Flavonoids and Isoflavonoids Biosynthesis in the Model Legume Lotus japonicus; Connections to Nitrogen Metabolism and Photorespiration
Abstract
1. Introduction
2. Flavonoid and Isoflavonoid Biosynthetic Pathways in Lotus
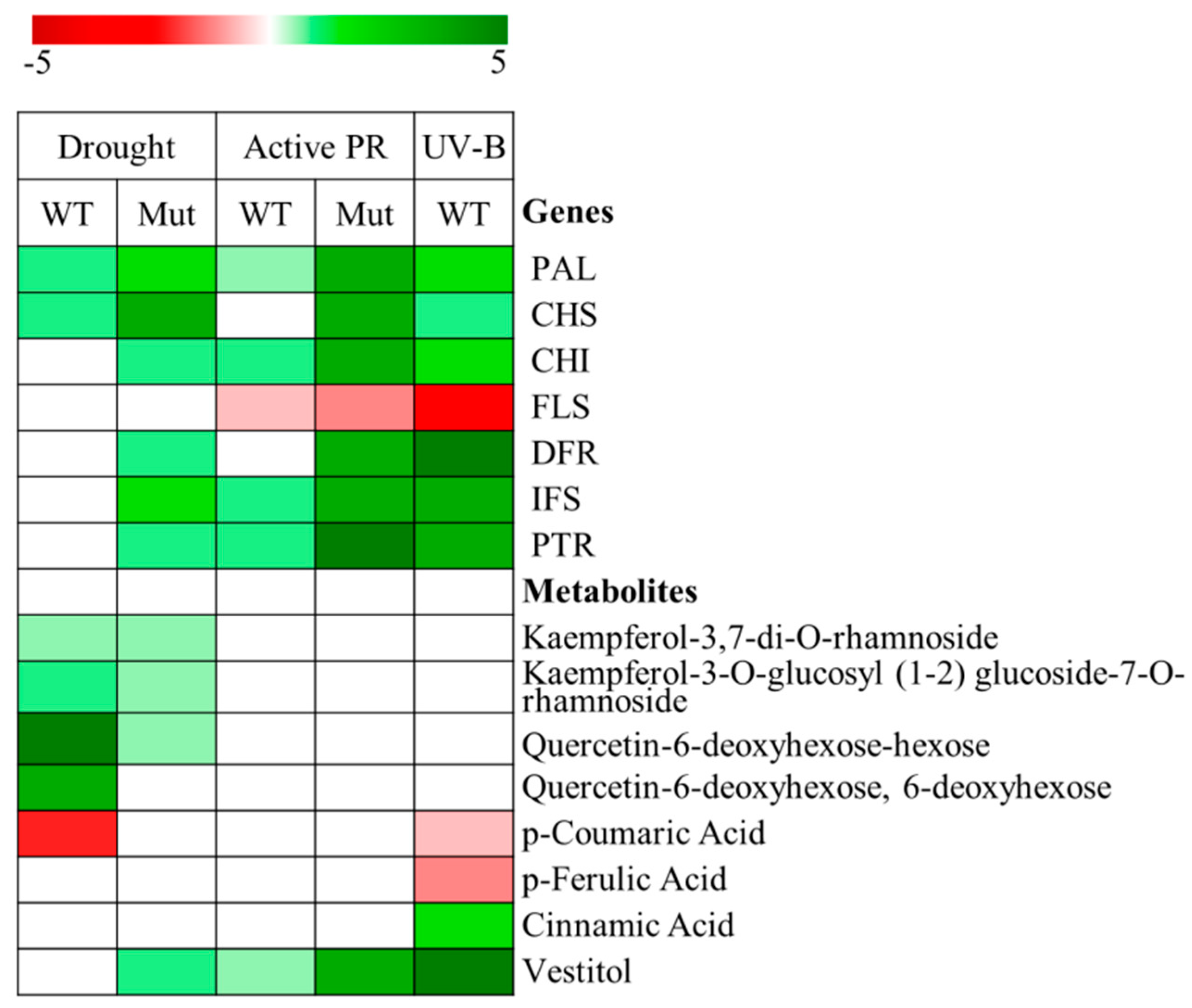
3. Differential Regulation of Flavonoid and Isoflavonoid Biosynthetic Pathways in Lotus japonicus
4. Co-Expression Analysis of Potential MYB Regulatory Genes in Lotus japonicus
5. The Importance of Isoflavonoid Biosynthesis in Soybean: Use of the Knowledge Obtained in the Model Legume Lotus japonicus for the Genetic Improvement of Soybean
6. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Jiang, W.; Xia, Y.; Wang, X.; Shen, G.; Pang, Y. Genistein-specific G6DT gene for the inducible production of wighteone in Lotus japonicus. Plant Cell Physiol. 2018, 59, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.; García-Calderón, M.; Pérez-Delgado, C.M.; Credali, A.; Pal’ove-Balang, P.; Estivill, G.; Repçák, M.; Vega, J.M.; Galván, F.; Márquez, A.J. Reassimilation of ammonium in Lotus japonicus. J. Exp. Bot. 2014, 19, 5557–5566. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.; Osbourn, A.; Ma, P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Theis, N.; Lerdau, M. The evolution of function in plant secondary metabolites. Int. J. Plant Sci. 2003, 164, S93–S102. [Google Scholar] [CrossRef]
- Izhaki, I. Emodin—A secondary metabolite with multiple ecological functions in higher plants. New Phytol. 2002, 155, 205–217. [Google Scholar] [CrossRef]
- De Geyter, N.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012, 17, 349–359. [Google Scholar] [CrossRef]
- Novelli, S.; Gismondi, A.; Di Marco, G.; Canuti, L.; Nanni, V.; Canini, A. Plant defense factors involved in Olea europea resistance against Xylella fastidiosa infection. J. Plant Res. 2019, 132, 439–455. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, D.P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Tohge, T.; Perez de Souza, L.; Fernie, A.R. Current understanding of the pathways of flavonoid biosynthesis in model and crop plants. J. Exp. Bot. 2017, 68, 4013–4028. [Google Scholar] [CrossRef]
- Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Evolution of secondary metabolites in legumes (Fabaceae). S. Afr. J. Bot. 2013, 89, 164–175. [Google Scholar] [CrossRef]
- Aoki, T.; Akashi, T.; Ayabe, S. Flavonoids of leguminous plants: Structure, biological activity, and biosynthesis. J. Plant Res. 2000, 113, 475–488. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef]
- Liu, C.; Murray, J.D. The role of Flavonoids in Nodulation Host-Range Specificity: An update. Plants 2016, 5, 33. [Google Scholar] [CrossRef]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signaling: Opportunities and challenges for improving plant-microbe interactions. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef]
- Larose, G.; Chenevert, R.; Moutuglis, P.; Gagne, S.; Piche, Y.; Vierheilig, H. Flavonoid levels in Medicago sativa are modulated by the developmental stage of the symbiosis and the root colonizing arbuscular mycorrhizal fungus. J. Plant Physiol. 2002, 159, 1329–1339. [Google Scholar] [CrossRef]
- Morandi, D.; Bailey, J.A.; Gianinazzi-Pearson, V. Isoflavonoid accumulation in soybean roots infected with vesicular-arbuscular mycorrhizal fungi. Physiol. Plant Pathol. 1984, 24, 357–364. [Google Scholar] [CrossRef]
- Wang, C.; Reid, J.B.; Foo, E. The art of self-control—Autoregulation of plant-microbe symbioses. Front. Plant Sci. 2018, 9, 988. [Google Scholar] [CrossRef]
- Harrison, M.; Dixon, R.A. Isoflavonoid accumulation and expression of defense gene transcripts during the establishment of vescicular-arbuscular mycorrhizal associations in roots of Medicago truncatula. Mol. Plant Microbe Interact. 1993, 6, 643–654. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Upadhyaya, H.D.; Chung, I.-M.; De Vita, P.; García-Lara, S.; Guajardo-Flores, D.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O.; Rajakumar, G.; Sahrawat, K.L.; et al. Exploiting phenylpropanoid derivatives to enhance the nutraceutical values of cereals and legumes. Front. Plant Sci. 2016, 7, 763. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C. Isoflavonoids of the leguminosae. Nat. Prod. Rep. 2007, 24, 417–464. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C. Isoflavonoids of the Leguminosae. Nat. Prod. Rep. 2009, 26, 776–802. [Google Scholar] [CrossRef]
- Veitch, N.C. Isoflavonoids of the leguminosae. Nat. Prod. Rep. 2013, 30, 988–1027. [Google Scholar] [CrossRef]
- Aerts, R.J.; Barry, T.N.; McNabb, W.C. Polyphenols and agriculture: Beneficial effects of proanthocyanidins in forages. Agric. Ecosyst. Environ. 1999, 75, 1–12. [Google Scholar] [CrossRef]
- McMahon, L.R.; McAllister, T.A.; Berg, B.P.; Majak, W.; Acharya, S.N.; Popp, J.D.; Coulman, B.E.; Wang, Y.; Cheng, K.-J. A review of the effects of forage condensed tannins on ruminal fermentation and bloat in grazing cattle. Can. J. Plant Sci. 2000, 80, 469–485. [Google Scholar] [CrossRef]
- Douglas, G.B.; Stienezen, M.; Waghorn, G.C.; Foote, A.G.; Purchas, R.W. Effect of condensed tannins in birdsfoot trefoil (Lotus corniculatus) and sulla (Hedysarum coronarium) on body weight, carcass fat depth, and wool growth of lambs in New Zealand. N. Z. J. Agric. Res. 1999, 42, 55–64. [Google Scholar] [CrossRef]
- Hancock, KR.; Collette, V.; Fraser, K.; Greig, M.; Xue, H.; Richardson, K.; Jones, C.; Rasmussen, S. Expression of the R2R3-MYB Transcription factor TaMYB14 from Trifolium arvense activates proanthocyanidin biosynthesis in the legumes Trifolium repens and Medicago sativa. Plant Physiol. 2012, 159, 1204–1220. [Google Scholar] [CrossRef]
- Jonker, A.; Yu, P. The role of proanthocyanidins complex in structure and nutrition interaction in alfalfa forage. Int. J. Mol. Sci. 2016, 17, 793. [Google Scholar] [CrossRef]
- Márquez, A.J. (Ed.) Lotus japonicus Handbook, 1st ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 1–384. [Google Scholar] [CrossRef]
- Mathesius, U.; Journet, E.P.; Sumner, L.W. (Eds.) The Medicago truncatula Handbook. Available online: http://www.noble.org/medicago-handbook/ (accessed on 28 November 2019).
- Stacey, G.; Libault, M.; Brechenmacher, L.; Wan, J.; May, G.D. Genetics and functional genomics of legume nodulation. Curr. Opin. Plant Biol. 2006, 9, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.K.; Tabata, S.; Parniske, M.; Stougaard, J. Lotus japonicus: Legume research in the fast lane. Trends Plant Sci. 2005, 10, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L.H.; Tirichine, L.; Jurkiewicz, A.; Sullivan, J.T.; Heckman, A.B.; Bek, A.S.; Ronson, C.W.; James, E.K.; Stougaard, J. The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 2010, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The rules of engagement in the legume-rhizobial symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef]
- Libault, M.; Joshi, T.; Benedito, V.A.; Xu, D.; Udvardi, M.K.; Stacey, G. Legume transcription factor genes: What makes legumes so special? Plant Physiol. 2009, 151, 991–1001. [Google Scholar] [CrossRef]
- Austin, M.B.; Noel, J.P. The chalcone synthase superfamily of type III polyketide synthases. Nat. Prod. Rep. 2003, 20, 79–110. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. It takes a garden. How work on diverse plant species has contributed to an understanding of flavonoid metabolism. Plant Physiol. 2001, 127, 1399–1404. [Google Scholar] [CrossRef]
- Yerlikaya, S.; Baloglu, M.C.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Zengin, G. Investigation of chemical profile, biological properties of Lotus corniculatus L. extracts and their apoptotic-autophagic effects onbreast cancer cells. J. Pharm. Biomed. Anal. 2019, 174, 286–299. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, J.; Chu, S.; Yan, H.-L.; Yu, D. Diversifying selection on flavanone 3-hydroxylase and isoflavone synthase genes in cultivated soybean and its wild progenitors. PLoS ONE 2013, 8, e54154. [Google Scholar] [CrossRef]
- Zavala, K.; Opazo, J.C. Lineage-specific expansion of the chalcone synthase gene family in rosids. PLoS ONE 2015, 10, e0133400. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Shimada, N.; Sato, S.; Akashi, T.; Nakamura, Y.; Tabata, S.; Ayabe, S.-I.; Aoki, T. Genome-wide analyses of the structural gene families involved in the legume-specific 5-deoxyisoflavonoid biosynthesis of Lotus japonicus. DNA Res. 2007, 14, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Daubhadel, S.; Gijyen, M.; Moy, P.; Farhangkhoee, M. Transcriptome analysis reveals a critical role of CHS7 and CHS8 genes for isoflavonoid synthesis in soybean seeds. Plant Physiol. 2007, 143, 326–338. [Google Scholar] [CrossRef]
- Lim, J.Y.; Jeon, H.Y.; Gil, Ch.S.; Kwon, S.-J.; Na, J.K.; Lee, Ch.; Eom, S.H. Isoflavone accumulation and the metabolic gene expression in response to persistent UV-B irradiation in soybean sprouts. Food Chem. 2020, 303, 125376. [Google Scholar] [CrossRef]
- Masunaka, A.; Hyakumachi, M.; Takenaka, S. Plant growth–promoting fungus, Trichoderma koningi suppresses isoflavonoid phytoalexin vestitol production for colonization on/in the roots of Lotus japonicus. Microbes Environ. 2011, 26, 128–134. [Google Scholar] [CrossRef]
- Shimada, N.; Sasaki, R.; Sato, S.; Kneko, T.; Tabata, S.; Aoki, T.; Ayabe, S.-I. A comprehensive analysis of six dihydroflavonol 4-reductases encoded by a gene cluster of the Lotus japonicus genome. J. Exp. Bot. 2005, 56, 2573–2585. [Google Scholar] [CrossRef]
- Paolocci, F.; Robbins, M.P.; Madeo, L.; Arcioni, S.; Martens, S.; Damiani, F. Ectopic expression of a basic helix-loop-helix gene transactivates parallel pathways of proanthocyanidin biosynthesis. Structure, expression analysis, and genetic control of leucoanthocyanidin 4-reductase and anthocyanidin reductase genes in Lotus corniculatus. Plant Physiol. 2007, 143, 504–516. [Google Scholar] [CrossRef]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef]
- Saslowsky, D.; Winkel-Shirley, B. Localization of flavonoid enzymes in Arabidopsis roots. Plant J. 2001, 27, 37–48. [Google Scholar] [CrossRef]
- Crosby, K.C.; Pietraszewska-Bogiel, A.; Gadella, T.W.J.; Winkel, B.S.J. Förster resonance energy transfer demonstrates a flavonoid metabolon in living plant cells that displays competitive interactions between enzymes. FEBS Lett. 2011, 585, 2193–2198. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Fernie, A.R. The spatial organization of metabolism within the plant cell. Annu. Rev. Plant Biol. 2013, 64, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Ngaki, M.N.; Louie, G.V.; Philippe, G.; Manning, G.; Pojer, F.; Bowman, M.E.; Li, L.; Larsen, E.; Wurtele, E.S.; Noel, J.P. Evolution of the chalcone-isomerase fold from fatty-acid binding to stereospecific catalysis. Nature 2012, 485, 530–533. [Google Scholar] [CrossRef]
- Nakayama, T.; Takahashi, S.; Waki, T. Formation of flavonoid metabolons: Functional significance of protein-protein interactions and impact on flavonoid chemodiversity. Front. Plant Sci. 2019, 10, 821. [Google Scholar] [CrossRef]
- Ralston, L.; Yu, O. Metabolons involving plant cytochrome P450s. Phytochem. Rev. 2006, 5, 459–472. [Google Scholar] [CrossRef]
- Diharce, J.; Golebiowski, J.; Fiorucci, S.; Antonczak, S. Fine-tuning of microsolvation and hydrogen bond interaction regulates substrate channelling in the course of flavonoid biosynthesis. Phys. Chem. Chem. Phys. 2016, 18, 10337–10345. [Google Scholar] [CrossRef]
- Gruber, M.; Skadhauge, B.; Yu, M.; Muir, A.; Richards, K. Variation in morphology, plant habit, proanthocyanidins, and flavonoids within a Lotus germplasm collection. Can. J. Plant Sci. 2008, 88, 121–132. [Google Scholar] [CrossRef]
- Escaray, F.J.; Passeri, V.; Babuin, F.M.; Marco, F.; Carrasco, P.; Damiani, F.; Pieckenstain, F.L.; Poalocci, F.; Ruiz, O. Lotus tenuis x L. corniculatus interspecific hybridization as a means to breed bloat-safe pastures and gain insight into the genetic control of proanthocyanidin biosynthesis in legumes. BMC Plant Biol. 2014, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, S.; Rumball, W.; Lane, G.F.; Fraser, K.; Foo, L.Y.; Yu, M.; Meagher, L.P. Variation of proanthocyanidins in Lotus species. J. Chem. Ecol. 2006, 32, 1797–1816. [Google Scholar] [CrossRef]
- García-Calderón, M.; Pons-Ferrer, T.; Mrázová, A.; Pal’ove-Balang, P.; Vilková, M.; Pérez-Delgado, C.M.; Vega, J.M.; Eliášová, A.; Repčák, M.; Márquez, A.J.; et al. Modulation of phenolic metabolism under stress conditions in a Lotus japonicus mutant lacking plastidic glutamine synthetase. Front. Plant Sci. 2015, 6, 760. [Google Scholar] [CrossRef] [PubMed]
- Mrázová, A.; Mrázová, A.; Belay, S.A.; Eliášová, A.; Perez-Delgado, C.; Kaducová, M.; Betti, M.; Vega, J.M.; Pal’ove-Balang, P. Expression, activity of phenylalanine-ammonia-lyase and accumulation of phenolic compounds in Lotus japonicus under salt stress. Biologia 2017, 72, 36–42. [Google Scholar] [CrossRef]
- Suzuki, H.; Sasaki, R.; Ogata, Y.; Nakamura, Y.; Sakurai, N.; Kitajima, M.; Takayama, H.; Kanaya, S.; Aoki, K.; Shibata, D.; et al. Metabolic profiling of flavonoids in Lotus japonicus using liquid chromatography Fourier transform ion cyclotron resonance mass spectrometry. Phytochemistry 2008, 69, 99–111. [Google Scholar] [CrossRef]
- Jay, M.; De Luca, V.; Ibrahim, R. Meta-methylation of flavonol rings A (8-) and B (3’-) is catalysed by two distinct O-methyltransferases in Lotus corniculatus. Z. Nat. C 1983, 38, 413–417. [Google Scholar] [CrossRef]
- Kaducová, M.; Monje-Rueda, M.D.; García-Calderón, M.; Pérez-Delgado, M.C.; Eliášová, A.; Gajdošová, S.; Petruľová, V.; Betti, M.; Márquez, A.J.; Paľove-Balang, P. Induction of isoflavonoid biosynthesis in Lotus japonicus after UV-B irradiation. J. Plant Physiol. 2019, 236, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Shimada, N.; Aoki, T.; Sato, S.; Nakamura, Y.; Tabata, S.; Ayabe, S.-I. A cluster of genes encodes the two types of chalcone isomerase involved in the biosynthesis of general flavonoids and legume-specific 5-deoxy(iso)flavonoids in Lotus japonicus. Plant Physiol. 2003, 131, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Waki, T.; Yoo, D.-C.; Fujino, N.; Mameda, R.; Dennesiouk, K.; Yamashita, S.; Motohashi, R.; Akashi, T.; Aoki, T.; Ayabe, S.-I.; et al. Identification of protein protein interactions of isoflavonoid biosynthetic enzymes with 2-hydroxyisoflavanone synthase in soybean (Glycine max (L.) Merr.). Biochem. Biophys. Res. Commun. 2016, 469, 546–551. [Google Scholar] [CrossRef]
- Dastmalchi, M.; Bernards, M.A.; Dhaubhadel, S. Twin anchors of the soybean isoflavonoid metabolon: Evidence for tethering of the complex to the endoplasmic reticulum by IFS and C4H. Plant J. 2016, 85, 689–706. [Google Scholar] [CrossRef]
- Winkel, B. The Subtleties of Subcellular Distribution. Pointing the way to underexplored functions for flavonoid enzymes and end products. In Recent Advances in Polyphenol Research; Halbwirth, H., Stich, K., Cheynier, V., Quideau, S., Eds.; Jonh Willey & Sons. Ltd.: Chichester, UK, 2019; Volume 6, pp. 89–108. [Google Scholar] [CrossRef]
- Jung, W.; Yu, O.; Cindy Lau, S.-M.; O’Keefe, D.P.; Odell, J.; Fader, G. McGonigle, B. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat. Biotechnol. 2000, 18, 208–212. [Google Scholar] [CrossRef]
- Lapčík, O. Isoflavonoids in non-leguminous taxa: A rarity or a rule? Phytochemistry 2007, 68, 2909–2916. [Google Scholar] [CrossRef]
- Wang, X. Structure, function and engineering of enzymes in isoflavonoid biosynthesis. Funct. Integr. Genom. 2011, 11, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Akashi, T.; Aoki, T.; Ayabe, S.-I. Molecular and biochemical characterization of 2-hydroxyisoflavanone dehydratase. Involvement of carboxylesterase-like proteins in leguminous isoflavone biosynthesis. Plant Physiol. 2005, 137, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Akashi, T.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Ayabe, S.-I.; Aoki, T. 2-Hydroxyisoflavanone dehydratase is a critical determinant of isoflavone productivity in hairy root cultures of Lotus japonicus. Plant Cell Physiol. 2007, 48, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, E.; Courot, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Dixon, R.A.; Wang, X. Crystal structure of vestitone reductase from alfalfa (Medicago sativa L.). J. Mol. Biol. 2007, 369, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Shimada, N.; Akashi, T.; Aoki, T.; Ayabe, S.i. Induction of isoflavonoids pathway in the model legume Lotus japonicus: Molecular characterization of enzymes involved in phytoalexin biosynthesis. Plant Sci. 2000, 160, 37–47. [Google Scholar] [CrossRef]
- Monje-Rueda, M.D.; García-Calderón, M.; Pal’Ove-Balang, P.; Márquez, A.J.; Betti, M. Functional analysis of two Lotus japonicus mutants affected in MYB transcription factors. (manuscript in preparation).
- Uchida, K.; Akashi, T.; Aoki, T. The missing link in leguminous pterocarpan biosynthesis is a dirigent domain-containing protein with isoflavanol dehydratase activity. Plant Cell Physiol. 2017, 58, 398–408. [Google Scholar] [CrossRef]
- Akashi, T.; Koshimizu, S.; Aoki, T.; Ayabe, S.-I. Identification of cDNA encoding pterocarpan reductase involved in isoflavan phytoalexin biosynthesis in Lotus japonicus by EST mining. FEBS Lett. 2006, 580, 5666–5670. [Google Scholar] [CrossRef]
- Kaducová, M.; Eliášová, A.; Truš, K.; Bačovčinová, M.; Sklenková, K.; Pal’ove-Balang, P. Induction of pterocarpane reductase expression and accumulation of vestitol in Lotus corniculatus on UV-B irradiation. (manuscript in preparation).
- Deavours, B.E.; Liu, C.-J.; Naoumkina, M.A.; Tang, Y.; Farang, M.A.; Sumner, L.W.; Noel, J.P.; Dixon, R.A. Functional analysis of members of the isoflavone and isoflavanone O-methyltransferase enzyme families from the model legume Medicago truncatula. Plant Mol. Biol. 2006, 62, 715–733. [Google Scholar] [CrossRef]
- Masai, M.; Arakawa, M.; Iwaya, K.; Aoki, T.; Nakagawa, T.; Ayabe, Sh.-I.; Uchiyama, H. Discriminative phytoalexin accumulation in Lotus japonicus against symbiotic and non-symbiotic microorganisms and related chemical signals. Biosci. Biotechnol. Biochem. 2013, 77, 1773–1775. [Google Scholar] [CrossRef][Green Version]
- Ueda, H.; Sugimoto, Y. Vestitol as a chemical barrier against intrusion of parasitic plant Striga hermonthica into Lotus japonicus roots. Biosci. Biotechnol. Biochem. 2010, 74, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Lanot, A.; Morris, P. Elicitation of isoflavan phytoalexines. In Lotus japonicus Handbook; Márquez, A.J., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 355–362. [Google Scholar] [CrossRef]
- Alseekh, S.; Perez de Souza, L.; Benina, M.; Fernie, A.R. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry 2020, 174, 112347. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Malnoy, M.; Goremykin, V.; Nikiforova, S.; Martens, S. A genome-wide phylogenetic reconstruction of family 1 UDPglycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land. Plant J. 2012, 69, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Shen, G.; Chang, Z.; Tang, Y.; Gao, H.; Pang, Y. Involvement of tree putative glucosyltransferases from the UGT72 family in flavonol glucoside/rhamnoside biosynthesis in Lotus japonicus seeds. J. Exp. Bot. 2017, 68, 597–612. [Google Scholar] [CrossRef]
- Modolo, L.V.; Blount, J.W.; Achnine, L.; Naoumkina, M.A.; Wang, X.Q.; Dixon, R.A. A functional genomics approach to (iso)flavonoid glycosylation in the model legume Medicago truncatula. Plant Mol. Biol. 2007, 64, 499–518. [Google Scholar] [CrossRef]
- Dhaubhadel, S.; Farhangkhoee, M.; Chapman, R. Identification and characterization of isoflavonoid specific glycosyltransferase and malonyltransferase from soybean seeds. J. Exp. Bot. 2008, 59, 981–994. [Google Scholar] [CrossRef]
- Yin, Q.; Shen, G.; Di, S.; Fan, C.; Chang, Z.; Pang, Y. Genome-wide Identification and Functional Characterization of UDP-glucosyltransferase Genes Involved in Flavonoid Biosynthesis in Glycine max. Plant Cell Physiol. 2017, 58, 1558–1572. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effect of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Pérez-Delgado, C.M.; Moyano, T.C.; García-Calderón, M.; Canales, J.; Gutiérrez, R.A.; Márquez, A.J.; Betti, M. Use of transcriptomics and co-expression networks to analyze the interconnections between nitrogen assimilation and photorespiratory metabolism. J. Exp. Bot. 2016, 67, 3095–3108. [Google Scholar] [CrossRef]
- Pérez-Delgado, C.M.; García-Calderón, M.; Sánchez, D.H.; Udvardi, M.K.; Kopka, J.; Márquez, A.J.; Betti, M. Transcriptomic and metabolic changes associated with photorespiratory ammonium accumulation in the model legume Lotus japonicus. Plant Physiol. 2013, 162, 1834–1848. [Google Scholar] [CrossRef]
- García-Calderón, M.; Chiurazzi, M.; Espuny, M.R.; Márquez, A.J. Photorespiratory metabolism and nodule function: Behaviour of Lotus japonicus mutants deficient in plastid glutamine synthetase. Mol. Plant Microbe Interact. 2012, 25, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Dugas, A.J.; Castañeda-Acosta, J.; Bonin, G.C.; Price, K.L.; Fischer, N.H.; Winston, G.W. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: Structure-activity relationships. J. Nat. Prod. 2000, 63, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhang, J.; Song, T.; Tian, J.; Yao, Y. Promotion of flavonoid biosynthesis in leaves and calli of ornamental crabapple (Malus sp.) by high carbon to nitrogen ratios. Front. Plant Sci. 2015, 6, 673. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Tattini, M. Flavonols: Old compounds for old roles. Ann. Bot. 2011, 108, 1225–1233. [Google Scholar] [CrossRef]
- Parry, A.D.; Tiller, S.A.; Edwards, R. The effects of heavy metals and root immersion on isoflavonoid metabolism in alfalfa (Medicago sativa). Plant Physiol. 1994, 106, 195–202. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Valliyodan, B.; Zhang, J.; Lenoble, M.E.; Yu, O.; Rogers, E.; Nguyen, H.T.; Sharp, R.E. Regulation of growth response to water stress in the soybean primary root. I. Proteomic analysis reveals region-specific regulation of phenylpropanoid metabolism and control of free iron in the elongation zone. Plant Cell Environ. 2010, 33, 223–243. [Google Scholar] [CrossRef]
- Zavala, J.A.; Mazza, C.A.; Dillon, F.M.; Chludil, H.D.; Ballaré, C.I. Soybean resistance to stink bugs (Nezara viridula and Piezodorus guildinii) increases with exposure to solar UV-B radiation and correlated with isoflavonoids content in pods under field-conditions. Plant Cell Environ. 2015, 38, 920–928. [Google Scholar] [CrossRef]
- Shelton, D.; Stranne, M.; Mikkelsen, L.; Pakseresht, N.; Welham, T.; Hiraka, H.; Tabata, S.; Sato, S.; Paquette, S.; Wang, T.L.; et al. Transcription factors of Lotus: Regulation of isoflavonoid biosynthesis requires coordinated changes in transcription factor activity. Plant Physiol. 2012, 159, 531–547. [Google Scholar] [CrossRef]
- Zabala, G.; Zou, J.; Tuteja, J.; González, D.O.; Clough, S.J.; Vodkin, L.O. Transcriptome changes in the phenylpropanoid pathway of Glycine max in response to Pseudomonas syringae infection. BMC Plant Biol. 2006, 6, 26. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, W.; Duan, X.; Dai, Ch.; Zhang, Y.; Cui, W.; Wang, R.; Shen, W. Hydrogen-rich water-alleviated ultraviolet-B-triggered oxidative damage is partially associated with the manipulation of the metabolism of (iso)flavonoids and antioxidant defence in Medicago sativa. Funct. Plant Biol. 2015, 42, 1141–1157. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, X.; Ji, Y.; Wang, S.; Chen, Y.; Luo, J.; Shen, Y.; Peng, L. Measurement of metabolite variations and analysis of related gene expression in Chinese liquorice (Glycyrrhiza uralensis) plants under UV-B irradiation. Sci. Rep. 2018, 8, 6144. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Tobler, M.A.; Barnes, P.W. Different irradiances of UV and PAR in the same ratios alter the flavonoid profiles of Arabidopsis thaliana wild types and UV-signalling pathway mutants. Photochem. Photobiol. Sci. 2019, 18, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, P.; Yang, R.; Zhou, T.; Gu, Z. UV-B mediates isoflavone accumulation and oxidative-antioxidant system responses in germinating soybean. Food Chem. 2019, 275, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Wang, X.; Li, Y.; Han, R. Lower levels of UV-B light trigger the adaptive responses by inducing plant antioxidant metabolism and flavonoid biosynthesis in Medicago sativa seedlings. Funct. Plant Biol. 2019, 46, 896–906. [Google Scholar] [CrossRef]
- Kim, B.G.; Kim, J.H.; Kim, J.; Lee, C.; Ahn, J.-H. Accumulation of flavonols in response to ultraviolet-B irradiation in soybean is related to induction of flavanone 3-hydroxylase and flavonol synthase. Mol. Cells 2008, 25, 247–252. [Google Scholar]
- Pérez-Delgado, C.M.; García-Calderón, M.; Márquez, A.J.; Betti, M. Reassimilation of photorespiratory ammonium in Lotus japonicus plants deficient in plastidic glutamine synthetase. PLoS ONE 2015, 10, e0130438. [Google Scholar] [CrossRef]
- Takanashi, K.; Takahashi, H.; Sakural, N.; Sugiyama, A.; Suzuki, H.; Shibata, D.; Nakazono, M.; Yazaki, K. Tissue-specific transcriptome analysis in nodules of Lotus japonicus. Mol. Plant Microb. Interact. 2012, 25, 869–876. [Google Scholar] [CrossRef]
- Wasson, A.P.; Pellerone, F.I.; Mathesius, U. Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 2006, 18, 1617–1629. [Google Scholar] [CrossRef]
- Zhang, J.; Subramanian, S.; Stacey, G.; Yu, O. Flavones and flavonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J. 2009, 57, 171–183. [Google Scholar] [CrossRef]
- Sugiyama, A.; Shitan, N.; Yazaki, K. Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume–Rhizobium symbiosis. Plant Physiol. 2007, 144, 2000–2008. [Google Scholar] [CrossRef]
- Nakagawa, T.; Kaku, H.; Shimoda, Y.; Sugiyama, A.; Shimamura, M.; Takanashi, K.; Yazaki, K.; Aoki, T.; Shibuya, N.; Kouchi, H. From defense to symbiosis: Limited alterations in the kinase domain of LysM receptor-like kinases are crucial for evolution of legume-Rhizobium symbiosis. Plant J. 2011, 65, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, Y.; Banba, M.; Shimoda, Y.; Chechetka, S.A.; Suzuri, R.; Okusako, Y.; Ooki, Y.; Toyokura, K.; Suzuki, A.; Uchiumi, T.; et al. Transcriptome profiling of Lotus japonicus roots during arbuscular mycorrhiza development and comparison with that of nodulation. DNA Res. 2007, 14, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Kouchi, H.; Shimomura, K.; Hata, S.; Hirota, A.; Wu, G.J.; Kumagai, H.; Tajima, S.; Suganuma, N.; Suzuki, A.; Aoki, T.; et al. Large-scale analysis of gene expression profiles during early stages of root nodule formation in a model legume Lotus japonicus. DNA Res. 2004, 11, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Colebatch, G.; Desbrosses, G.; Ott, T.; Krusell, L.; Montanari, O.; Kloska, S.; Kopka, J.; Udvardi, M.K. Global changes in transcription orchestrate metabolic differentiation during symbiotic nitrogen fixation in Lotus japonicus. Plant J. 2004, 39, 487–512. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB transcription factors: Their role in drought response mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef]
- Chezem, W.R.; Clay, N.K. Regulation of plant secondary metabolism and associated specialized cell development by MYBs and bHLs. Phytochemistry 2016, 131, 26–43. [Google Scholar] [CrossRef]
- Hichri, I.; Heppel, S.C.; Pillet, J.; Léon, C.; Czemmel, S.; Delrot, S.; Lauvergeat, V.; Bogs, J. The basic helix-loop-helix transcription factor MYC1 is involved in the regulation of the flavonoid biosynthesis pathway in grapevine. Mol. Plant 2010, 3, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Iwasaka, R.; Kaneko, T.; Sato, S.; Tabata, S.; Sakuta, M. Functional differentiation of Lotus japonicus TT2s, R2R3-MYB Transcription factors comprising a multigene family. Plant Cell Physiol. 2008, 49, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Escaray, F.J.; Passeri, V.; Perea-Garcia, A.; Antonelli, C.J.; Damiani, F.; Ruiz, O.A.; Paolocci, F. The R2R3-MYB TT2b and the bHLH TT8 genes are the major regulators of proanthocyanidin biosynthesis in the leaves of Lotus species. Planta 2017, 246, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kume, N.; Nakaya, Y.; Yamagami, A.; Nakano, T.; Sakuta, M. Comparative analysis of the triplicate proathocyanidin regulators in Lotus japonicus. Plant Cell Physiol. 2010, 51, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Kunihiro, S.; Tanabe, D.; Niwa, Y.; Kitamura, K.; Abe, J.; Yamada, T. Isolation and molecular characterization of a Lotus japonicus R2R3-MYB subgroup 7 transcription factor gene. Plant Biotechnol. 2017, 34, 45–49. [Google Scholar] [CrossRef]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef]
- Zhu, L.; Shan, H.; Chen, S.M.; Jiang, J.F.; Gu, C.S.; Zhou, G.Q.; Chen, Y.; Song, A.P.; Chen, F.D. The heterologous expression of the Chrysanthemum R2R3-MYB transcription factor CmMYB1 alters lignin composition and represses flavonoid synthesis in Arabidopsis thaliana. PLoS ONE 2013, 8, e65680. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, I.M.; Heim, M.A.; Weisshaar, B.; Uhrig, J.F. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J. 2004, 40, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Babuin, M.F.; Campestre, M.P.; Rocco, R.; Bordenave, C.D.; Escaray, F.J.; Antonelli, C.; Calzadilla, P.; Gárriz, A.; Serna, E.; Carrasco, P.; et al. Response to long-term NaHCO3-derived alkalinity in model Lotus japonicus ecotypes Gifu B-129 and Miyakojima MG-20: Transcriptomic profiling and physiological characterization. PLoS ONE 2014, 9, e97106. [Google Scholar] [CrossRef]
- Saito, K.; Hirai, M.Y.; Yonekura-Sakakibara, K. Decoding genes with coexpression networks and metabolomics—‘majority report by precogs’. Trends Plant Sci. 2007, 13, 36–43. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Tohge, T.; Matsuda, F.; Nakabayashi, R.; Takayama, H.; Niida, R.; Watanabe-Takahashi, A.; Inoue, E.; Saito, K. Comprehensive flavonol profiling and transcriptome coexpression analysis leading to decoding gene-metabolite correlations in Arabidopsis. Plant Cell 2008, 20, 2160–2176. [Google Scholar] [CrossRef]
- Tohge, T.; Kusano, M.; Fukushima, A.; Saito, K.; Fernie, R. Transcriptional and metabolic programs following exposure of plants to UV-B irradiation. Plant Signal. Behav. 2011, 6, 12. [Google Scholar] [CrossRef][Green Version]
- Doncheva, N.T.; Assenov, Y.; Domingues, F.S.; Albrecht, M. Topological analysis and interactive visualization of biological networks and protein structures. Nat. Protoc. 2012, 7, 670–685. [Google Scholar] [CrossRef]
- Lea, U.L.; Slimestad, R.; Smedvig, P.; Lillo, C. Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. Planta 2007, 225, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Lillo, C.; Lea, U.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K.M.; Lea, U.S.; Slimestad, R.; Verheul, M.; Lillo, C. Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental-triggered flavonoid synthesis. J. Plant Physiol. 2008, 165, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Klejdus, B.; Bačkor, M.; Repčák, M. Phenylalanine ammonia-lyase activity and phenolic compounds accumulation in nitrogen-deficient Matricaria chamomilla leaf rosettes. Plant Sci. 2007, 172, 393–399. [Google Scholar] [CrossRef]
- Muzika, R.-M. Terpenes and phenolics in response to nitrogen fertilization: A test of the carbon/nutrient balance hypothesis. Chemoecology 1993, 4, 3–7. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. Effects of nitrogen fertilization on synthesis of primary and secondary metabolites in three varieties of Kacip Fatimah (Labisia Pumila Blume). Int. J. Mol. Sci. 2011, 12, 5238–5254. [Google Scholar] [CrossRef]
- Lattanzio, V.; Cardinali, A.; Ruta, C.; Morone Fortunato, I.; Lattanzio, V.M.T.; Linsalata, V.; Cicco, N. Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ. Exp. Bot. 2009, 65, 54–62. [Google Scholar] [CrossRef]
- Chu, S.; Wang, J.; Zhu, Y.; Liu, S.; Zhou, X.; Zhang, H.; Wang, C.; Yang, W.; Tian, Z.; Cheng, H.; et al. An R2R3-type MYB transcription factor, GmMYB29, regulates isoflavone biosynthesis in soybean. PLoS Genet. 2017, 13, e1006770. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, L.; Ma, Y.; Wei, Z.; Hong, H.; Liu, Z.; Lei, J.; Liu, Y.; Guan, R.; Guo, Y.; et al. Development and utilization of a new chemically-induced soybean library with a high mutation density. J. Integr. Plant Biol. 2017, 59, 60–74. [Google Scholar] [CrossRef]
- Li, X.-W.; Li, J.-W.; Zhai, Y.; Zhao, X.; Zhang, H.-J.; Su, L.T.; Wang, Y.; Wang, Q.-Y. A R2-R3-MYB transcription factor, GmMYB12B2, affects the expression levels of flavonoid biosynthesis genes encoding key enzymes in transgenic Arabidopsis plants. Gene 2013, 532, 72–79. [Google Scholar] [CrossRef]
- Han, X.; Yin, Q.; Liu, J.; Jiang, W.; Di, S. GmMYB58 and GmMYB205 are seed-specific activators for isoflavonoid biosynthesis in Glycine max. Plant Cell Rep. 2017, 36, 1889–1902. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, T.; Wu, P.; Guo, W.; Su, L.; Wang, Y.; Liu, Y.; Yan, F.; Wang, Q. Isolation and characterization of GmMYBJ3, an R2-R3-MYB transcription factor that affects isoflavonoids biosynthesis in soybean. PLoS ONE 2017, 12, e017990. [Google Scholar] [CrossRef]
- Bian, S.; Li, R.; Xia, S.; Liu, Y.; Jin, D.; Xie, X.; Dhaubhadel, S.; Zhai, L.; Wang, J.; Li, X. Soybean CCA1-like MYB transcription factor GmMYB133 modulates isoflavonoid biosynthesis. Biochem. Biophys. Res. Commun. 2018, 507, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Anguraj Vadivel, A.K.; Renaud, J.; Kagale, S.; Dhaubhadel, S. GmMYB176 regulates multiple steps of isoflavonoid biosynthesis in soybean. Front. Plant Sci. 2019, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yuan, L.; Xu, L.; Xu, Z.; Huang, Y.; He, X.; Ma, H.; Yi, J.; Zhang, D. Over-expression of GmMYB39 leads to an inhibition of the isoflavonoid biosynthesis in soybean (Glycine max L.). Plant Biotechnol. Rep. 2013, 7, 445–455. [Google Scholar] [CrossRef]
- Yan, J.; Wang, B.; Zhong, Y.; Yao, L.; Cheng, L.; Wu, T. The soybean R2R3 MYB transcription factor GmMYB100 negatively regulates plant flavonoid biosynthesis. Plant Mol. Biol. 2015, 89, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D. Soy isoflavones-benefits and risks from nature’s selective estrogen receptor modulators (SERMs). J. Am. Coll. Nutr. 2001, 20 (Suppl. 5), 354S–362S. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, Y.; Wang, Z.; Gou, Z.; Lyu, J.; Li, W.; Yu, Y.; Shu, L.; Zhao, Y.; Ma, Y.; et al. Resequencing of 302 wild and cultivated accessions identifies genes related to domestication and improvement in soybean. Nat. Biotechnol. 2015, 33, 408–414. [Google Scholar] [CrossRef]
- Gandhi, A.P. Quality of soybean and its food products. Int. Food Res. J. 2009, 16, 11–19. [Google Scholar]
- Messina, M. Soy and health update: Evaluation of the clinical and epidemiological literature. Nutrients 2016, 8, 754. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Kang, J.; Gu, H.; Qin, G. AtMYB14 regulates cold tolerance in Arabidopsis. Plant Mol. Biol. Rep. 2013, 31, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Cominelli, E.; Galbiati, M.; Vavasseur, A.; Conti, L.; Sala, T.; Vuylsteke, M.; Leonhardt, N.; Dellaporta, S.L.; Tonelli, C. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr. Biol. 2005, 15, 1196–1200. [Google Scholar] [CrossRef] [PubMed]
- Kirik, V.; Kolle, K.; Wohlfarth, T.; Miséra, S.; Baumlein, H. Ectopic expression of a novel MYB gene modifies the architecture of the Arabidopsis inflorescence. Plant J. 1998, 13, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, S.; An, X.; Liu, X.; Qin, H.; Wang, D. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J. Genet. Genom. 2009, 36, 17–29. [Google Scholar] [CrossRef]
- Wang, F.; Ren, X.; Zhang, F.; Qi, M.; Zhao, H.; Chen, X.; Ye, Y.; Yang, J.; Li, S.; Zhang, Y.; et al. A R2R3-type MYB transcription factor gene from soybean, GmMYB12, is involved in flavonoids accumulation and abiotic stress tolerance in transgenic Arabidopsis. Plant Biotechnol. Rep. 2019, 13, 219–233. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Calderón, M.; Pérez-Delgado, C.M.; Palove-Balang, P.; Betti, M.; Márquez, A.J. Flavonoids and Isoflavonoids Biosynthesis in the Model Legume Lotus japonicus; Connections to Nitrogen Metabolism and Photorespiration. Plants 2020, 9, 774. https://doi.org/10.3390/plants9060774
García-Calderón M, Pérez-Delgado CM, Palove-Balang P, Betti M, Márquez AJ. Flavonoids and Isoflavonoids Biosynthesis in the Model Legume Lotus japonicus; Connections to Nitrogen Metabolism and Photorespiration. Plants. 2020; 9(6):774. https://doi.org/10.3390/plants9060774
Chicago/Turabian StyleGarcía-Calderón, Margarita, Carmen M. Pérez-Delgado, Peter Palove-Balang, Marco Betti, and Antonio J. Márquez. 2020. "Flavonoids and Isoflavonoids Biosynthesis in the Model Legume Lotus japonicus; Connections to Nitrogen Metabolism and Photorespiration" Plants 9, no. 6: 774. https://doi.org/10.3390/plants9060774
APA StyleGarcía-Calderón, M., Pérez-Delgado, C. M., Palove-Balang, P., Betti, M., & Márquez, A. J. (2020). Flavonoids and Isoflavonoids Biosynthesis in the Model Legume Lotus japonicus; Connections to Nitrogen Metabolism and Photorespiration. Plants, 9(6), 774. https://doi.org/10.3390/plants9060774






