S.; Sahu, P.P.; Srivastava, P.S.; Prasad, M.N.V. NAC proteins: Regulation and role in stress tolerance. Trends Plant Sci. 2012, 17, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Park, M.; Kitazumi, A.; Herath, V.; Mohanty, B.; Yun, S.J.; Reyes, B.G.D.L. Cis-regulatory signatures of orthologous stress-associated bZIP transcription factors from rice, sorghum and Arabidopsis based on phylogenetic footprints. BMC Genom. 2012, 13, 497. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Garg, V.; Kudapa, H.; Doddamani, D.; Pazhamala, L.T.; Khan, A.W.; Thudi, M.; Lee, S.; Varshney, R.K. Genome-wide dissection of AP2/ERF and HSP90 gene families in five legumes and expression profiles in chickpea and pigeonpea. Plant Biotechnol. J. 2016, 14, 1563–1577. [Google Scholar] [CrossRef]
- Wang, J.; Sun, N.; Deng, T.; Zhang, L.; Zuo, K. Genome-wide cloning, identification, classification and functional analysis of cotton heat shock transcription factors in cotton (Gossypium hirsutum). BMC Genom. 2014, 15, 961. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.; Ma, X.; Luo, D.; Gong, Z.; Lu, M. The plant heat stress transcription factors (HSFs): Structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef]
- Akerfelt, M.; Morimoto, R.I.; Sistonen, L. Heat shock factors: Integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010, 11, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Nover, L.; Bharti, K.; Doring, P.; Mishra, S.K.; Ganguli, A.; Scharf, K. Arabidopsis and the heat stress transcription factor world: How many heat stress transcription factors do we need? Cell Stress Chaperon. 2001, 6, 177–189. [Google Scholar] [CrossRef]
- Bienz, M.; Pelham, H.R.B. Mechanisms of heat-shock gene activation in higher eukaryotes. Adv. Genet. 1987, 24, 31–72. [Google Scholar]
- Damberger, F.F.; Pelton, J.G.; Harrison, C.J.; Nelson, H.C.M.; Wemmer, D.E. Solution structure of the DNA-binding domain of the heat shock transcription factor determined by multidimensional heteronuclear magnetic resonance spectroscopy. Protein Sci. 1994, 3, 1806–1821. [Google Scholar] [CrossRef] [PubMed]
- Vuister, G.W.; Kim, S.; Orosz, A.; Marquardt, J.L.; Wu, C.; Bax, A. Solution structure of the DNA-binding domain of Drosophila heat shock transcription factor. Nat. Struct. Mol. Biol. 1994, 1, 605–614. [Google Scholar] [CrossRef]
- Peteranderl, R.; Rabenstein, M.; Shin, Y.; Liu, C.W.; Wemmer, D.E.; King, D.S.; Nelson, H.C.M. Biochemical and biophysical characterization of the trimerization domain from the heat shock transcription factor. Biochemistry 1999, 38, 3559–3569. [Google Scholar] [CrossRef]
- Scharf, K.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. BBA-Gene Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef]
- Baniwal, S.K.; Bharti, K.; Chan, K.Y.; Fauth, M.; Ganguli, A.; Kotak, S.; Mishra, S.K.; Nover, L.; Port, M.; Scharf, K. Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 2004, 29, 471–487. [Google Scholar] [CrossRef]
- Shim, D.; Hwang, J.; Lee, J.; Lee, S.; Choi, Y.; An, G.; Martinoia, E.; Lee, Y. Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 2009, 21, 4031–4043. [Google Scholar] [CrossRef]
- Ikeda, M.; Mitsuda, N.; Ohmetakagi, M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible hsfs but positively regulate the acquired thermotolerance. Plant Physiol. 2011, 157, 1243–1254. [Google Scholar] [CrossRef]
- Ma, J.; Xu, Z.; Wang, F.; Tan, G.; Li, M.; Xiong, A. Genome-wide analysis of HSF family transcription factors and their responses to abiotic stresses in two Chinese cabbage varieties. Acta Physiol. Plant. 2014, 36, 513–523. [Google Scholar] [CrossRef]
- Xue, G.P.; Sadat, S.; Drenth, J.; Mcintyre, C.L. The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J. Exp. Bot. 2014, 65, 539–557. [Google Scholar] [CrossRef]
- Ogawa, D.; Yamaguchi, K.; Nishiuchi, T. High-level overexpression of the Arabidopsis HsfA2 gene confers not only increased themotolerance but also salt/osmotic stress tolerance and enhanced callus growth. J. Exp. Bot. 2007, 58, 3373–3383. [Google Scholar] [CrossRef] [PubMed]
- Banti, V.; Mafessoni, F.; Loreti, E.; Alpi, A.; Perata, P. The heat-inducible transcription factor HSFA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol. 2010, 152, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liao, H.; Charng, Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011, 34, 738–751. [Google Scholar] [CrossRef]
- Bechtold, U.; Albihlal, W.S.; Lawson, T.; Fryer, M.J.; Sparrow, P.A.C.; Richard, F.; Persad, R.; Bowden, L.; Hickman, R.; Martin, C. Arabidopsis HEAT SHOCK TRANSCRIPTION FACTORA1b overexpression enhances water productivity, resistance to drought, and infection. J. Exp. Bot. 2013, 64, 3467–3481. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Tripp, J.; Winkelhaus, S.; Tschiersch, B.; Theres, K.; Nover, L.; Scharf, K.-D. In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev. 2002, 16, 1555–1567. [Google Scholar] [CrossRef]
- Scharf, K.-D.; Heider, H.; Höhfeld, I.; Lyck, R.; Schmidt, E.; Nover, L. The tomato Hsf system: HsfA2 needs interaction with HsfA1 for efficient nuclear import and may be localized in cytoplasmic heat stress granules. Mol. Cell Biol. 1998, 18, 2240–2251. [Google Scholar] [CrossRef]
- Giorno, F.; Woltersarts, M.; Grillo, S.; Scharf, K.; Vriezen, W.H.; Mariani, C. Developmental and heat stress-regulated expression of HsfA2 and small heat shock proteins in tomato anthers. J. Exp. Bot. 2010, 61, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, H.; Zhao, H.; Gao, T.; Song, A.; Jiang, J.; Chen, F.; Chen, S. Chrysanthemum CmHSFA4 gene positively regulates salt stress tolerance in transgenic chrysanthemum. Plant Biotechnol. J. 2018, 16, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Giorno, F.; Guerriero, G.; Baric, S.; Mariani, C. Heat shock transcriptional factors in Malus domestica: Identification, classification and expression analysis. BMC Genom. 2012, 13, 639. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Niu, C.; Yang, C.; Jinn, T. The heat stress factor HSFA6b Connects ABA signaling and ABA-mediated heat responses. Plant Physiol. 2016, 172, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, J.; Ji, Q.; Wang, C.; Luo, L.; Yuan, Y.; Wang, Y.; Wang, J. Genome-wide analysis of heat shock transcription factor families in rice and Arabidopsis. J. Genet. Genom. 2008, 35, 105–118. [Google Scholar] [CrossRef]
- Chauhan, H.; Khurana, N.; Agarwal, P.; Khurana, P. Heat shock factors in rice (Oryza sativa L.): Genome-wide expression analysis during reproductive development and abiotic stress. Mol. Genet. Genom. 2011, 286, 171–187. [Google Scholar] [CrossRef]
- Wang, F.; Dong, Q.; Jiang, H.; Zhu, S.; Chen, B.; Xiang, Y. Genome-wide analysis of the heat shock transcription factors in Populus trichocarpa and Medicago truncatula. Mol. Biol. Rep. 2012, 39, 1877–1886. [Google Scholar] [CrossRef]
- Lohani, N.; Golicz, A.A.; Singh, M.; Bhalla, P.L. Genome-wide analysis of the Hsf gene family in Brassica oleracea and a comparative analysis of the Hsf gene family in B. oleracea, B. rapa and B. napus. Funct. Integr. Genom. 2019, 19, 515–531. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Li, Z.; Luo, S.; Sun, B. Effects of heat stress on gene expression in eggplant ( Solanum melongema L.) seedlings. Afr. J. Biotechnol. 2011, 10, 18078–18084. [Google Scholar]
- Berz, J.; Simm, S.; Schuster, S.; Scharf, K.; Schleiff, E.; Ebersberger, I. HEATSTER: A database and web server for identification and classification of heat stress transcription factors in plants. Bioinform. Biol. Insights 2019, 13, 117793221882136. [Google Scholar] [CrossRef]
- Agarwal, P.; Khurana, P. Functional characterization of HSFs from wheat in response to heat and other abiotic stress conditions. Funct. Integr. Genom. 2019, 19, 497–513. [Google Scholar] [CrossRef]
- Duan, S.; Liu, B.; Zhang, Y.; Li, G.; Guo, X. Genome-wide identification and abiotic stress-responsive pattern of heat shock transcription factor family in Triticum aestivum L. BMC Genom. 2019, 20, 257. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; De Peer, Y.V.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucl. Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chou, S.; Murshid, A.; Prince, T.L.; Schreiner, S.; Stevenson, M.A.; Calderwood, S.K. The role of heat shock factors in stress-induced transcription. Methods Mol. Biol. 2011, 787, 21–32. [Google Scholar] [PubMed]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Gho, H.J.; Nguyen, M.X.; Kim, S.-R.; An, G. Genome-wide expression analysis of HSP70 family genes in rice and identification of a cytosolic HSP70 gene highly induced under heat stress. Funct. Integr. Genom. 2013, 13, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Snyman, M.; Cronjé, M. Modulation of heat shock factors accompanies salicylic acid-mediated potentiation of Hsp70 in tomato seedlings. J. Exp. Bot. 2008, 59, 2125–2132. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pan, S.K.; Cheng, S.F.; Zhang, B.; Bachem, C.W.B.; De Boer, J.M.; Borm, T.J.A.; Kloosterman, B.; Van Eck, H.J.; Datema, E. Genome sequence and analysis of the tuber crop potato. Nature 2011, 475, 189–195. [Google Scholar]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar]
- Hirakawa, H.; Shirasawa, K.; Miyatake, K.; Nunome, T.; Negoro, S.; Ohyama, A.; Yamaguchi, H.; Sato, S.; Isobe, S.; Tabata, S. Draft genome sequence of eggplant (Solanum melongena L.): The representative solanum species indigenous to the old world. DNA Res. 2014, 21, 649–660. [Google Scholar] [CrossRef]
- Kim, S.; Park, M.; Yeom, S.; Kim, Y.; Lee, J.M.; Lee, H.; Seo, E.; Choi, J.Y.; Cheong, K.; Kim, K. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef]
- Sierro, N.; Battey, J.N.D.; Ouadi, S.; Bakaher, N.; Bovet, L.; Willig, A.; Goepfert, S.; Peitsch, M.C.; Ivanov, N.V. The tobacco genome sequence and its comparison with those of tomato and potato. Nat. Commun. 2014, 5, 3833. [Google Scholar] [CrossRef]
- Mittal, D.; Chakrabarti, S.; Sarkar, A.; Singh, A.; Grover, A. Heat shock factor gene family in rice: Genomic organization and transcript expression profiling in response to high temperature, low temperature and oxidative stresses. Plant Physiol. Bioch. 2009, 47, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Dossa, K.; Diouf, D.; Cisse, N. Genome-wide investigation of hsf genes in sesame reveals their segmental duplication expansion and their active role in drought stress response. Front. Plant Sci. 2016, 7, 1522. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, S.; Liu, R.; Lu, J.; Lu, L.; Zhang, C.; Liu, Z.; Luo, C.; Zhang, L.; Yant, L. Genome-wide identification, phylogenetic and expression analysis of the heat shock transcription factor family in bread wheat (Triticum aestivum L.). BMC Genom. 2019, 20, 505. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, J.; Han, X.; Zhang, Y.; Zhuo, R. Identification, expression analysis of the Hsf family, and characterization of class A4 in Sedum Alfredii hance under cadmium stress. Int. J. Mol. Sci. 2018, 1216. [Google Scholar] [CrossRef]
- Lv, L.L.; Feng, X.F.; Li, W.; Li, K. High temperature reduces peel color in eggplant (Solanum melongena) as revealed by RNA-seq analysis. Genome 2019, 62, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.; Meng, Y.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucl. Acids Res. 2017, 45. [Google Scholar] [CrossRef]
- Elgebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A. The Pfam protein families database in 2019. Nucl. Acids Res. 2019, 47. [Google Scholar]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, 193–196. [Google Scholar] [CrossRef]
- Zimmermann, L.; Stephens, A.; Nam, S.; Rau, D.; Kübler, J.; Lozajic, M.; Gabler, F.; Söding, J.; Lupas, A.; Alva, V. A completely reimplemented MPI bioinformatics toolkit with a new HHpred server at its core. J. Mol. Biol. 2018, 430, 2237–2243. [Google Scholar] [CrossRef]
- Peter, J.A.C.; Tiago, A.; Jeffrey, T.C.; Brad, A.C.; Cymon, J.C.; Andrew, D.; Iddo, F.; Thomas, H.; Frank, K.; Bartek, W.; et al. Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 2009, 25, 1422–1423. [Google Scholar]
- Chen, C.; Xia, R.; Chen, H.; He, Y. TBtools, a Toolkit for Biologists integrating various HTS-data handling tools with a user-friendly interface. bioRxiv 2018, 289660. [Google Scholar]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.C.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucl. Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Hasebe, M.; Tomita, M.; Yanagawa, H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 2009, 106, 10171–10176. [Google Scholar] [CrossRef]
- Kotak, S.; Port, M.; Ganguli, A.; Bicker, F.; Von Koskulldoring, P. Characterization of C-terminal domains of Arabidopsis heat stress transcription factors (Hsfs) and identification of a new signature combination of plant class A Hsfs with AHA and NES motifs essential for activator function and intracellular localization. Plant J. 2004, 39, 98–112. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Pang, Q.; Li, Z.; Luo, S.; Chen, R.; Jin, Q.; Li, Z.; Li, D.; Sun, B.; Sun, G. Selection and stability analysis of reference gene for qRT-PCR in eggplant under high temperature stress. Acta Hortic. Sin. 2017, 44, 475–486. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).