Calcium Improves Germination and Growth of Sorghum bicolor Seedlings under Salt Stress
Abstract
1. Introduction
2. Results
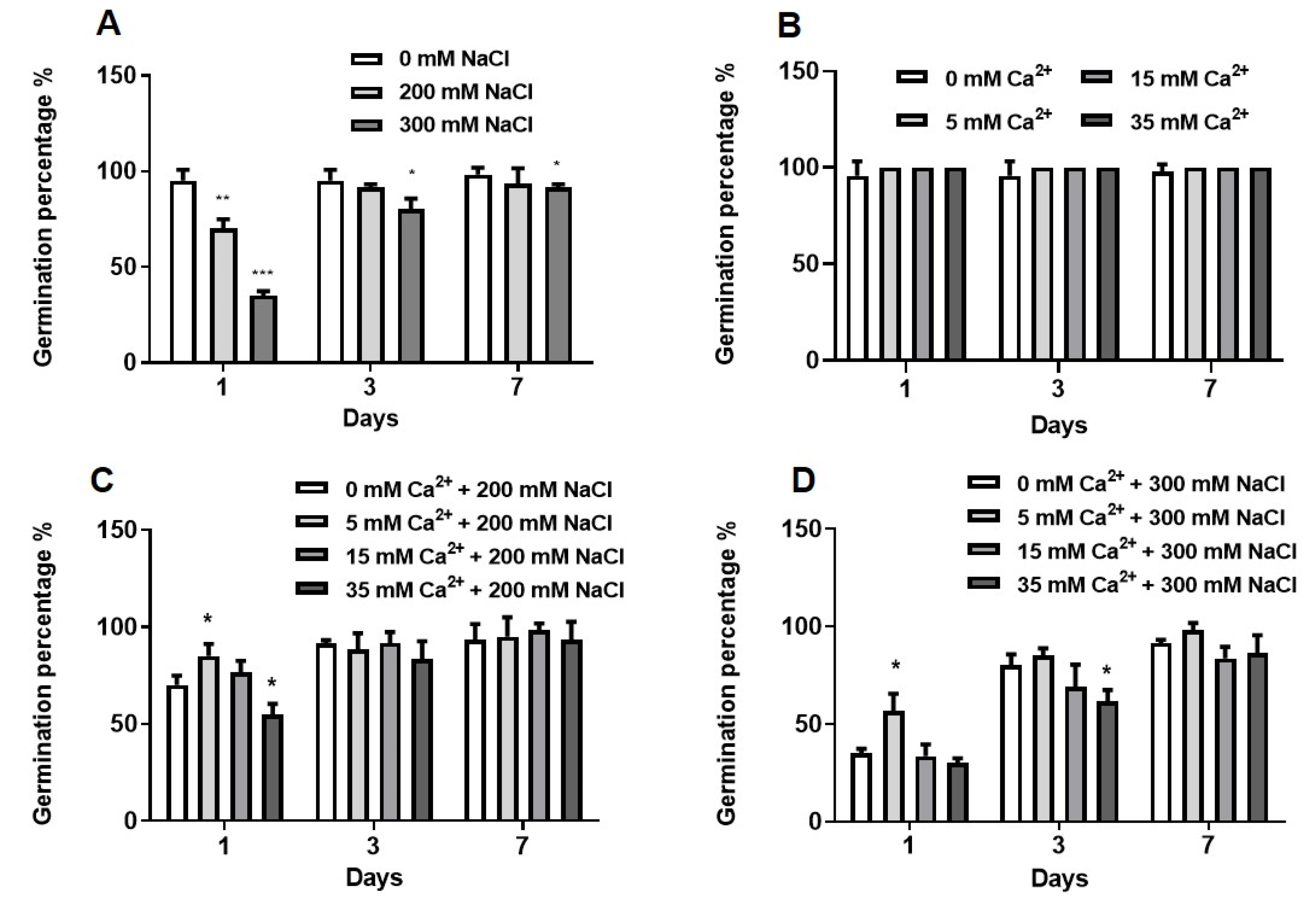
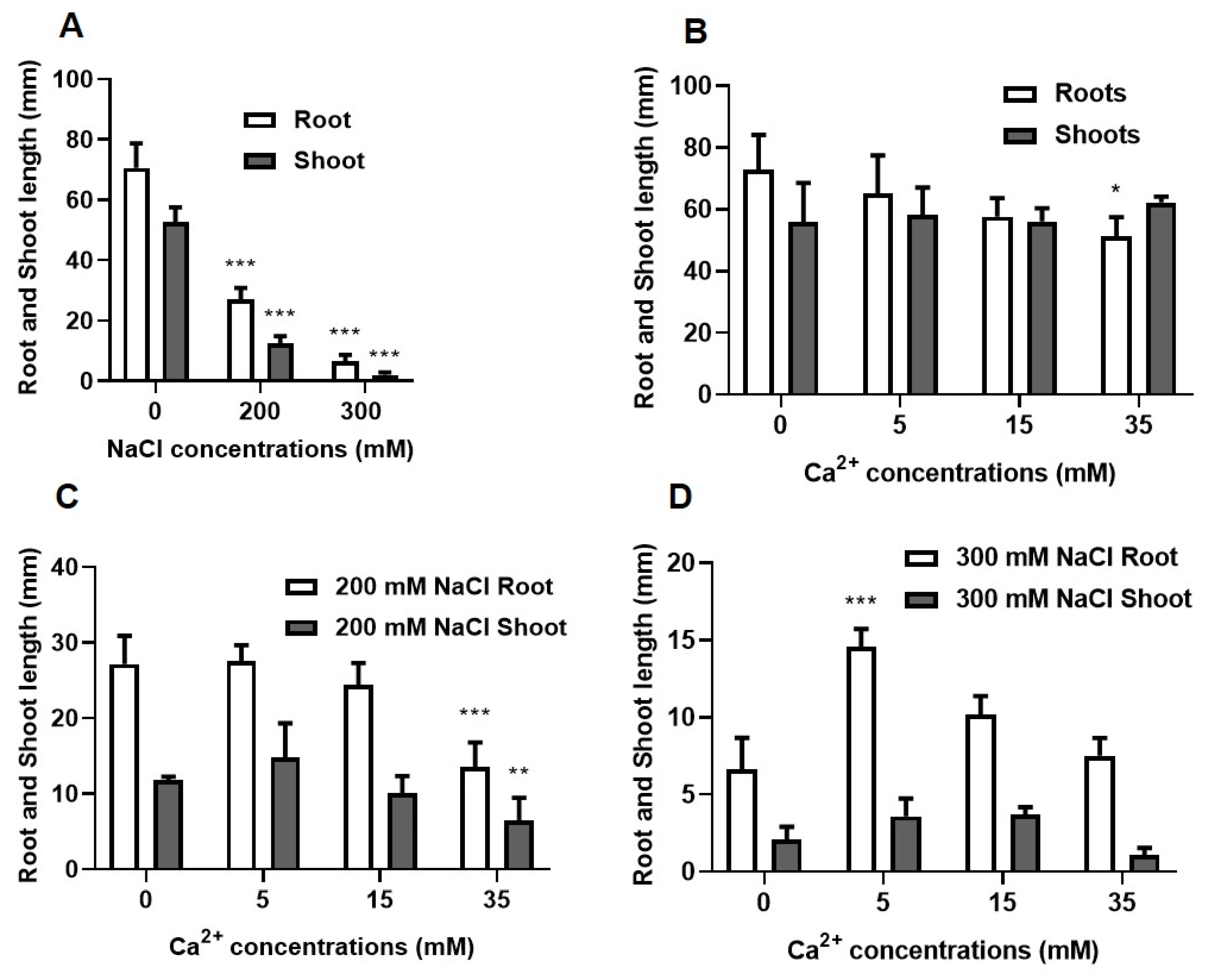
2.1. Physiological Analysis of the Effect of NaCl and Ca2+ on Seed Germination and Growth
2.1.1. Germination Assays
2.1.2. Growth Analysis
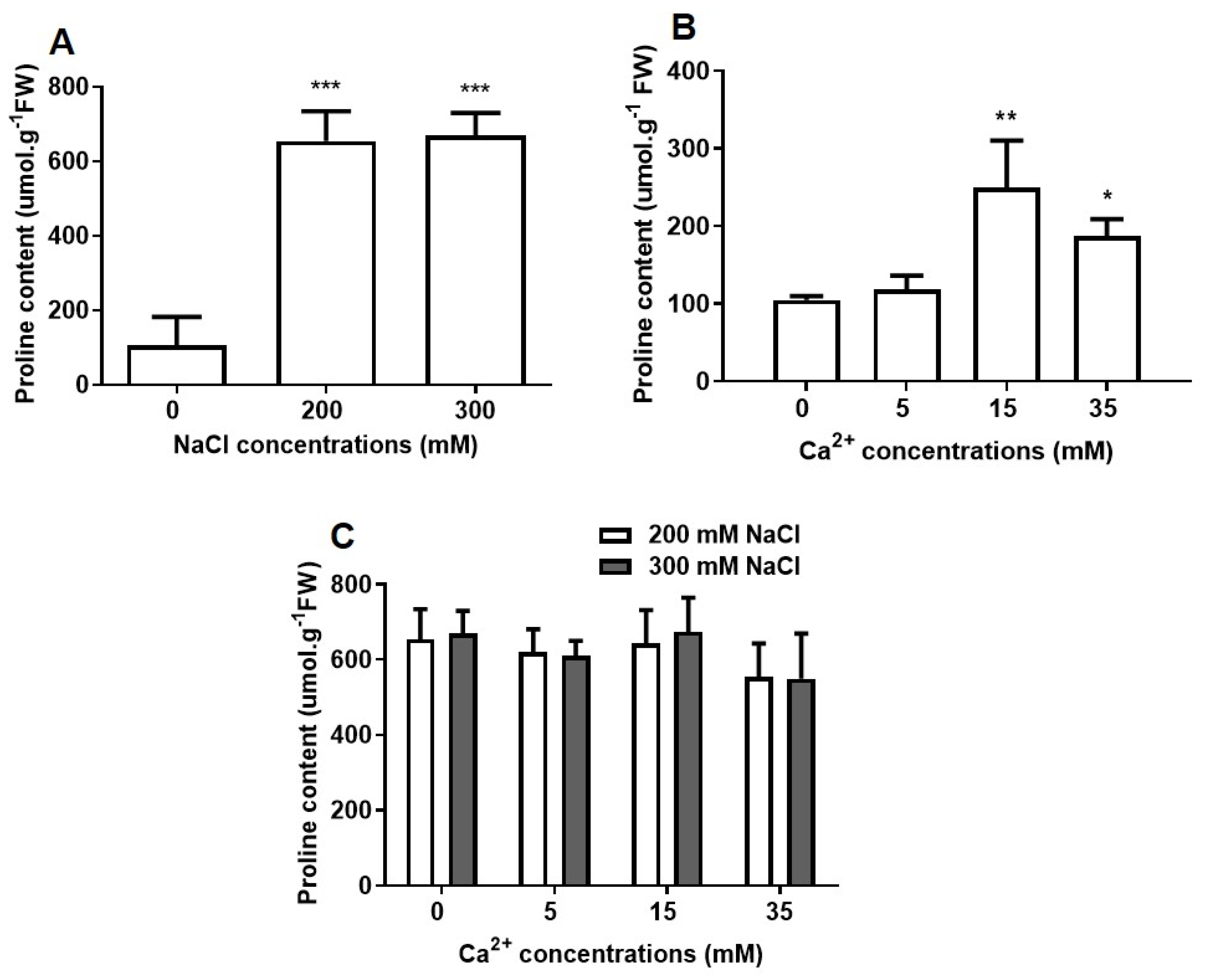
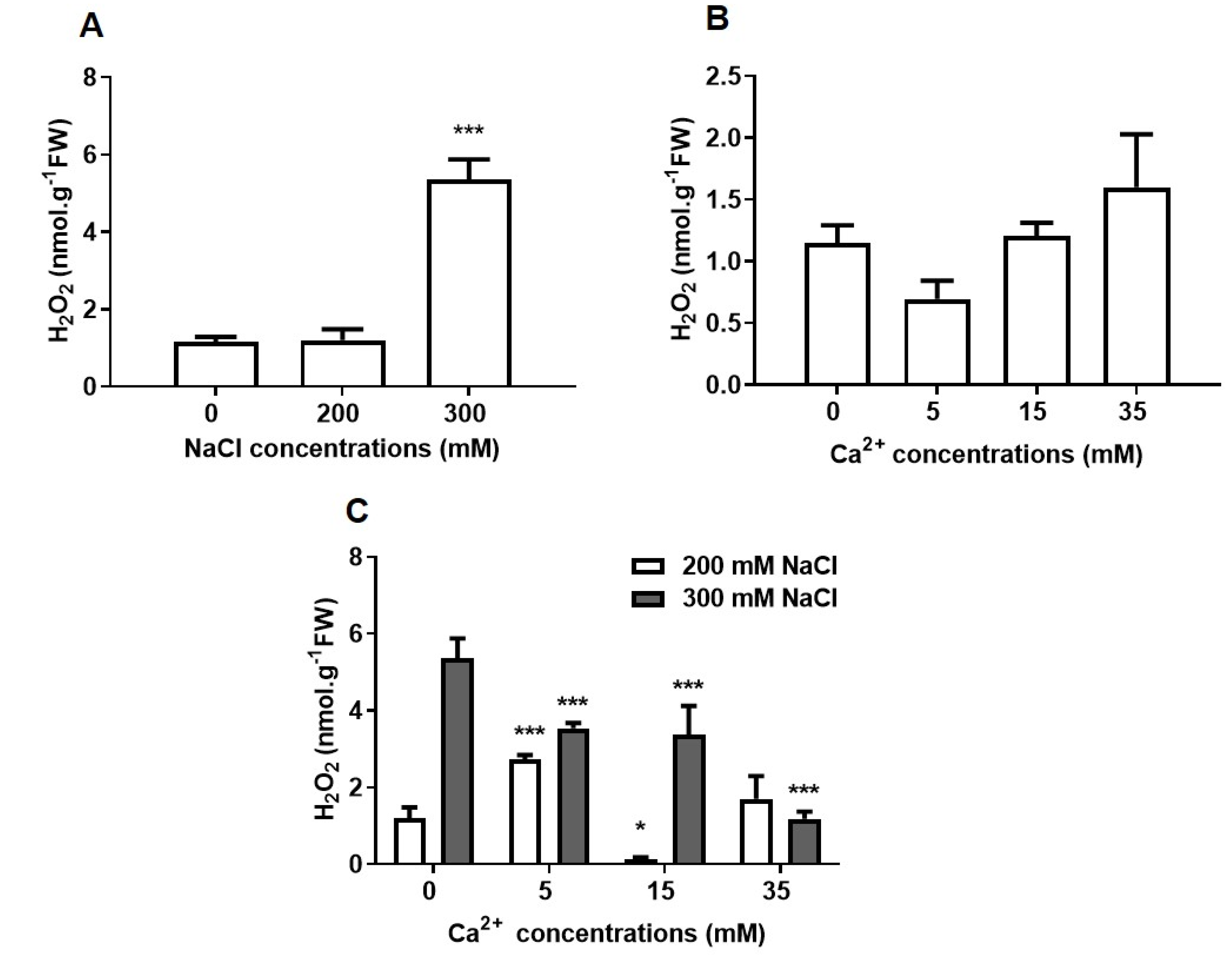
2.2. Biochemical Analysis of the Effect of NaCl and Ca2+ on Seed Germination
2.2.1. Proline Accumulation
2.2.2. Oxidative Stress
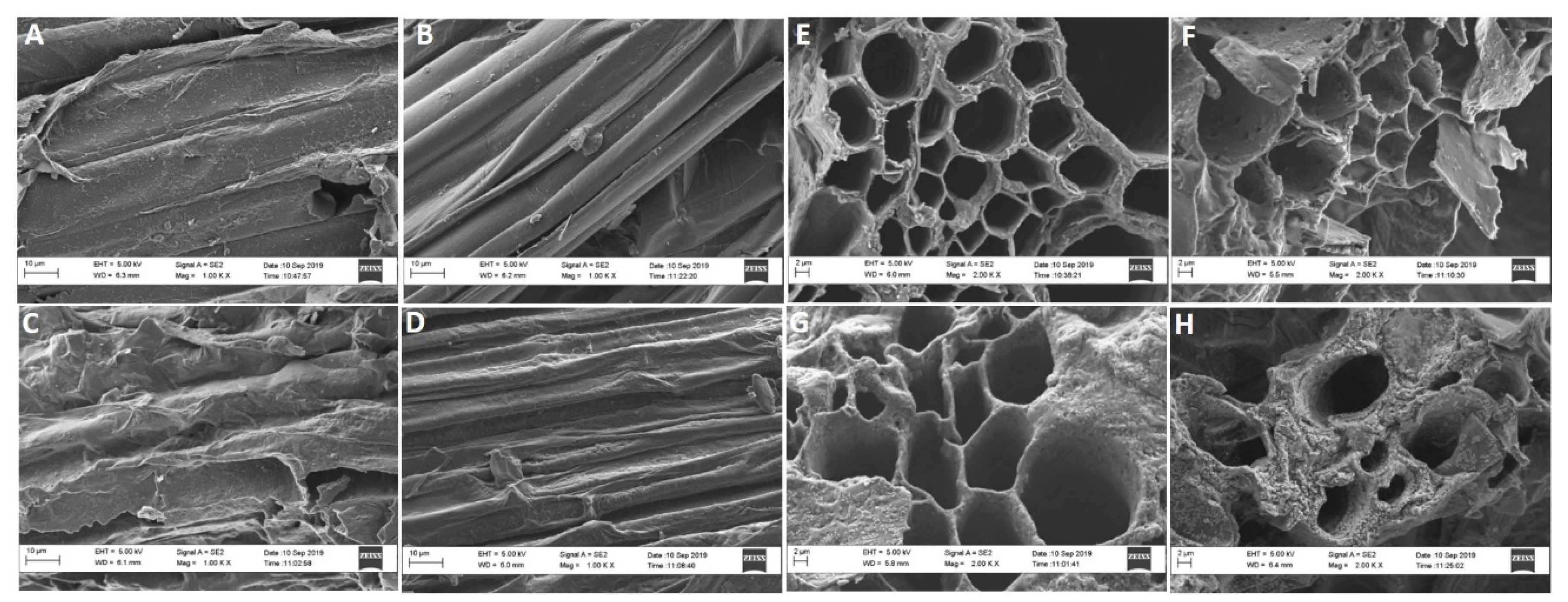
2.2.3. Membrane Structure
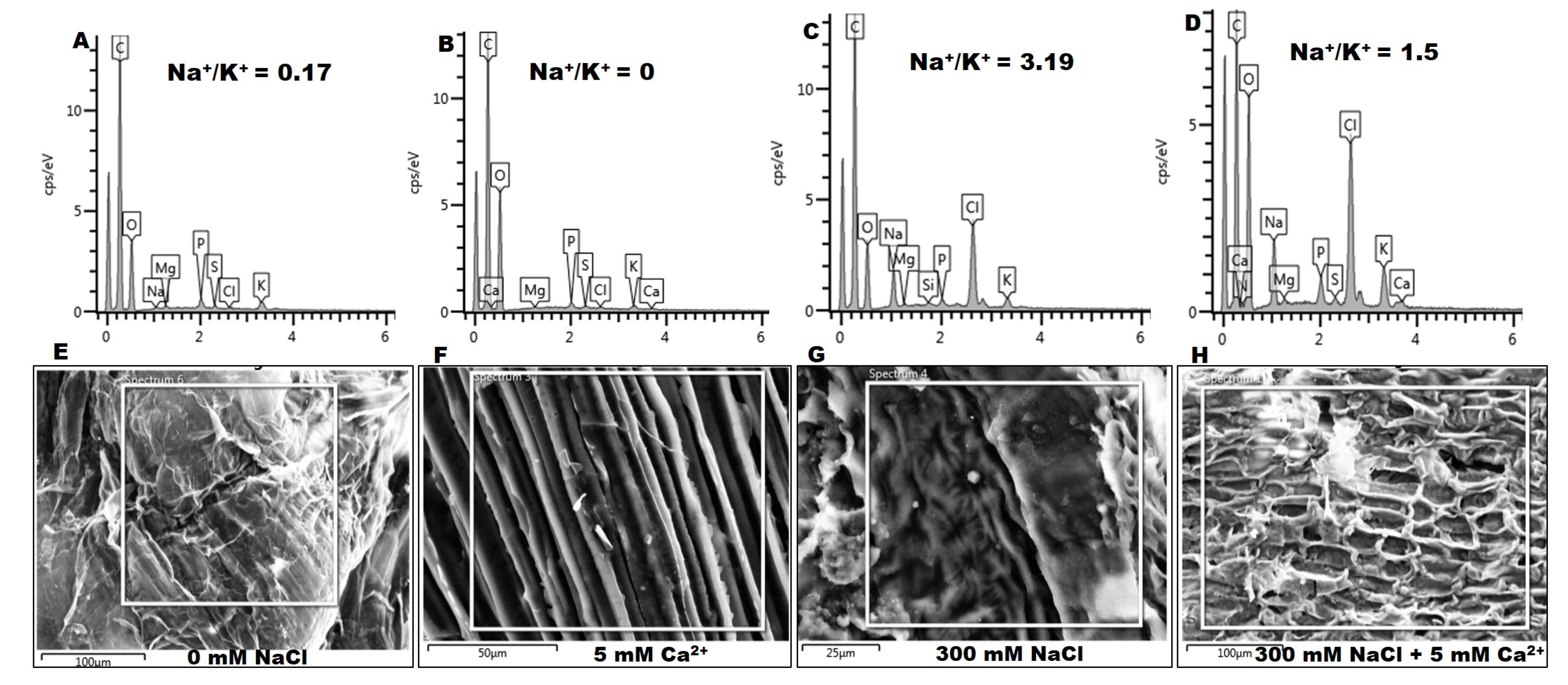
2.2.4. Na+ and K+ Content
2.3. Transcriptional Analysis of the Effect of NaCl and Ca2+ on Germination
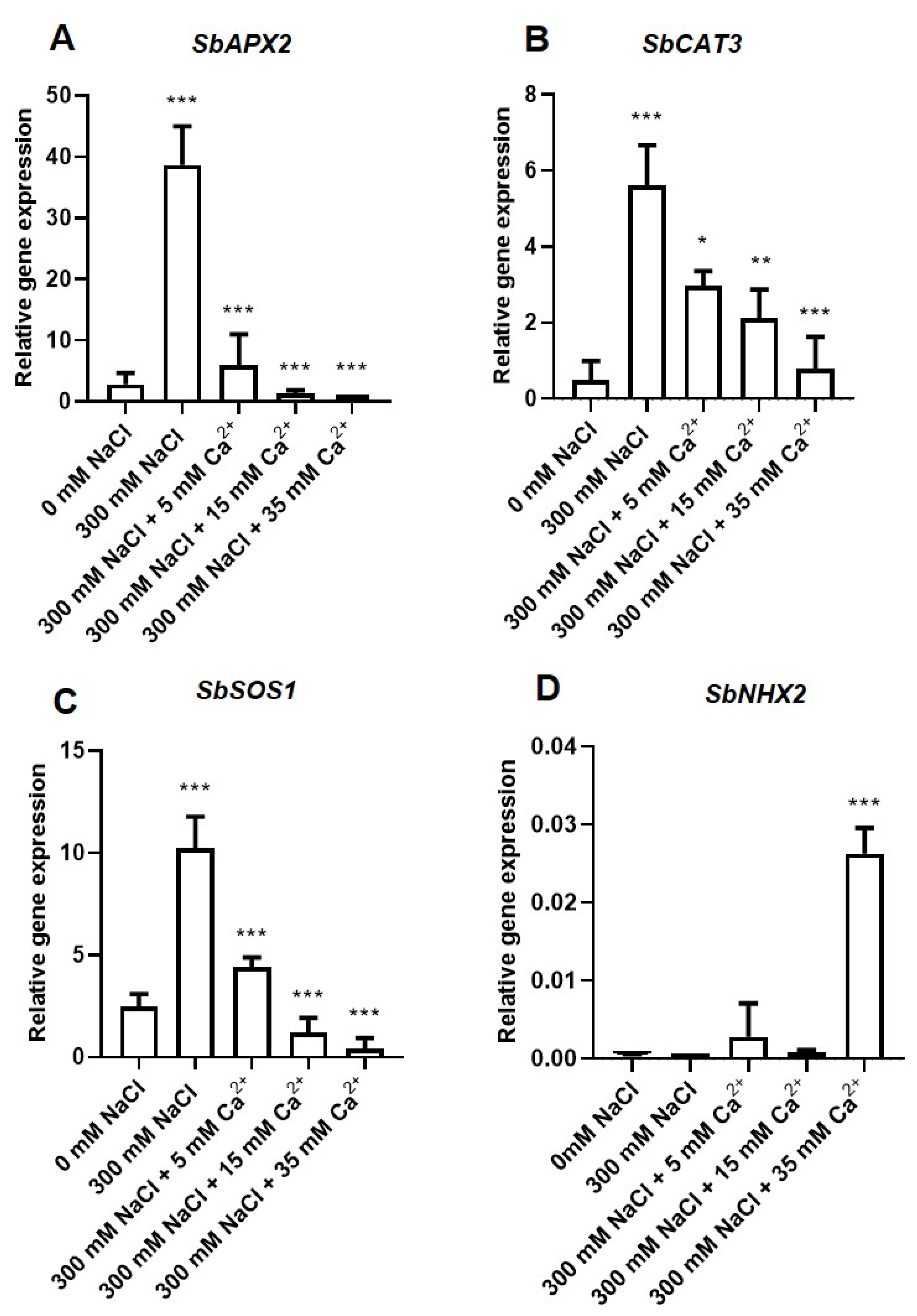
2.3.1. Transcription Analysis of the Antioxidant Genes
2.3.2. Transcription Analysis of the Na+/H+ (NHX) Antiporter Coding Genes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Seed Preparation and Growth Conditions
5.2. Physiological analysis
5.2.1. Germination Assays
5.2.2. Growth Attributes
5.3. Biochemical Analysis
5.3.1. Proline Content
5.3.2. Hydrogen Peroxide Content
5.3.3. Anatomical and Element Analysis
5.4. Quantitative Real-Time PCR
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gan, V.; Stobbe, E.H.; Njue, C. Evaluation of selected nonlinear regression models in quantifying seedling emergence rate of spring wheat. Crop Sci. 1996, 36, 165–168. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium and salt-stress signaling in plants: Shedding light on SOS pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Houle, G.; Morel, L.; Reynolds, C.E.; Siegel, J. The Effect of salinity on different developmental stages of an endemic annual plant, Aster laurentianus (Asteraceae). Am. J. Bot. 2001, 88, 62–67. [Google Scholar] [CrossRef]
- Serrano, R.; Mulet, J.M.; Rios, G.; Marquez, J.A.; De Larrinoa, I.F.; Leube, M.P.; Mendizabal, I.; Pascual-Ahuir, A.; Proft, M.; Ros, R.; et al. A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot. 1999, 50, 1023–1036. [Google Scholar] [CrossRef]
- Yermiyahu, U.; Nir, S.; Ben-Hayyim, G.; Kafkafi, U.; Kinraide, T.B. Root elongation in saline solution related to calcium binding to root cell plasma membranes. Plant Soil 1997, 191, 67–76. [Google Scholar] [CrossRef]
- Wu, G.Q.; Wang, S.M. Calcium regulates K+/Na+ homeostasis in rice (Oryza sativa L.) under saline conditions. Plant Soil Environ. 2012, 58, 121–127. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 1–18. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Surender, P.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kishor, P.B.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ishitani, M.; Kim, C.; Zhu, J.K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6896–6901. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- OlÍas, R.; Eljakaoui, Z.; Li, J.; De Morales, P.A.; Marin-Manzano, M.C.; Pardo, M.; Belver, A. The plasma membrane Na+/H+ antiporter SOS1 is essential for salt tolerance in tomato and affects the partitioning of Na+ between plant organs. Plant Cell Environ. 2009, 32, 904–916. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Rengel, Z. The role of calcium in salt toxicity. Plant Cell Environ. 1992, 15, 625–632. [Google Scholar] [CrossRef]
- Liu, B.; Luo, C.; Li, X.; Gray, L.; Zhang, F.; Liu, M.; Ju, J.; Lei, B. Research on the threshold of aluminum toxicity and the alleviation effects of exogenous calcium, phosphorus and nitrogen on the growth of Chinese fir seedlings under aluminum stress. Commun. Soil Sci. Plant Anal. 2014, 45, 126–139. [Google Scholar] [CrossRef]
- Demidchik, V.; Davenport, R.J.; Tester, M. Non-selective cation channels in plants. Annu. Rev. Plant Biol. 2002, 53, 67–107. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Shabala, L.; Van Volkenburgh, E.; Newman, I. Effect of divalent cations on ion fluxes and leaf photochemistry in salinized barley leaves. J. Exp. Bot. 2005, 56, 1369–1378. [Google Scholar] [CrossRef]
- Salahshoor, F.; Kazemi, F. Effect of calcium on reducing salt stress in seed germination and early growth stage of Festuca ovina L. Plant Soil Environ. 2016, 62, 460–466. [Google Scholar] [CrossRef]
- Fan, H.; Ding, L.; Xu, Y.; Du, C. Seed germination, seedling growth and antioxidant system responses in cucumber exposed to Ca (NO3)2. Hort. Environ. Biotechnol. 2017, 58, 548–559. [Google Scholar] [CrossRef]
- Boursier, P.; Läuchli, A. Growth responses and mineral nutrient relations of salt-stressed sorghum. Crop Sci. 1990, 30, 1226–1233. [Google Scholar] [CrossRef]
- Krishnamurthy, L.; Serraj, R.; Hash, C.T.; Dakheel, A.J.; Reddy, B.V.S. Screening sorghum genotypes for salinity tolerant biomass production. Euphytica 2007, 156, 15–24. [Google Scholar] [CrossRef]
- Alves Da Costa, P.H.; De Azevedo Neto, A.D.; Bezerra, M.A.; Prisco, J.T.; Gomes-Filho, E. Antioxidant-enzymatic system of two sorghum genotypes differing in salt tolerance. Braz. J. Plant Physiol. 2005, 17, 353–361. [Google Scholar] [CrossRef]
- El Omari, R.; Ben, R.; Mrid, R.; Chibi, F.; Nhiri, M. Involvement of phosphoenolpyruvate carboxylase and antioxidants enzymes in nitrogen nutrition tolerance in Sorghum bicolor plants. Russ. J. Plant Physiol. 2016, 63, 719–726. [Google Scholar] [CrossRef]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar]
- Almansouri, M.; Kinet, J.M.; Lutts, S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil 2001, 231, 243–254. [Google Scholar] [CrossRef]
- Misra, N.; Dwivedi, U.N. Genotypic difference in salinity tolerance of green gram cultivars. Plant Sci. 2004, 166, 1135–1142. [Google Scholar] [CrossRef]
- Mohammadi, G.R. The influence of NaCl priming on seed germination and seedling growth of canola (Brassica napus L.) under salinity conditions. Am. Eurasian J. Agric. Environ. Sci. 2009, 5, 696–700. [Google Scholar]
- Duan, D.Y.; Li, W.R.; Liu, X.J.; Ouyang, H.; An, P. Seed germination and seedling growth of Suaeda salsa under salt stress. Ann. Bot. Fennici. 2007, 44, 161–169. [Google Scholar]
- Qu, X.X.; Huang, Z.Y.; Baskin, J.M.; Baskin, C.C. Effect of temperature, light and salinity on seed germination and radicle growth of the geographically widespread halophyte shrub Halocnemum strobilaceum. Ann. Bot. 2008, 101, 293–299. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R. Effects of salt stress levels on five maize (Zea mays L.) cultivars at germination stage. Afr. J. Biotechnol. 2011, 10, 12909–12915. [Google Scholar]
- Khan, H.A.; Ayub, C.M.; Pervez, M.A.; Bilal, R.M.; Shahid, M.A.; Ziaf, K. Effect of seed priming with NaCl on salinity tolerance of hot pepper (Capsicum annuum L.) at seedling stage. Soil Environ. 2009, 28, 81–87. [Google Scholar]
- Pandey, M.; Penna, S. Time course of physiological, biochemical and gene expression changes under short-term salt stress in Brassica juncea L. Crop J. 2017, 219–230. [Google Scholar] [CrossRef]
- Zehra, A.; Gul, B.; Ansari, R.; Khan, M.A. Role of calcium in alleviating effect of salinity on germination of Phragmites karka seeds. S. Afr. J. Bot. 2012, 78, 122–128. [Google Scholar] [CrossRef]
- Sewelam, N.; Dowidar, S.; Abo-Kassem, E.A.; Sobhy, S. Study of the interactive effects of calcium and abscisic acid on drought stressed Triticum aestivum seedlings. Egypt. J. Bot. 2017, 57, 215–232. [Google Scholar] [CrossRef][Green Version]
- Faiza, S.; Bilquees, G.U.L.; Wei-qiang, L.I.; Xiao-jing, L.I.U.; Ajmal, K.M. Effect of calcium and light on the germination of Urochondra setulosa under different salts. J. Zhejiang Univ. Sci. B 2007, 8, 20–26. [Google Scholar]
- Bonilla, I.; Bolaños, L. Boron and calcium increase Pisum sativum seed germination and seedling development under salt stress. Plant Soil 2004, 267, 97–107. [Google Scholar] [CrossRef]
- Ahmad, P.; Allah, E.F.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Siddique, K.H.M. Exogenous application of calcium to 24-epibrassinosteroid pretreated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci. Rep. 2018, 8, 13515. [Google Scholar] [CrossRef]
- Tanveer, K.; Gilani, S.; Hussain, Z.; Ishaq, R.; Adeel, M.; Ilyas, M. Effect of salt stress on tomato plant and the role of calcium. J. Plant Nutr. 2020, 43, 28–35. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Tariq, S.; Saleem, M.; Khan, M.A.; Hassan, S.W.U.; Sadef, Y. Calcium and zinc mediated growth and physio-biochemical changes in mungbean grown under saline conditions. J. Plant Nutr. 2020, 43, 512–525. [Google Scholar] [CrossRef]
- Aroca, R.; Porcel, R.; Ruiz-Lozano, J.M. Regulation of root water uptake under abiotic stress conditions. J. Exp. Bot. 2012, 63, 43–57. [Google Scholar] [CrossRef]
- Cramer, G.R. Sodium-calcium interactions under salinity stress. In Salinity: Environment-Plants-Molecules, 2nd ed.; Läuchli, A., Lüttge, U., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 205–227. [Google Scholar]
- Takemura, T.; Hanagata, N.; Sugihara, K.; Baba, S.; Karube, I.; Dubinsky, Z. Physiological and biochemical responses to salt stress in the mangrove, Bruguiera gymnorrhiza. Aquat. Bot. 2000, 68, 15–28. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K. Interactive effects of salt and alkali stresses on seed germination, germination recovery, and seedling growth of a halophyte Spartina alterniflora (Poaceae). S. Afr. J. Bot. 2010, 76, 380–387. [Google Scholar] [CrossRef]
- Zaman, S.; Saleh, M.A. Mutagenic effect of ethylene glycol on somatic cells of wheat (Triticum Aestivum L.). Life Earth Sci. 2005, 1, 43–49. [Google Scholar]
- El-Haddad, E.S.H.M.; O’Leary, J.W. Effect of salinity and K/Na ratio of irrigation water on growth and solute content of Atriplex amnicola and Sorghum bicolor. Irrig. Sci. 1994, 14, 127–133. [Google Scholar] [CrossRef]
- Heidari, M. Antioxidant activity and osmolyte concentration of sorghum (Sorghum bicolor) Asiana and wheat (Triticum aestivum) genotypes under salinity stress. Asian J. Plant Sci. 2009, 8, 240–244. [Google Scholar] [CrossRef]
- Watanabe, S.; Kojima, K.; Ide, Y.; Sasaki, S. Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell. Tiss. Org. 2000, 63, 199–206. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 215–223. [Google Scholar] [CrossRef]
- Verslues, P.E.; Sharma, S. Proline metabolism and its implications for plant-environment interaction. Arab. Book Am. Soc. Plant Biol. 2010, 8, 140. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Cha-um, S.; Singh, H.P.; Samphumphuang, T.; Kirdmanee, C. Calcium-alleviated salt tolerance in indica rice (Oryza sativa L. spp. indica): Physiological and morphological changes. Aust. J. Crop. Sci. 2012, 6, 176–182. [Google Scholar]
- Tahjib-Ul-Arif, M.; Roy, P.R.; Sohag, A.M.M.; Afrin, S.; Rady, M.M.; Hossain, M.A. Exogenous calcium supplementation improves salinity tolerance in BRRI Dhan28; a salt-susceptible high-yielding Oryza Sativa cultivar. J. Crop Sci. Biotechnol. 2018, 21, 383–394. [Google Scholar] [CrossRef]
- Morgan, S.H.; Maity, P.J.; Geilfus, C.M.; Lindberg, S.; Muhling, K.H. Leaf ion homeostasis and plasma membrane H+-ATPase activity in Vicia faba change after extra calcium and potassium supply under salinity. Plant Physiol. Biochem. 2014, 82, 244–253. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutase—mentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Allen, R.D. Overexpression of an Arabidopsis peroxisomal ascorbate peroxidase gene in tobacco increases protection against oxidative stress. Plant Cell Physiol. 1999, 40, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Filiz, E.; Ozyigit, I.I.; Saracoglu, I.A.; Uras, M.E.; Sen, U.; Yalcin, B. Abiotic stress-induced regulation of antioxidant genes in different Arabidopsis ecotypes: Microarray data evaluation. Biotechnol. Biotechnol. Equip. 2018, 33, 1–16. [Google Scholar] [CrossRef]
- Rossatto, T.; Do Amaral, M.N.; Benitez, L.C.; Vighi, I.L.; Braga, E.J.B.; Junior, A.M.D.M.; Maia, M.A.C.; Pinto, L.D.S. Gene expression and activity of antioxidant enzymes in rice plants, cv. BRS AG, under saline stress. Physiol. Mol. Biol. Plants 2017, 23, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.C.; Becana, M.; Sato, S.; James, E.K.; Tabata, S.; Spaink, H.P. Characterization of genomic clones and expression analysis of the three types of superoxide dismutases during nodule development in Lotus japonicus. Mol. Plant Microbe Interact. 2007, 20, 262–275. [Google Scholar] [CrossRef]
- Soleimani, Z.; Afshar, A.S.; Nematpour, F.S. Responses of antioxidant gene and enzymes to salinity stress in the Cuminum cyminum L. Russ. J. Plant Physiol. 2017, 64, 361–367. [Google Scholar] [CrossRef]
- Xu, S.; Sa, Z.S.; Cao, Z.Y.; Xuan, W.; Huang, B.K.; Ling, T.F.; Hu, Q.Y.; Shen, W.B. Carbon monoxide alleviates wheat seed germination inhibition and counteracts lipid peroxidation mediated by salinity. J. Integr. Plant Biol. 2006, 48, 1168–1176. [Google Scholar] [CrossRef]
- Jabeen, N.; Ahmad, R. The activity of antioxidant enzymes in response to salt stress in safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) seedlings raised from seed treated with chitosan. J. Sci. Food Agric. 2013, 93, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Moshaei, M.R.; Nematzadeh, G.A.; Askari, H.; Nejad, A.S.M.; Pakdin, A. Quantitative gene expression analysis of some sodium ion transporters under salinity stress in Aeluropus littoralis. Saudi J. Biol. Sci. 2014, 21, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Purty, R.S.; Sharma, M.P.; Singla-Pareek, S.L.; Pareek, A. Physiological responses among Brassica species under salinity stress show strong correlation with transcript abundance for SOS pathway-related genes. J. Plant Physiol. 2009, 166, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Sairam, R.K.; Bhattacharya, R.C. Differential expression of salt overly sensitive pathway genes determines salinity stress tolerance in Brassica genotypes. Plant Physiol. Biochem. 2012, 51, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Sathee, L.; Sairam, R.K.; Chinnusamy, V.; Jha, S.K. Differential transcript abundance of salt overly sensitive (SOS) pathway genes is a determinant of salinity stress tolerance of wheat. Acta Physiol. Plant 2015, 37, 1–10. [Google Scholar]
- Liu, J.; Zhu, J.K. An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc. Natl. Acad. Sci. USA 1997, 94, 14960–14964. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Flores, P.; Navarro, J.M.; Martínez, V. Effects of Ca2+, K+ and cGMP on Na+ uptake in pepper plants. Plant Sci. 2003, 165, 1043–1049. [Google Scholar] [CrossRef]
- Kumari, P.H.; Kumar, S.A.; Ramesh, K.; Reddy, P.S.; Nagaraju, M.; Prakash, A.B.; Shah, T.; Henderson, A.; Srivastava, R.K.; Rajasheker, G.; et al. Genome-Wide Identification and Analysis of Arabidopsis Sodium Proton Antiporter (NHX) and Human Sodium Proton Exchanger (NHE) Homologs in Sorghum bicolor. Genes (Basel) 2018, 9, 236. [Google Scholar] [CrossRef]
- Ohnishi, M.; Fukada-Tanaka, S.; Hoshino, A.; Takada, J.; Inagaki, Y.; Iida, S. Characterization of a novel Na+/H+ antiporter gene InNHX2 and comparison of InNHX2 with InNHX1, which is responsible for blue flower coloration by increasing the vacuolar pH in the Japanese morning glory. Plant Cell Physiol. 2005, 46, 259–267. [Google Scholar] [CrossRef]
- Hanana, M.; Cagnac, O.; Yamaguchi, T.; Hamdi, S.; Ghorbel, A.; Blumwald, E. A grape berry (Vitis vinifera L.) cation/proton antiporter is associated with berry ripening. Plant Cell Physiol. 2007, 48, 804–911. [Google Scholar] [CrossRef]
- Yokoi, S.; Quintero, F.J.; Cubero, B.; Ruiz, M.T.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002, 30, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, A.; Nakamura, A.; Tagiri, A.; Tanaka, H.; Miyao, A.; Hirochika, H.; Tanaka, Y. Function, Intracellullar Localization and the Importance in Salt Tolerance of a Vascuolar Na+/H+ Antiporter from Rice. Plant Cell Physiol. 2004, 45, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Su, Q.; An, L.J.; Wu, S. Characterization and expression of a vacuolar Na+/H+ antiporter gene from the monocot halophyte Aeluropus littoralis. Plant Physiol. Biochem. 2008, 46, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Joshi, M.; Yadav, N.S.; Jha, B. Cloning and characterization of the Salicornia brachiata Na+/H+ antiporter gene SbNHX1 and its expression by abiotic stress. Mol. Biol. Rep. 2011, 38, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.D.; Perez, P.G.; Deng, Y.W.; Li, Z.Z.; Huang, D.F. Isolation and characterization of a vacuolar Na+/H+ antiporter gene from Cucumis melo L. Afr. J. Biotechnol. 2011, 10, 1752–1759. [Google Scholar]
- Adabnejad, H.; Kavousi, H.R.; Hamidi, H.; Tavassolian, I. Assessment of the vacuolar Na+/H+ antiporter (NHX1) transcriptional changes in Leptochloa fusca L. in response to salt and cadmium stresses. Mol. Biol. Res. Commun. 2015, 4, 133–142. [Google Scholar]
- Mulaudzi-Masuku, T.; Mutepe, R.D.; Mukhoro, O.C.; Faro, A.; Ndimba, B. Identification and characterization of a heat-inducible Hsp70 gene from Sorghum bicolor which confers tolerance to thermal stress. Cell Stress Chaperons 2015, 7, 793–804. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulaudzi, T.; Hendricks, K.; Mabiya, T.; Muthevhuli, M.; Ajayi, R.F.; Mayedwa, N.; Gehring, C.; Iwuoha, E. Calcium Improves Germination and Growth of Sorghum bicolor Seedlings under Salt Stress. Plants 2020, 9, 730. https://doi.org/10.3390/plants9060730
Mulaudzi T, Hendricks K, Mabiya T, Muthevhuli M, Ajayi RF, Mayedwa N, Gehring C, Iwuoha E. Calcium Improves Germination and Growth of Sorghum bicolor Seedlings under Salt Stress. Plants. 2020; 9(6):730. https://doi.org/10.3390/plants9060730
Chicago/Turabian StyleMulaudzi, Takalani, Kaylin Hendricks, Thembeka Mabiya, Mpho Muthevhuli, Rachel Fanelwa Ajayi, Noluthando Mayedwa, Christoph Gehring, and Emmanuel Iwuoha. 2020. "Calcium Improves Germination and Growth of Sorghum bicolor Seedlings under Salt Stress" Plants 9, no. 6: 730. https://doi.org/10.3390/plants9060730
APA StyleMulaudzi, T., Hendricks, K., Mabiya, T., Muthevhuli, M., Ajayi, R. F., Mayedwa, N., Gehring, C., & Iwuoha, E. (2020). Calcium Improves Germination and Growth of Sorghum bicolor Seedlings under Salt Stress. Plants, 9(6), 730. https://doi.org/10.3390/plants9060730





