1. Introduction
Acmella oleracea (L.) R. K. Jansen (commonly called jambu), Asteraceae, is a flowering Brazilian species largely diffused worldwide. It is grown as an ornamental or medicinal plant and it is also known as the “Toothache plant”. It is used as medicinal plant in different parts of the world in particular to treat oral pain because of its analgesic properties. When the leaves and flowers are chewed, they cause a tingling sensation to tongue and lips due to the presence of alkylamides, the most abundant in
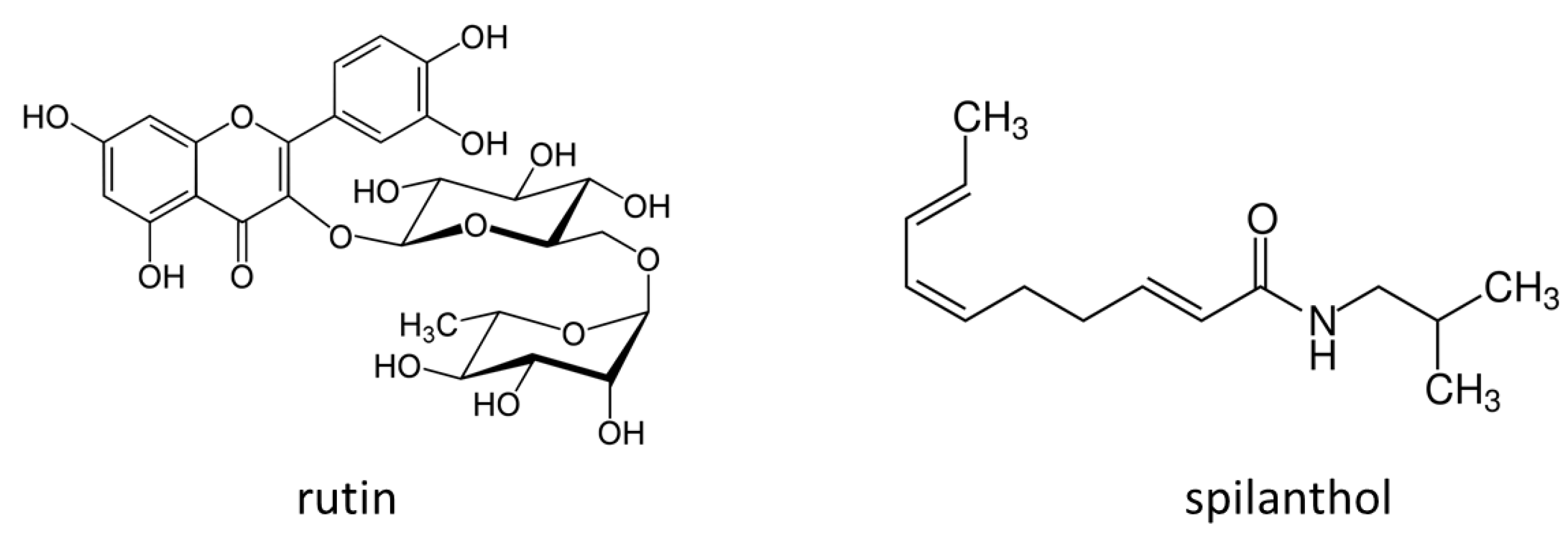
A. oleracea being spilanthol. This isobutylamide derivative presents local anesthetic action and this effect justifies the traditional use for treating toothache [
1,
2,
3,
4].
Jambu is attracting greater interest in the field of herbal medicine and nutraceuticals due to its potential analgesic, antioxidant, and anti-inflammatory activities, and several patent applications have been filed, showing the industrial potential of this plant for cosmetic and pharmaceutical applications [
2,
5].
In this regard, the cultivation of species for the use in cosmetic and pharmaceutical applications can be a challenge due to the need for standardized and high yield content of active constituents [
6,
7]. Different agronomic practices can be used with the aim to increase the secondary metabolites or the yield/ha of cultivated plants. The search for natural products by consumers is also related to more sustainable ways of production as well as to agricultural practices that limit the use of chemicals.
There is increasing interest in sustainable ways of production, as well as in the agricultural practices that limit the use of chemicals, in food but also in the area of natural products for pharmaceutical, nutraceutical, and cosmetic uses.
Biostimulants are products that are applied to plants or soils to regulate and enhance the crop’s physiological processes, thus making them more efficient [
8,
9,
10,
11,
12,
13]. To our knowledge, limited information related to the use of biostimulants in the cultivation of medicinal and officinal species has been published so far. Many natural substances have been investigated as biostimulants in crops, and four types of constituents have been generally considered, namely triacontanol-containing products, plant-derived protein hydrolysates, seaweeds, and microorganisms [
8,
9,
10,
11,
12,
13,
14]. In this study, a Triacontanol-based mixture (Tria), an extract from plant tissues (LL017), and a seaweed extract (Swe) were used at two different application doses (Low and High). Previous published research reported the triacontanol-mediated improvement of several parameters in various crops, such as growth, yield, photosynthesis, protein synthesis, uptake of water and nutrients, nitrogen-fixation, enzymatic activities and contents of free amino acids, reducing sugars, soluble proteins, and active constituents as essential oil. The application of plant-derived extracts stimulates plant uptake of macro- and micronutrients, and helps in rapid plant growth and biomass accumulation, interfering with carbon and nitrogen metabolic activities [
8,
9,
10]. Seaweed extracts were largely used in the agricultural practice for their plant growth-promoting effects and for their ameliorating effect on crop tolerance to abiotic stresses such as salinity, extreme temperatures, nutrient deficiency, and drought [
8,
15].
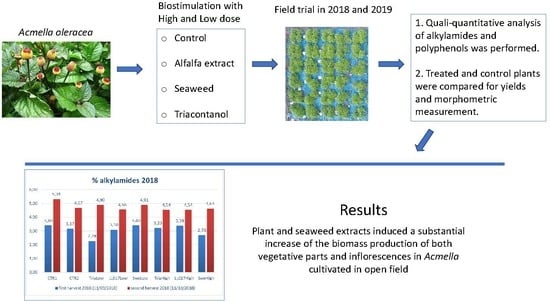
The selected biostimulant treatments were considered in open field conditions to investigate the morphometric changes in plants grown during the hot season. Normal fertilization (UTC 100% fertilized) CTR1 and double fertilization (UTC 200% fertilized) CTR2 were used as control conditions.
The aim of the study was to investigate the morphological modification and the levels of bioactive compounds in A. oleracea plants cultivated treated with different biostimulants. Secondary metabolites content was chemically characterized with different analytical methods in A. oleracea. N-Alkylamides are considered to be the most significant class of metabolites of this plant, and mostly contain a poly-unsaturated aliphatic fatty acid chain and a shorter substituent at the amide side. Plant biomass and flower production were monitored in both control and biostimulant treated A. oleracea plants. Nuclear magnetic resonance (NMR) and liquid chromatography coupled with mass spectrometry (LC-DAD-MSn) were used as analytical approaches to assess chemical constituents, allowing the annotation and the quantification of different alkylamides and polyphenols studying their modification in treated and control groups.
3. Discussion
Biostimulants aim to increase plant production and/or their quality by improving their fitness. Their mode of action is not well understood yet, but much evidence supports the beneficial effects of biostimulation. These aspects are of interest especially if plants considered are used for cosmetic, nutraceutical, and pharmaceutical applications.
Our results on the application of six different biostimulant treatments on A. oleracea plants showed a good impact on plant growth and production, with a limited effect on the biosynthesis of secondary metabolites.
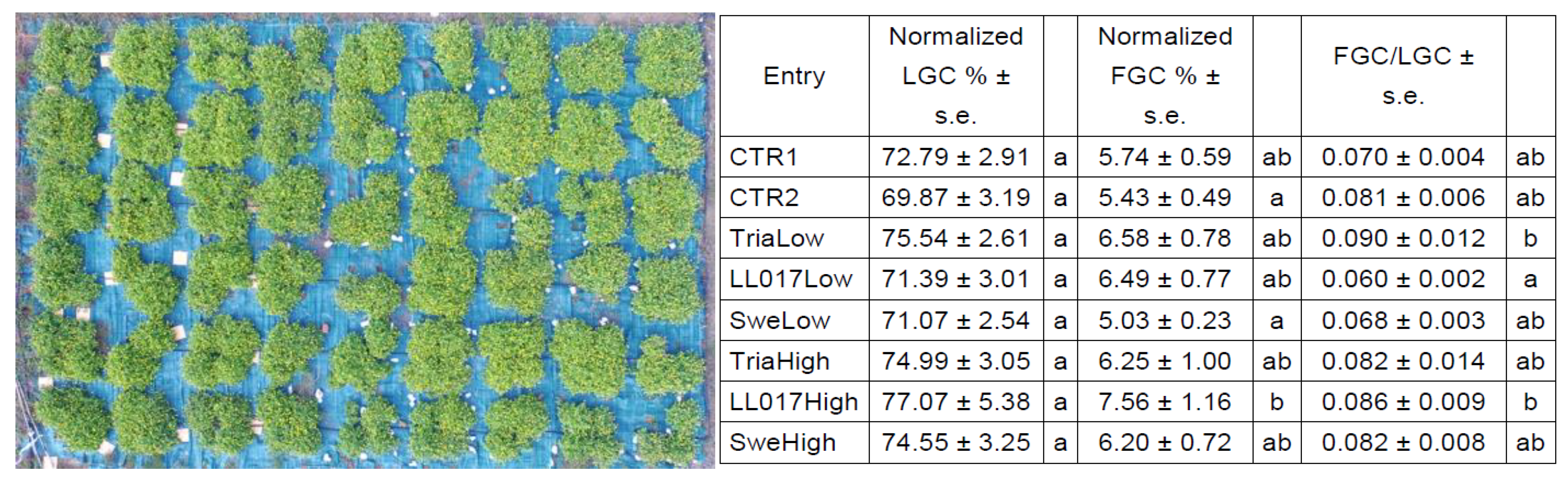
Triacontanol-based mixture (Tria), plant tissues extract (LL017), and seaweed extract (Swe) at all dosages improved plant growth, especially in 2019, when treated plants nearly reached the entry with double nutrition in terms of dimensions at the end of the season. This result is significant, as plant growth positively affects flower production, an energy-consuming process. Our results indicate that all treated entries produced a higher flower biomass compared to the untreated control (CTR1), with TriaLow that reached +84.54%. Moreover, biostimulants filled the gap between single and double fed plants in terms of flower production. Regarding 2018 plant traits, the plants were very inhomogeneous and data were not used. Nevertheless, LL017High and TriaLow gave the highest indexes of flowers on total canopy.
Regarding the biosynthesis of bioactive compounds, the obtained results indicate limited influence of biostimulant treatments on the % (
w/
w) of alkylamides and polyphenols. Higher levels of alkylamides were observed in the field trial compared to other reference standard plant material that was cultivated in different geographic areas. For
A. oleracea in the experimental conditions, the considered biostimulant treatments resulted in a content of secondary metabolites that was not significantly improved considering their % amount (
w/
w) at harvest times compared to control plants. Results indicate additional information since the open field trials gave higher metabolites content than plants cultivated in pots in greenhouse conditions (
Tables S3 and S4). This might be due to a much lower level of environmental stimuli in the greenhouse, that maintained plants in less stressed conditions, with a subsequently lower level of secondary metabolites synthesis.
A. oleracea flowers are the plant part that is more required for alkylamides extraction, and for this reason, on the basis of our results, we suggest that TriaLow is the best treatment to be carried out to increase the production of this plant material. Moreover, results indicate that triacontanol fills the gap between normal and over-fertilized plants, since TriaLow produced more flowers than CTR2, the double fertilized entry. Overall results, combining morphological traits and chemical data, indicate that the quantity of alkylamides is very similar among treated and non-treated plants, and a higher biomass production induced by biostimulants is very desirable in terms of final yield for extractable metabolites.
For the both years the last harvest of A. oleracea showed decreased levels of polyphenols, suggesting a strong influence of the harvest time.
4. Materials and Methods
4.1. Open Field Experimental Site and Plant Cultivation
A. oleracea plants were cultivated in 2018 and 2019 in Landlab research station (Quinto Vicentino-VI-Italy, 45.57° N, 11.62° E, 33 masl) in an open field with native soil substrate, sandy-loam, pH 7–7.5. Seeds were sown on peat to provide plants that were transplanted in the field once established. Mulching on plots was provided in order to avoid the overlap of plants from different plots. Once transplanted at the beginning of the summer, plants were trimmed in order to make the canopy uniform. Solid fertilizer (ILSA Professional NPK (8-6-14) solid granules at 160 kg/ha (double NPK) or 80 kg/ha (base NPK)) was applied three times to satisfy the nutrient demand of plants, that was estimated in 80 kg/ha N, 60 kg/ha P, 140 kg/ha K for 100% fertilized and 160 kg/ha N, 120 kg/ha P, and 280 kg/ha K for 200% fertilized. Plants were cultivated in randomized complete block plots, with six replicates per entry and four plants per replicate. Irrigation was provided by means of plant-by-plant drip system in order to maintain moisture in the substrate.
4.2. Biostimulant Treatments
Three different types of biostimulant were prepared and used on the basis of literature search. Triacontanol-based biostimulant obtained by combining enzymatic hydrolysate from Fabaceae species with supercritical extract rich in triacontanol, an extraction from plant tissues, and a seaweed-based product (Acadian Seaplants, Canada).
The triacontanol-based biostimulant was prepared using a mixture of a plant hydrolysate and a combination of concentrated solution of triacontanol obtained by extraction from the same species. Two different products were prepared, one containing 6.5 ppm of triacontanol and one containing 100 ppm of triacontanol with a previously validated method [
24]. Briefly, for liquid products 1000 mg of material were weighed, the internal standard (IS) solution was added (1000 uL of a 500 ug/mL solution), and extracted in a flask with 50 mL of dichloromethane. Extraction was performed in ultrasound bath for 15 min. An Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA, USA) coupled to a Sedere Sedex 60 ELSD detector (Olivet, France) was used. In order to elute highly lipophilic compounds from the reverse phase column used as stationary phase (Agilent Extend C-18 4.6 × 150 mm, 5 µm), a gradient of acetonitrile (A) and methanol/methyl tertbutyl ether 10/90 (B) was used as mobile phase. Gradient conditions were optimized in order to perform the analysis in 30 min and to reach the best separation of TRIA and the IS. The gradient started with 10% B, in 15 min it went to 40% B, then isocratic up to 26 min. Flow was 1 mL/min, injection was 10 µL.
The aminoacidic analysis in triacontanol based biostimulant was performed by HPLC-MS/MS as we previously described [
25]. Briefly, samples were extracted in an ultrasound bath with a solution of diluted HCl (0.5 M) for 10 min at room temperature. Standard solutions were prepared, weighing the exact amount of each amino acid in diluted HCl solution (0.5 M) in four different concentrations from 1 to 10 ug/mL. For hydrolysis, samples were added of 15% trichloroacetic acid (10 mL) and left at 80 °C for one night in sealed vials. pH of solution was then adjusted with ammonia solution (33%) to 2.0 and solutions were used for analysis. For analysis, an Agilent Z-HILIC Column was used as stationary phase (3 × 100 mm, 4 micron), eluents were acetonitrile, (A) and water 0.5% formic acid (B). The gradient started with 1 min to 95% A, then in 11 min to 70% A, then 14 min to 40% A, then at 14.5 min back to 95% A. Flow rate was 0.450 mL/min. Each amino acid transition was optimized with corresponding standard solution. The amino acid profile of triacontanol based biostimulant was reported in
Table 6.
The plant extract contained 8.9 ppm of triacontanol and an amino acid profile reported in
Table 7.
Seaweed biostimulant composition was analyzed for polysaccharides and content was 10% (w/w). Products were also analyzed for the content of gibberellic, gibberellinic, indol-3-acetic acid, and none of the products contained these constituents at the detection limit (<5 ug/mL).
Entries considered in the trials were:
UTC 100% fertilized; CTR1
UTC 200% fertilized; CTR2
100% fertilized + 6.5 ppm Triacontanol UniPd 3 L/ha; TriaLow
100% fertilized + LL017 3 L/ha; LL017Low
100% fertilized + Acadian SWE 1.5 L/ha; SweLow
100% fertilized + 100 ppm Triacontanol UniPd 3 L/ha; TriaHigh
100% fertilized + LL017 50 L/ha; LL017High
100% fertilized + Acadian SWE 15 L/ha; SweHigh
Plants were treated once per week starting from the full establishment of the plants, until the last harvest, for a total of 15 times in 2018 and 13 times in 2019.
Products were applied with foliar application using a hand sprayer on well-developed plants at 3 L/ha for 6.5 (low) or 100 (high) ppm Triacontanol, 3 (low) or 50 (high) L/ha LL017, and 1.5 (low) or 15 (high) L/ha Acadian SWE in 500 L/ha of water as carrier.
Monitoring of weather conditions: local weather conditions were monitored by using the weather station Vantage pro2 (Davis Instruments Corp. Inc., CA), located in Landlab (45.57° N, 11.62° E, 33 masl), that is 20 m from the trial location.
4.3. Biometric Traits of the Plants in Open Field
Living ground cover (LGC) and flower ground cover (FGC) for plants grown in 2018 in open field were assessed from pictures taken by a drone (Phantom 3 Advanced, DJI, China).
The image was analyzed using WinCAM NDVI (Regents Instruments Inc., CA) to generate data that quantified the covering of plants and flowers. Data were normalized on the number of plants per plot in case of the death of some plants.
Biometrics of plants grown in the field in 2019 were assessed in terms of height, diameter, and flower yield by using a meter and a scale, respectively. Assessment were carried out after one, four, and eight treatments.
4.4. Acmella oleracea Harvest
Fully developed inflorescences were manually harvested every four treatments, once a month. Samples from each entry were maintained separately and they were dried at 40 °C in the oven for 48 h.
4.5. NMR Analysis
NMR analysis was used to assess the general phytochemical contents of the samples. Dried plant material (500 mg) (sample C/213365 28/02/18) was extracted with methanol, the solvent was removed under vacuum and dissolved in 700 microliter of deuterated methanol and used for the analysis.
4.6. NMR Quantification of Alkylamides
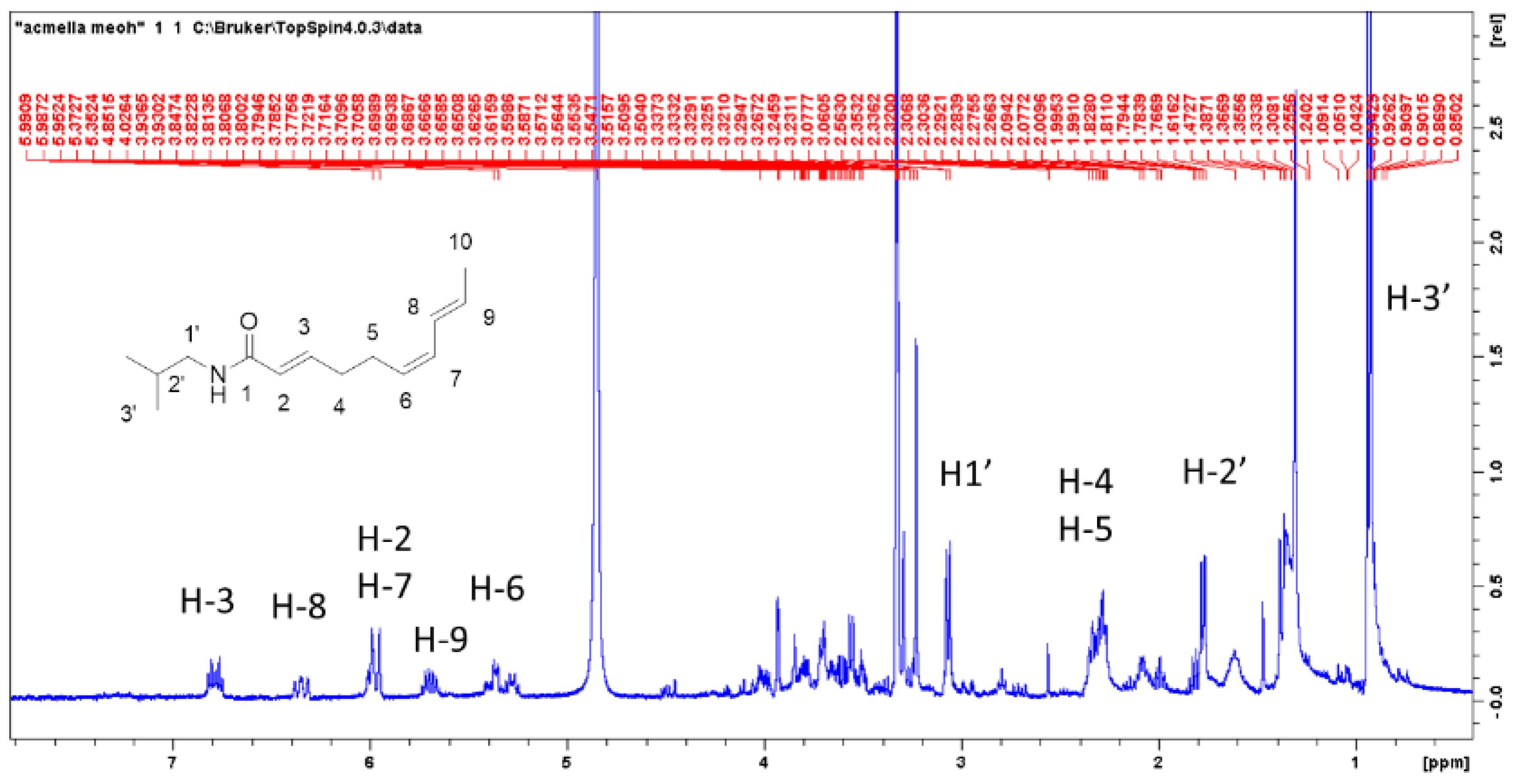
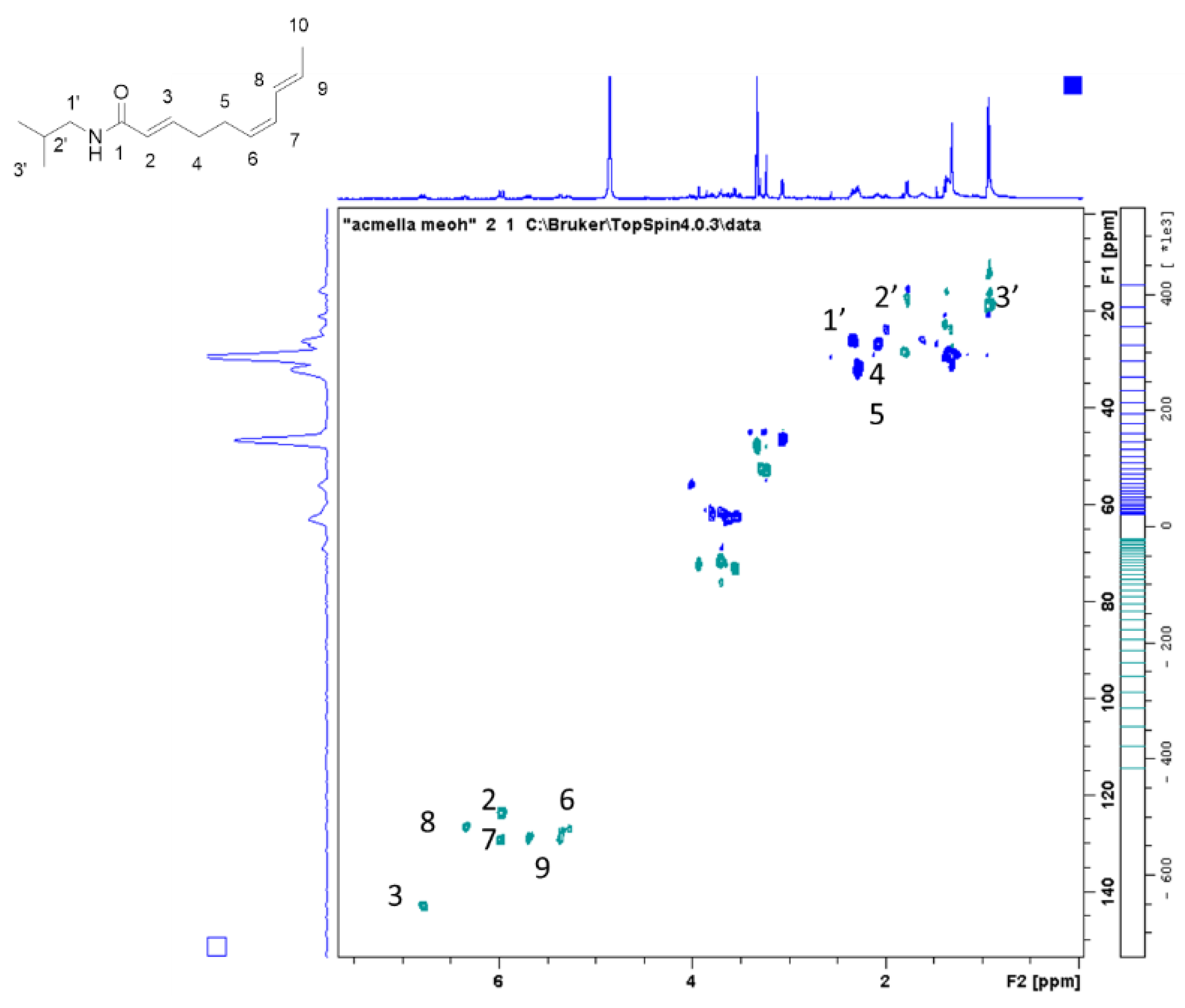
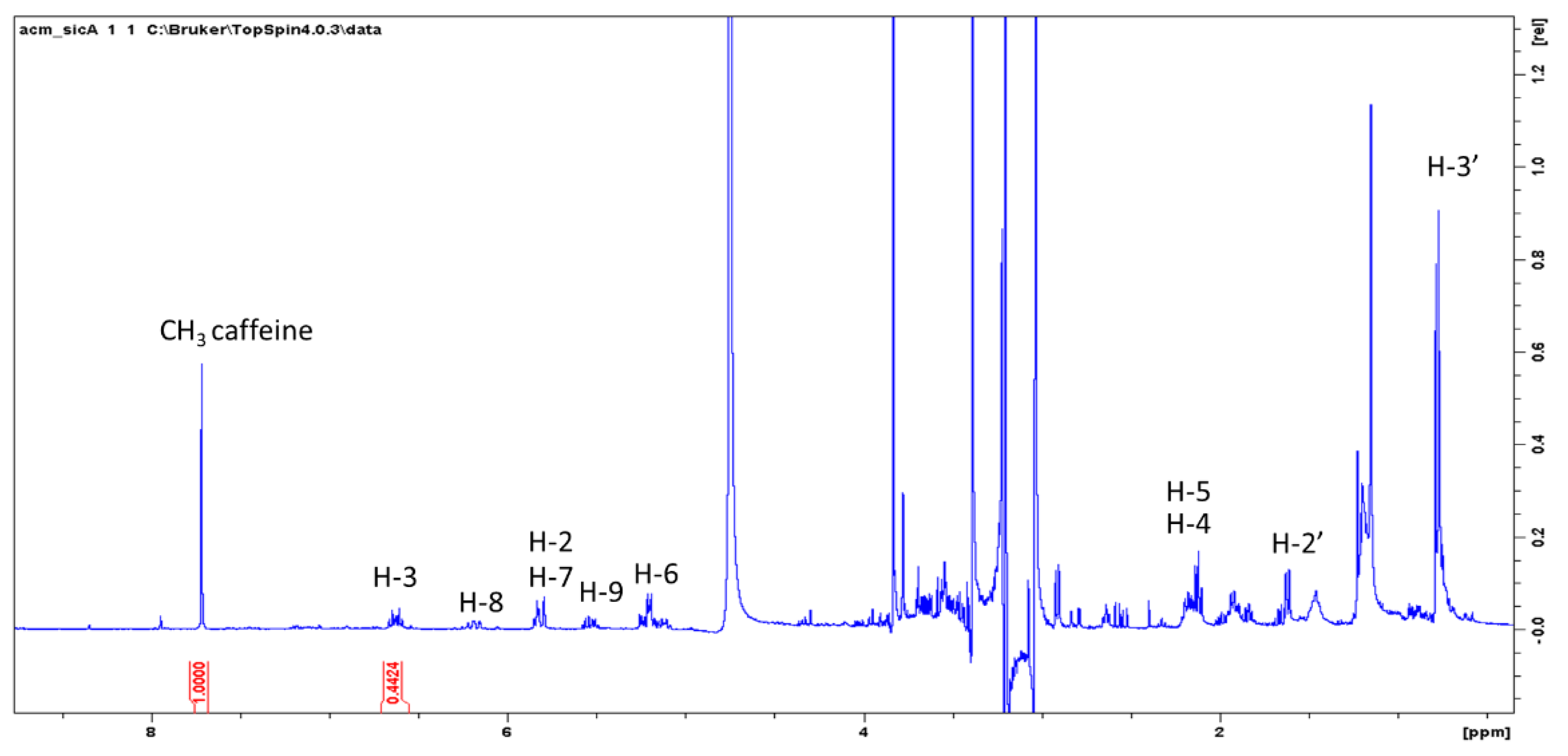
Preliminary investigation on the possibility to quantify spilanthol by
1H-NMR were performed using caffeine as internal standard as previously reported for other plant constituents [
17,
18,
19]. The extraction was performed weighing 100 mg of dried plant material, adding 1 mg of caffeine, and extracting in methanol in ultrasound bath for 10 min with 25 mL of methanol. Liquid was decanted and collected, a further 20 mL of methanol was added, and the mixture was sonicated for 10 min. Liquids were collected, dried under vacuum, and the residue dissolved with deuterated methanol. Alternatively, 100 mg of plant material can be directly extracted with methanol and internal standard (1–3 mg) and the mixture used for
1H-NMR determination using solvent suppression.
An example of this determination is reported. Imidazole signal of caffeine can be used as reference while H-3 of spilanthol can be used for the measurement.
4.7. Qualitative Analysis of Acmella oleracea by LC-DAD-MSn
A total of 25 mg of Acmella oleracea powder was extracted with 15 mL of methanol, sonicated for 10 min, and supernatant was filtered. A second extraction was performed with another 10 mL of methanol, sonicated for 10 min. Solution was pulled with a final volume of 25 mL. Solution was used for LC analysis.
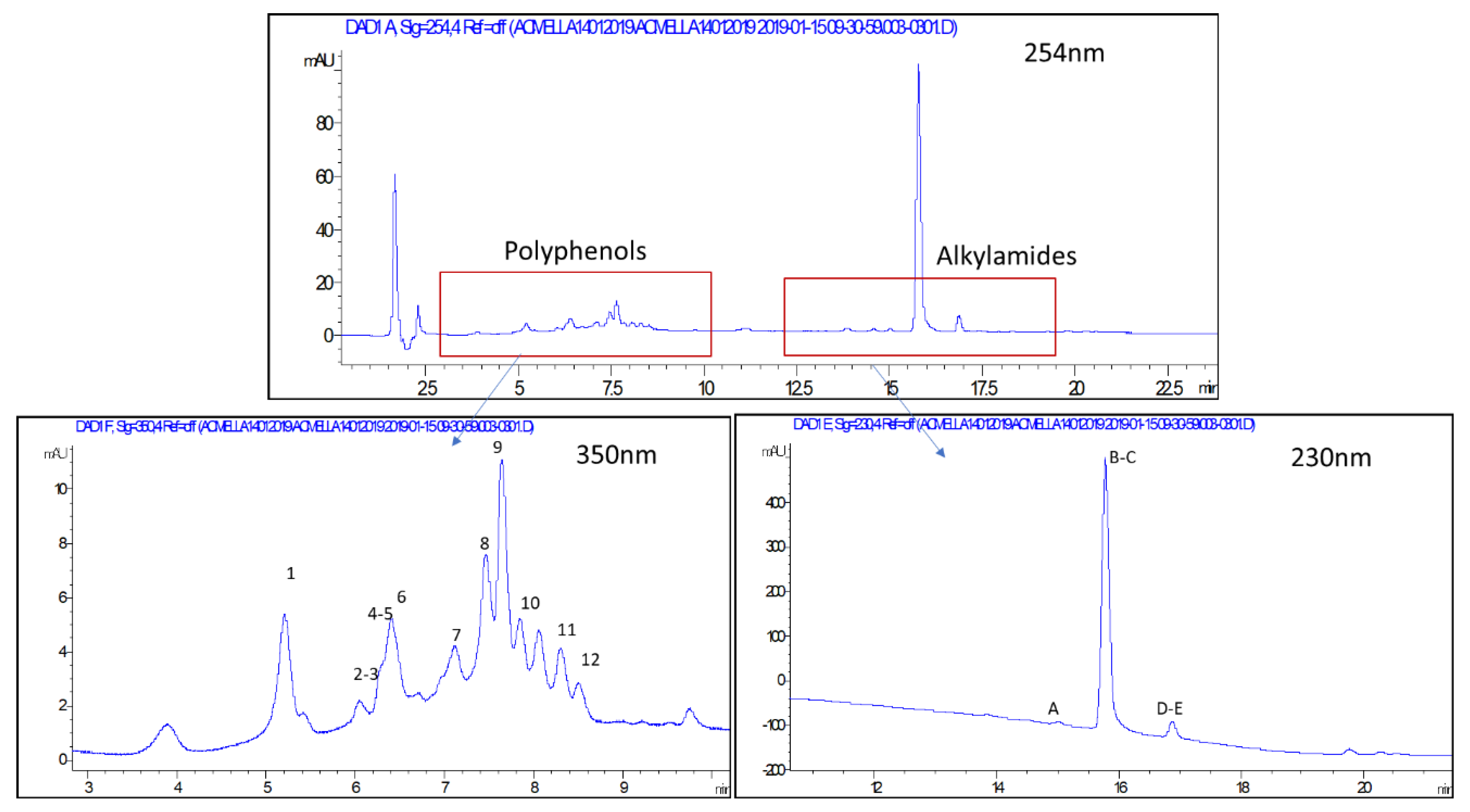
LC-DAD-MSn allowed to obtain detailed fingerprinting of the extract due to the typical UV spectra of separated compounds and due to specific information obtained by the MSn approach. After preliminary trials due to the presence of compounds that are easily ionized in negative ion mode in the first part of the chromatogram and due to the preferable analysis of alkylamides in positive ion mode the spectra were acquired up to 15 min in negative and after 15 min in positive ion mode.
The instrumentation was Agilent 1260 chromatograph (Santa Clara, CA, USA) equipped with 1260 diode array detector (DAD) and Varian MS-500 ion trap mass spectrometer equipped with ESI source. At the end of the column, two “T” splitters separated the flow rate: half of the liquid was split to DAD and half to Agilent/Varian MS-500 ion trap mass spectrometer. UV-Vis spectra were acquired in the range of 190–400 nm. The LC separation was obtained on an Agilent C-18 XDB 3.0 × 150 mm (3 micron), as mobile phases acetonitrile (A) and water 0.1% formic acid (B). Flow rate was 0.4 mL/min. Gradient starts with 10% A, then 75% A in 15 min, then 10 min isocratic, then 90% A in 1 min. The MS parameters were different in negative mode (from 0 to 15 min) and in positive mode (from 15 to 31 min), in order to improve the ionization of polyphenol ions and alkylamide derivatives, respectively. The parameters in negative mode were as follows: capillary voltage 85 volt, RF loading 100%; nebulizer gas pressure, 25 psi; drying gas pressure, 25 psi; drying gas temperature, 265 °C; needle voltage, ±5000 V; spray shield voltage, 600 V. Mass spectra was acquired in negative mode in the spectral range 50–1500 Da. The parameters in positive mode were as follows: capillary voltage 85 volt, RF loading 85%; nebulizer gas pressure, 25 psi; drying gas pressure, 25 psi; drying gas temperature, 265 °C; needle voltage, ±4000 V; spray shield voltage, 600 V. Mass spectra were acquired in negative mode in the spectral range 50–900 Da. Data obtained was used to tentatively assign an identification to secondary metabolite compound on the base of literature references in the MSn pathway.
For polyphenol Acmella samples were quantified with DAD detector. Rutin was used as standard compound for flavonoids, while chlorogenic acid was used as standard compounds for caffeic acid derivatives, and stock solutions were prepared at concentration of 60 ug/mL in methanol. Then dilutions were prepared in methanol in the range between 6 and 60 ug/mL and the calibration curve was as follows, y = 32.5x + 0.3 (R2 = 0.993) for rutin, y = 17x + 0.5 (R2 = 0.993) for chlorogenic acid. Peaks with UV spectra with maximum at 350 nm, ascribable to flavonoid structure, were selected and area was obtained at 350 nm. Peaks with UV spectra with maximum at 330 nm, ascribable to caffeoyl derivatives, were selected and the area was obtained at 330 nm. Quantitative data of the various samples were reported as sum of all the different quantified constituents.
For alkylamides, quantification was performed with a DAD detector. Since spilanthol was not available as reference standard, standardized commercial extract of Acmella was quantified by NMR. This extract was then used as reference for the quantification in cultivated samples using LC approach. Standardized extract was extracted with the same procedure of cultivated samples and serial dilutions were performed in methanol in the range of 5–50 ug/mL. Calibration of spilantol was as follows: y = 73,575x + 20.152 (R2 = 0.993). Peaks with UV spectra with maximum at 230 nm, ascribable to alkylamide structure, were selected and area was obtained at 230 nm.