Some Accessions of Amazonian Wild Rice (Oryza glumaepatula) Constitutively Form a Barrier to Radial Oxygen Loss along Adventitious Roots under Aerated Conditions
Abstract
1. Introduction
2. Results
2.1. Discovery of Wild Rice Accessions That Constitutively Form a Radial Oxygen Loss (ROL) Barrier
2.2. Assessment of Inducible ROL Barrier Formation
2.3. Assessment of Aerenchyma Formation
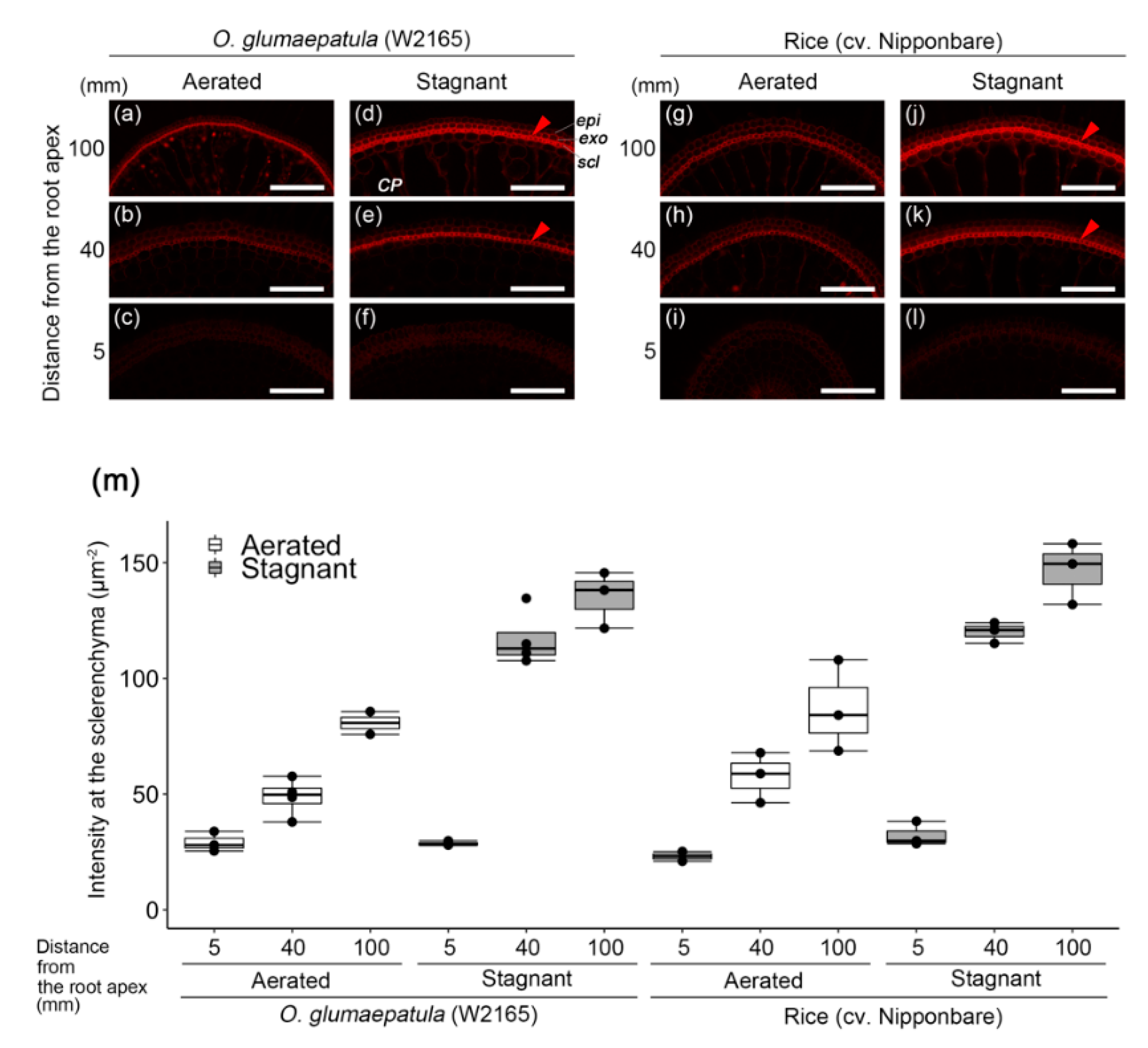
2.4. Suberin and Lignin Accumulation in W2165
2.5. Apoplastic Barrier Assay in W2165
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Growth Conditions
4.3. ROL Barrier Formation
4.4. Aerenchyma Formation
4.5. Histochemical Staining
4.6. Quantification of Fluorescence Intensity
4.7. Permeability Test
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jackson, M.B.; Fenning, T.M.; Drew, M.C.; Saker, L.R. Stimulation of ethylene production and gas-space (aerenchyma) formation in adventitious roots of Zea mays L. by small partial pressures of oxygen. Planta 1985, 165, 486–492. [Google Scholar] [CrossRef]
- Colmer, T.D. Long-distance transport of gases in plants: A perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ. 2003, 26, 17–36. [Google Scholar] [CrossRef]
- Shiono, K.; Takahashi, H.; Colmer, T.D.; Nakazono, M. Role of ethylene in acclimations to promote oxygen transport in roots of plants in waterlogged soils. Plant Sci. 2008, 175, 52–58. [Google Scholar] [CrossRef]
- Nishiuchi, S.; Yamauchi, T.; Takahashi, H.; Kotula, L.; Nakazono, M. Mechanisms for coping with submergence and waterlogging in rice. Rice 2012, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, O.; Sauter, M.; Colmer, T.D.; Nakazono, M. Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytol. 2020. In Press. [Google Scholar] [CrossRef]
- Yamauchi, T.; Colmer, T.D.; Pedersen, O.; Nakazono, M. Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiol. 2018, 176, 1118–1130. [Google Scholar] [CrossRef]
- Abiko, T.; Miyasaka, S.C. Aerenchyma and barrier to radial oxygen loss are formed in roots of Taro (Colocasia esculenta) propagules under flooded conditions. J. Plant Res. 2020, 133, 49–56. [Google Scholar] [CrossRef]
- Manzur, M.E.; Grimoldi, A.A.; Insausti, P.; Striker, G.G. Radial oxygen loss and physical barriers in relation to root tissue age in species with different types of aerenchyma. Funct. Plant Biol. 2015, 42, 9–17. [Google Scholar] [CrossRef]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef]
- Naseer, S.; Lee, Y.; Lapierre, C.; Franke, R.; Nawrath, C.; Geldner, N. Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc. Natl. Acad. Sci. USA 2012, 109, 10101–10106. [Google Scholar] [CrossRef]
- Graça, J. Suberin: The biopolyester at the frontier of plants. Front. Chem. 2015, 3. [Google Scholar] [CrossRef]
- Schreiber, L.; Franke, R.B. Endodermis and exodermis in roots. In eLS; John Wiley & Sons Ltd.: Chichester, UK, 2011; pp. 1–7. [Google Scholar] [CrossRef]
- Ejiri, M.; Shiono, K. Prevention of radial oxygen loss is associated with exodermal suberin along adventitious roots of annual wild species of Echinochloa. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Enstone, D.E.; Peterson, C.A.; Ma, F. Root endodermis and exodermis: Structure, function, and responses to the environment. J. Plant Growth Regul. 2003, 21, 335–351. [Google Scholar] [CrossRef]
- Ranathunge, K.; Lin, J.; Steudle, E.; Schreiber, L. Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCl permeabilities in rice (Oryza sativa L.) roots. Plant Cell Environ. 2011, 34, 1223–1240. [Google Scholar] [CrossRef]
- Ranathunge, K.; Schreiber, L.; Franke, R. Suberin research in the genomics era—New interest for an old polymer. Plant Sci. 2011, 180, 399–413. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W. Rice: Sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Ann. Bot. 2005, 96, 625–638. [Google Scholar] [CrossRef]
- Colmer, T.D.; Gibberd, M.R.; Wiengweera, A.; Tinh, T.K. The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. J. Exp. Bot. 1998, 49, 1431–1436. [Google Scholar] [CrossRef]
- Mongon, J.; Konnerup, D.; Colmer, T.D.; Rerkasem, B. Responses of rice to Fe2+ in aerated and stagnant conditions: Growth, root porosity and radial oxygen loss barrier. Funct. Plant Biol. 2014, 41, 922–929. [Google Scholar] [CrossRef]
- Ranathunge, K.; Schreiber, L.; Bi, Y.M.; Rothstein, S.J. Ammonium-induced architectural and anatomical changes with altered suberin and lignin levels significantly change water and solute permeabilities of rice (Oryza sativa L.) roots. Planta 2016, 243, 231–249. [Google Scholar] [CrossRef]
- Colmer, T.D.; Kotula, L.; Malik, A.I.; Takahashi, H.; Konnerup, D.; Nakazono, M.; Pedersen, O. Rice acclimation to soil flooding: Low concentrations of organic acids can trigger a barrier to radial oxygen loss in roots. Plant Cell Environ. 2019, 42, 2183–2197. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.P.; Galwey, N.W.; Colmer, T.D. Waterlogging tolerance in the tribe Triticeae: The adventitious roots of Critesion marinum have a relatively high porosity and a barrier to radial oxygen loss. Plant Cell Environ. 2001, 24, 585–596. [Google Scholar] [CrossRef]
- McDonald, M.P.; Galwey, N.W.; Colmer, T.D. Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dryland grass species. Plant Cell Environ. 2002, 25, 441–451. [Google Scholar] [CrossRef]
- Colmer, T.D. Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Ann. Bot. 2003, 91, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Shiono, K.; Ogawa, S.; Yamazaki, S.; Isoda, H.; Fujimura, T.; Nakazono, M.; Colmer, T.D. Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Ann. Bot. 2011, 107, 89–99. [Google Scholar] [CrossRef]
- Kotula, L.; Ranathunge, K.; Schreiber, L.; Steudle, E. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J. Exp. Bot. 2009, 60, 2155–2167. [Google Scholar] [CrossRef]
- Atwell, B.J.; Wang, H.; Scafaro, A.P. Could abiotic stress tolerance in wild relatives of rice be used to improve Oryza sativa? Plant Sci. 2014, 215, 48–58. [Google Scholar] [CrossRef]
- Malik, A.I.; Islam, A.K.M.R.; Colmer, T.D. Transfer of the barrier to radial oxygen loss in roots of Hordeum marinum to wheat (Triticum aestivum): Evaluation of four H. marinum–wheat amphiploids. New Phytol. 2011, 190, 499–508. [Google Scholar] [CrossRef]
- Konnerup, D.; Malik, A.l.; Islam, A.K.M.R.; Colmer, T.D. Evaluation of root porosity and radial oxygen loss of disomic addition lines of Hordeum marinum in wheat. Funct. Plant Biol. 2017, 44, 400–409. [Google Scholar] [CrossRef]
- Abiko, T.; Kotula, L.; Shiono, K.; Malik, A.I.; Colmer, T.D.; Nakazono, M. Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant Cell Environ. 2012, 35, 1618–1630. [Google Scholar] [CrossRef]
- Garthwaite, A.J.; von Bothmer, R.; Colmer, T.D. Diversity in root aeration traits associated with waterlogging tolerance in the genus Hordeum. Funct. Plant Biol. 2003, 30, 875–889. [Google Scholar] [CrossRef]
- Watanabe, K.; Takahashi, H.; Sato, S.; Nishiuchi, S.; Omori, F.; Malik, A.I.; Colmer, T.D.; Mano, Y.; Nakazono, M. A major locus involved in the formation of the radial oxygen loss barrier in adventitious roots of teosinte Zea nicaraguensis is located on the short-arm of chromosome 3. Plant Cell Environ. 2017, 40, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Nagayama, H.; Kurakazu, T.; Sanchez, P.L.; Doi, K.; Yamagata, Y.; Yasui, H. Introgression lines of rice (Oryza sativa L.) carrying a donor genome from the wild species, O. glumaepatula Steud. and O. meridionalis Ng. Breed. Sci. 2010, 60, 597–603. [Google Scholar] [CrossRef]
- Laan, P.; Berrevoets, M.J.; Lythe, S.; Armstrong, W.; Blom, C.W.P.M. Root morphology and aerenchyma formation as indicators of the flood-tolerance of Rumex species. J. Ecol. 1989, 77, 693–703. [Google Scholar] [CrossRef]
- Visser, E.J.W.; Bögemann, G.M. Aerenchyma formation in the wetland plant Juncus effusus is independent of ethylene. New Phytol. 2006, 171, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Omori, F.; Takamizo, T.; Kindiger, B.; Bird, R.M.; Loaisiga, C.H.; Takahashi, H. QTL mapping of root aerenchyma formation in seedlings of a maize x rare teosinte “Zea nicaraguensis” cross. Plant Soil 2007, 295, 103–113. [Google Scholar] [CrossRef]
- Malik, A.I.; English, J.P.; Colmer, T.D. Tolerance of Hordeum marinum accessions to O2 deficiency, salinity and these stresses combined. Ann. Bot. 2009, 103, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Mano, Y.; Omori, F. Relationship between constitutive root aerenchyma formation and flooding tolerance in Zea nicaraguensis. Plant Soil 2013, 370, 447–460. [Google Scholar] [CrossRef]
- Insalud, N.; Bell, R.W.; Colmer, T.D.; Rerkasem, B. Morphological and physiological responses of rice (Oryza sativa) to limited phosphorus supply in aerated and stagnant solution culture. Ann. Bot. 2006, 98, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, M.; Shimamoto, Y.; Morishima, H. Population genetic structure of wild rice Oryza glumaepatula distributed in the Amazon flood area influenced by its life-history traits. Mol. Ecol. 1998, 7, 1371–1381. [Google Scholar] [CrossRef]
- Huang, X.; Kurata, N.; Wei, X.; Wang, Z.X.; Wang, A.; Zhao, Q.; Zhao, Y.; Liu, K.; Lu, H.; Li, W.; et al. A map of rice genome variation reveals the origin of cultivated rice. Nature 2012, 490, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Waters, D.L.E.; Nock, C.J.; Ishikawa, R.; Rice, N.; Henry, R.J. Chloroplast genome sequence confirms distinctness of Australian and Asian wild rice. Ecol. Evol. 2012, 2, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Shiono, K.; Yamauchi, T.; Yamazaki, S.; Mohanty, B.; Malik, A.I.; Nagamura, Y.; Nishizawa, N.K.; Tsutsumi, N.; Colmer, T.D.; Nakazono, M. Microarray analysis of laser-microdissected tissues indicates the biosynthesis of suberin in the outer part of roots during formation of a barrier to radial oxygen loss in rice (Oryza sativa). J. Exp. Bot. 2014, 65, 4795–4806. [Google Scholar] [CrossRef] [PubMed]
- Kulichikhin, K.; Yamauchi, T.; Watanabe, K.; Nakazono, M. Biochemical and molecular characterization of rice (Oryza sativa L.) roots forming a barrier to radial oxygen loss. Plant Cell Environ. 2014, 37, 2406–2420. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.D.L.C.; Kotula, L.; Veneklaas, E.J.; Colmer, T.D. Root-zone hypoxia reduces growth of the tropical forage grass Urochloa humidicola in high-nutrient but not low-nutrient conditions. Ann. Bot. 2019, 124, 1019–1032. [Google Scholar] [CrossRef]
- Shiono, K.; Ando, M.; Nishiuchi, S.; Takahashi, H.; Watanabe, K.; Nakamura, M.; Matsuo, Y.; Yasuno, N.; Yamanouchi, U.; Fujimoto, M.; et al. RCN1/OsABCG5, an ATP-binding cassette (ABC) transporter, is required for hypodermal suberization of roots in rice (Oryza sativa). Plant J. 2014, 80, 40–51. [Google Scholar] [CrossRef]
- Wiengweera, A.; Greenway, H.; Thomson, C.J. The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Ann. Bot. 1997, 80, 115–123. [Google Scholar] [CrossRef]
- Armstrong, W.; Wright, E.J. Radial oxygen loss from roots: the theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiol. Plant. 1975, 35, 21–26. [Google Scholar] [CrossRef]
- Armstrong, W. Polarographic oxygen electrodes and their use in plant aeration studies. Proc. R. Soc. B Biol. Sci. 1994, 102, 511–527. [Google Scholar] [CrossRef]
- Lux, A.; Morita, S.; Abe, J.; Ito, K. An improved method for clearing and staining free-hand sections and whole-mount samples. Ann. Bot. 2005, 96, 989–996. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Kendrick, B.; Peterson, C.A. Efficient lipid staining in plant material with sudan red 7B or fluoral yellow 088 in polyethylene glycol-glycerol. Biotech. Histochem. 1991, 66, 111–116. [Google Scholar] [CrossRef]
- Kapp, N.; Barnes, W.J.; Richard, T.L.; Anderson, C.T. Imaging with the fluorogenic dye Basic Fuchsin reveals subcellular patterning and ecotype variation of lignification in Brachypodium distachyon. J. Exp. Bot. 2015, 66, 4295–4304. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, D.; Mizuta, Y.; Sato, Y.; Higashiyama, T. ClearSee: A rapid optical clearing reagent for whole-plant fluorescence imaging. Development 2015, 142, 4168–4179. [Google Scholar] [CrossRef] [PubMed]
- Ursache, R.; Andersen, T.G.; Marhavý, P.; Geldner, N. A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J. 2018, 93, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]



| Species | Accession | Aerenchyma Formation (% aerenchyma/cotex) | ||
|---|---|---|---|---|
| Aerated | Stagnant | t-Test | ||
| O. glumaepatula | W2165 | 48 ± 3 c | 46 ± 5 A | n.s. |
| W2149 | 32 ± 5 a,b | 52 ± 4 A | * | |
| W1183 | 21 ± 1 a | 49 ± 6 A | * | |
| O. rufipogon | W1962 | 38 ± 2 b,c | 52 ± 2 A | * |
| O. sativa | Nipponbare | 48 ± 2 c | 64 ± 4 A | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ejiri, M.; Sawazaki, Y.; Shiono, K. Some Accessions of Amazonian Wild Rice (Oryza glumaepatula) Constitutively Form a Barrier to Radial Oxygen Loss along Adventitious Roots under Aerated Conditions. Plants 2020, 9, 880. https://doi.org/10.3390/plants9070880
Ejiri M, Sawazaki Y, Shiono K. Some Accessions of Amazonian Wild Rice (Oryza glumaepatula) Constitutively Form a Barrier to Radial Oxygen Loss along Adventitious Roots under Aerated Conditions. Plants. 2020; 9(7):880. https://doi.org/10.3390/plants9070880
Chicago/Turabian StyleEjiri, Masato, Yuto Sawazaki, and Katsuhiro Shiono. 2020. "Some Accessions of Amazonian Wild Rice (Oryza glumaepatula) Constitutively Form a Barrier to Radial Oxygen Loss along Adventitious Roots under Aerated Conditions" Plants 9, no. 7: 880. https://doi.org/10.3390/plants9070880
APA StyleEjiri, M., Sawazaki, Y., & Shiono, K. (2020). Some Accessions of Amazonian Wild Rice (Oryza glumaepatula) Constitutively Form a Barrier to Radial Oxygen Loss along Adventitious Roots under Aerated Conditions. Plants, 9(7), 880. https://doi.org/10.3390/plants9070880





