Silicon Alters Leaf Surface Morphology and Suppresses Insect Herbivory in a Model Grass Species
Abstract
1. Introduction
2. Results
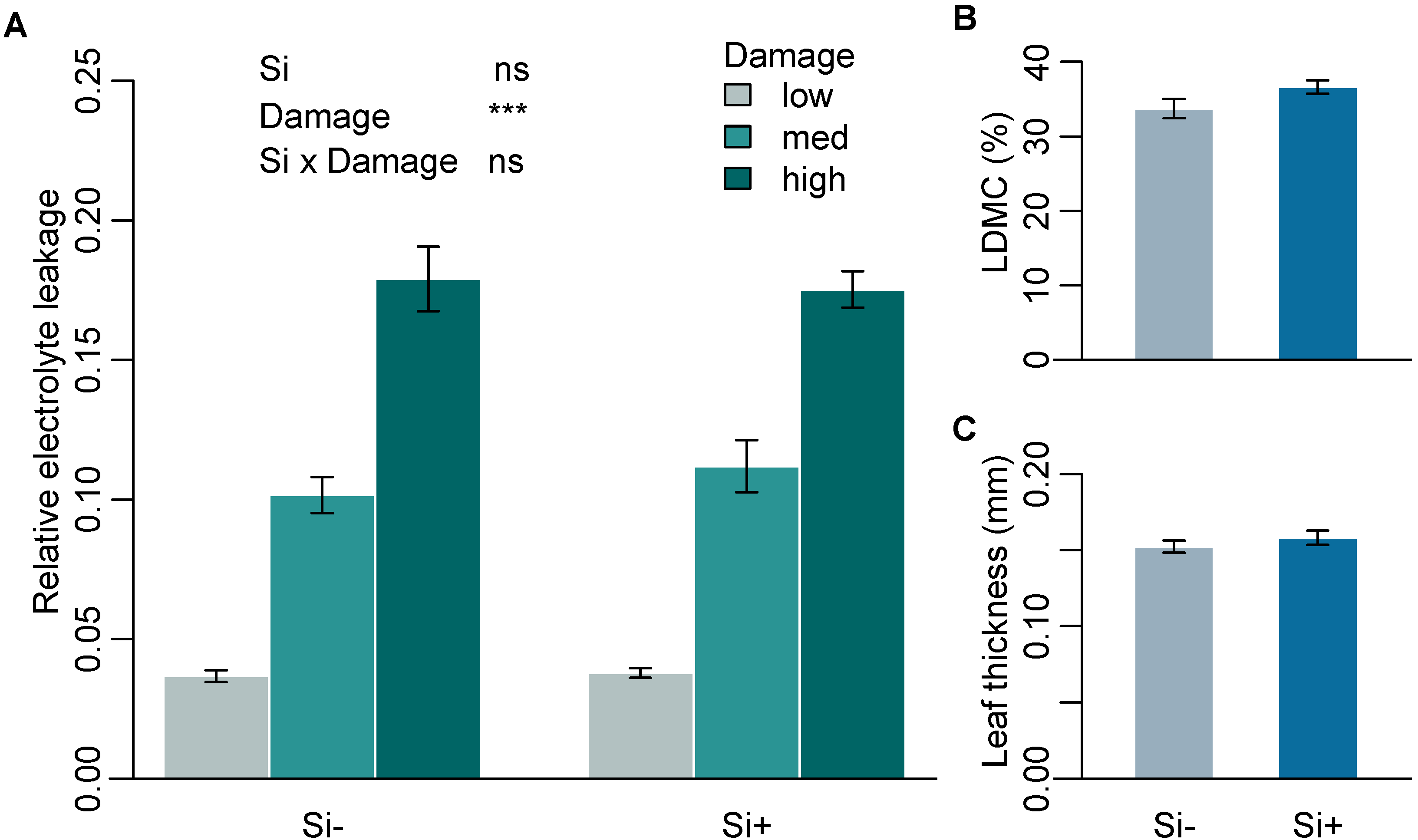
2.1. Effect of Si on Leaf Mechanical Traits
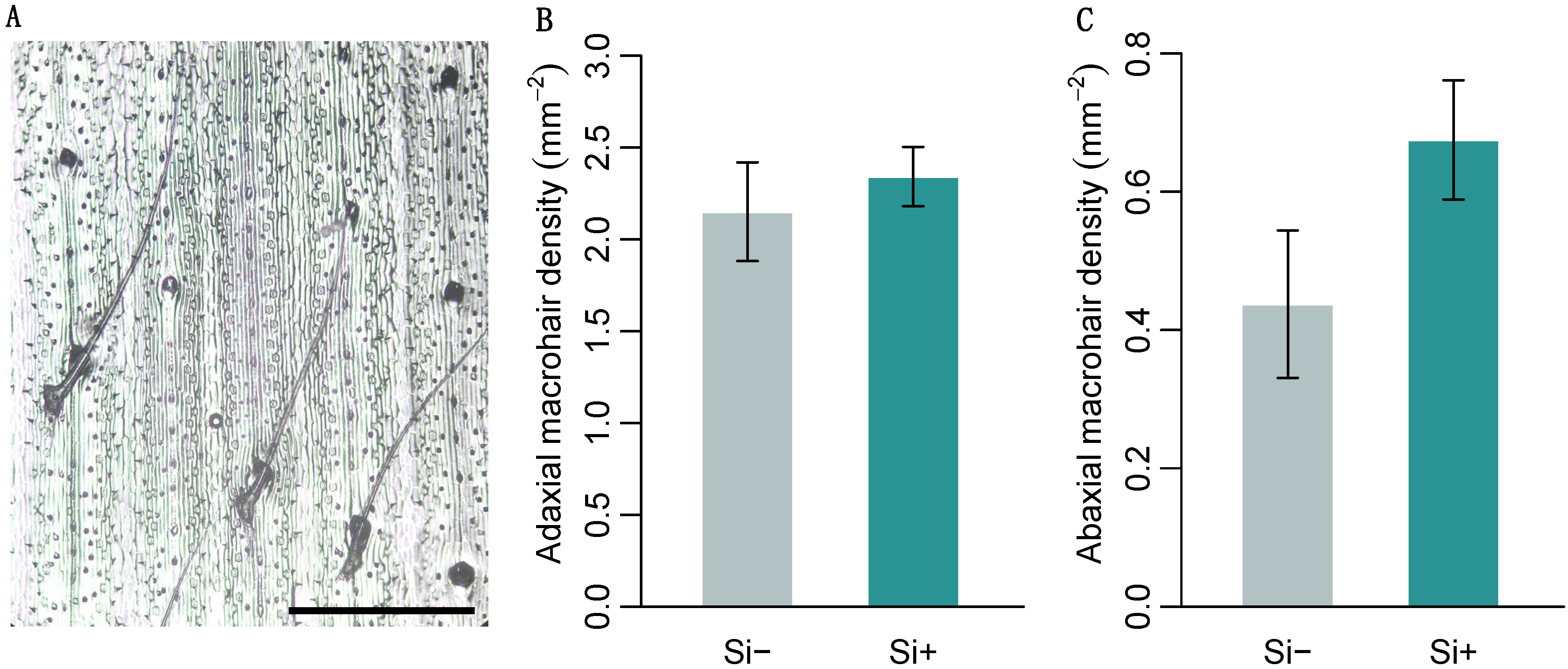
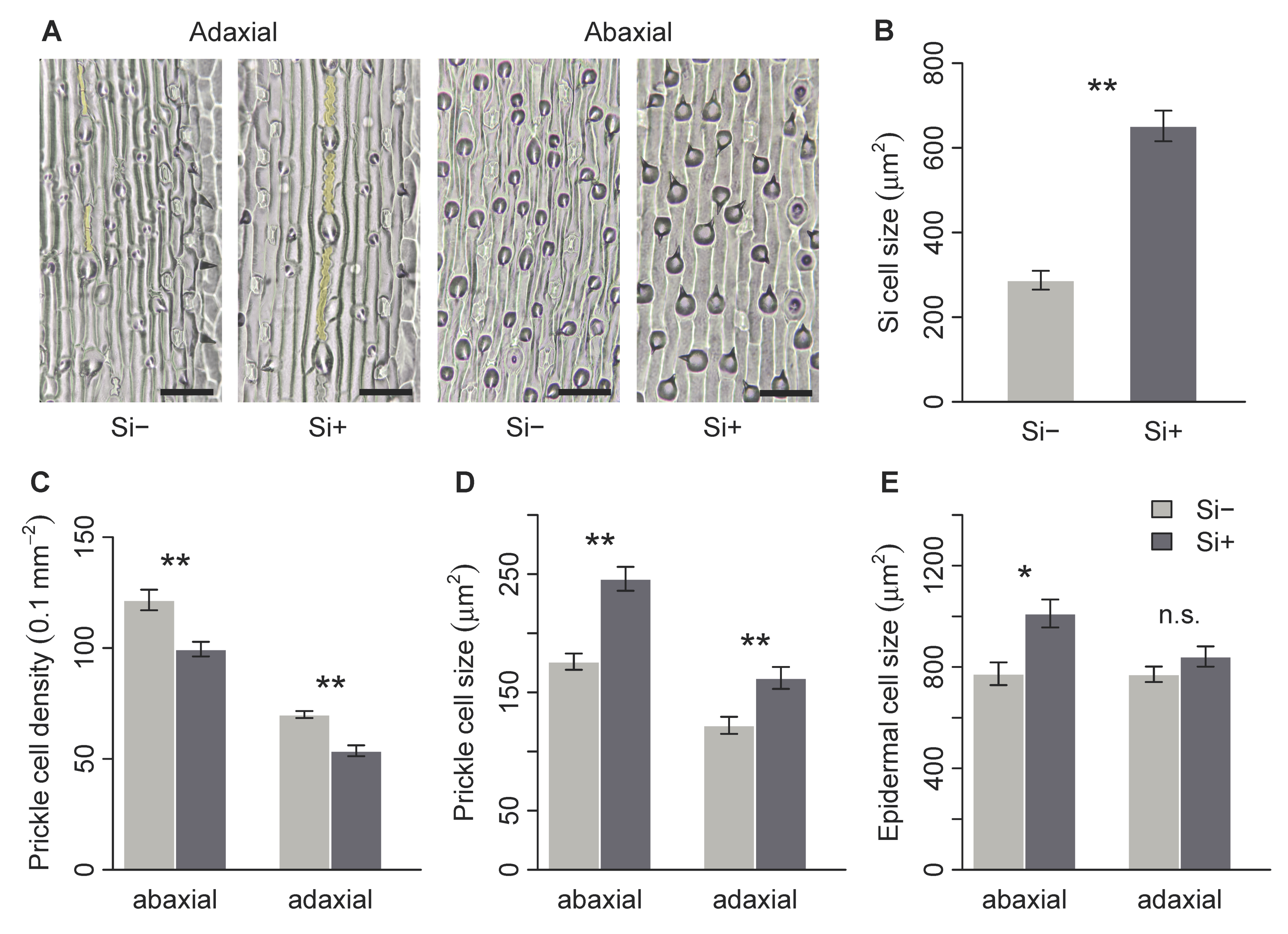
2.2. Effect of Si on Leaf Surface Morphology
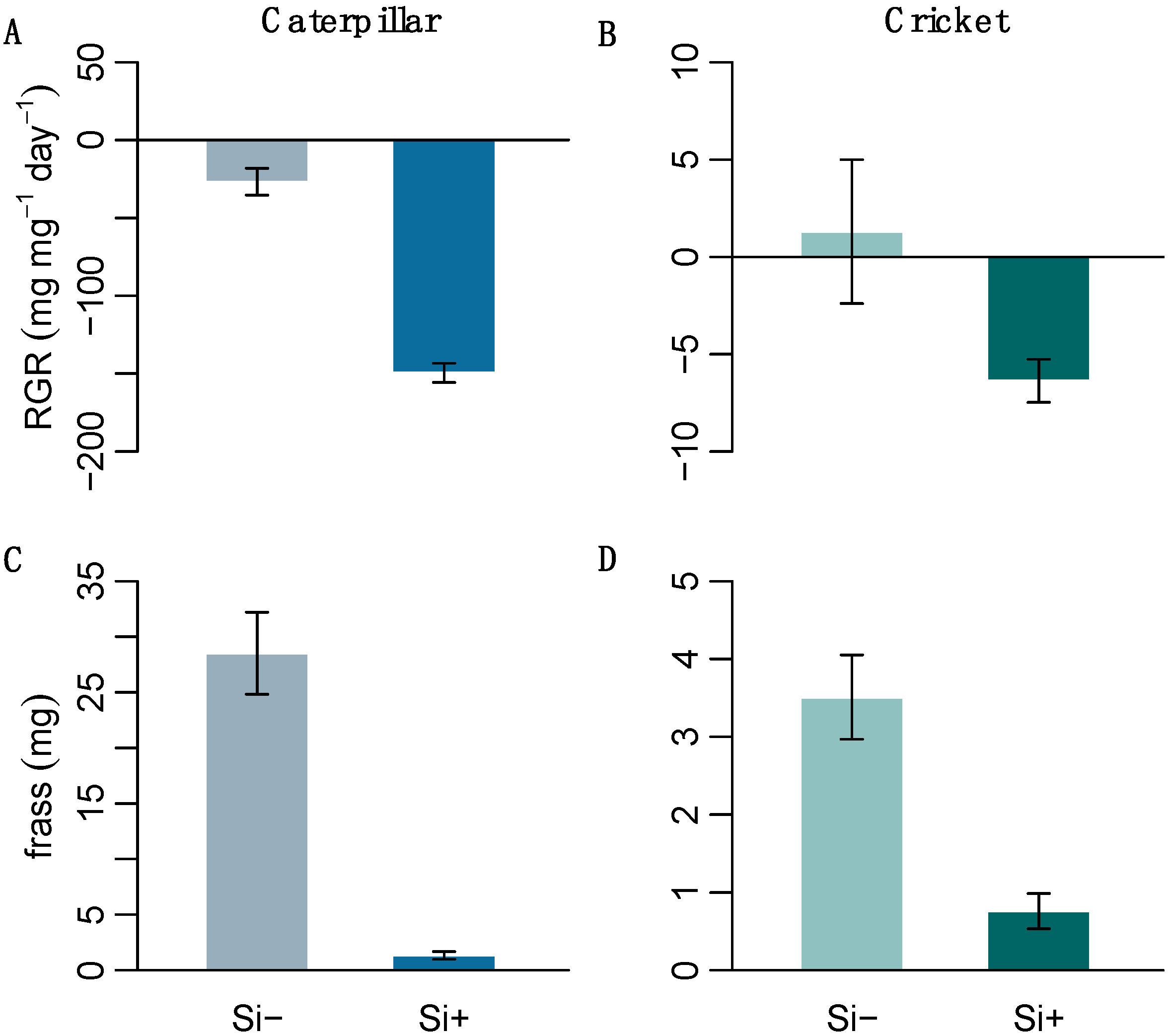
2.3. Effect of Si on Herbivore Performance
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Growth Conditions
5.2. Experimental Design
5.3. Leaf Mechanical Traits
5.4. Leaf Surface Morphology
5.5. Silicon
5.6. Insect Performance
5.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Debona, D.; Rodrigues, F.A.; Datnoff, L.E. Silicon’s role in abiotic and biotic plant stresses. Annu. Rev. Phytopathol. 2017, 55, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Sun, W.; Zhu, Y.-G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Fauteux, F.; Chain, F.; Belzile, F.; Menzies, J.G.; Bélanger, R.R. The protective role of silicon in the Arabidopsis–powdery mildew pathosystem. Proc. Natl. Acad. Sci. USA 2006, 103, 17554–17559. [Google Scholar] [CrossRef]
- Johnson, S.N.; Ryalls, J.M.W.; Barton, C.V.M.; Tjoelker, M.G.; Wright, I.J.; Moore, B.D. Climate warming and plant biomechanical defences: Silicon addition contributes to herbivore suppression in a pasture grass. Funct. Ecol. 2019, 33, 587–596. [Google Scholar] [CrossRef]
- Ye, M.; Song, Y.; Long, J.; Wang, R.; Baerson, S.R.; Pan, Z.; Zhu-Salzman, K.; Xie, J.; Cai, K.; Luo, S.; et al. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [CrossRef]
- Kumar, S.; Soukup, M.; Elbaum, R. Silicification in grasses: Variation between different cell types. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Hunt, J.W.; Dean, A.P.; Webster, R.E.; Johnson, G.N.; Ennos, A.R. A novel mechanism by which silica defends grasses against herbivory. Ann. Bot. 2008, 102, 653–656. [Google Scholar] [CrossRef]
- Massey, F.P.; Hartley, S.E. Physical defences wear you down: Progressive and irreversible impacts of silica on insect herbivores. J. Anim. Ecol. 2009, 78, 281–291. [Google Scholar] [CrossRef]
- Hartley, S.E.; Fitt, R.N.; McLarnon, E.; Wade, R.N. Defending the leaf surface: Intra- and inter-specific differences in silicon deposition in grasses in response to damage and silicon supply. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.H.; Rashid, I.; Hussain, B.; Reshi, Z.A.; Assad, R.; Sofi, I.A. Silicon supplementation of rescuegrass reduces herbivory by a grasshopper. Front. Plant Sci. 2019, 10, 671. [Google Scholar] [CrossRef]
- Głazowska, S.; Murozuka, E.; Persson, D.P.; Castro, P.H.; Schjoerring, J.K. Silicon affects seed development and leaf macrohair formation in Brachypodium distachyon. Physiol. Plant. 2018, 163, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Kariyat, R.R.; Smith, J.D.; Stephenson, A.G.; De Moraes, C.M.; Mescher, M.C. Non-glandular trichomes of Solanum carolinense deter feeding by Manduca sexta caterpillars and cause damage to the gut peritrophic matrix. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162323. [Google Scholar] [CrossRef] [PubMed]
- Andama, J.B.; Mujiono, K.; Hojo, Y.; Shinya, T.; Galis, I. Non-glandular silicified trichomes are essential for rice defense against chewing herbivores. Plant Cell Environ. 2020, pce.13775. [Google Scholar] [CrossRef]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2018, 221, 67–85. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Khan, A.L.; Waqas, M.; Jeong, H.-J.; Kim, D.-H.; Shin, J.S.; Kim, J.-G.; Yeon, M.-H.; Lee, I.-J. Regulation of jasmonic acid biosynthesis by silicon application during physical injury to Oryza sativa L. J. Plant Res. 2014, 127, 525–532. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Zhang, W.; Zhang, F. Do lignification and silicification of the cell wall precede silicon deposition in the silica cell of the rice (Oryza sativa L.) leaf epidermis? Plant Soil 2013, 372, 137–149. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, L.; Higgs, D. Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J. Plant Nutr. 2006, 29, 1469–1480. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Johnson, S.N.; Reynolds, O.L.; Gurr, G.M.; Esveld, J.L.; Moore, B.D.; Tory, G.J.; Gherlenda, A.N. When resistance is futile, tolerate instead: Silicon promotes plant compensatory growth when attacked by above- and belowground herbivores. Biol. Lett. 2019, 15, 20190361. [Google Scholar] [CrossRef] [PubMed]
- Massey, F.P.; Ennos, A.R.; Hartley, S.E. Silica in grasses as a defence against insect herbivores: Contrasting effects on folivores and a phloem feeder. J. Anim. Ecol. 2006, 75, 595–603. [Google Scholar] [CrossRef]
- Brkljacic, J.; Grotewold, E.; Scholl, R.; Mockler, T.; Garvin, D.F.; Vain, P.; Brutnell, T.; Sibout, R.; Bevan, M.; Budak, H.; et al. Brachypodium as a model for the grasses: Today and the future. Plant Physiol. 2011, 157, 3–13. [Google Scholar] [CrossRef]
- Vogel, J. Genetics and genomics of Brachypodium; Springer Science+Business Media: New York, NY, USA, 2016; ISBN 978-3-319-26942-9. [Google Scholar]
- Deshmukh, R.K.; Vivancos, J.; Ramakrishnan, G.; Guérin, V.; Carpentier, G.; Sonah, H.; Labbé, C.; Isenring, P.; Belzile, F.J.; Bélanger, R.R. A precise spacing between the NPA domains of aquaporins is essential for silicon permeability in plants. Plant J. 2015, 83, 489–500. [Google Scholar] [CrossRef]
- Hall, C.R.; Mikhael, M.; Hartley, S.E.; Johnson, S.N. Elevated atmospheric CO2 suppresses jasmonate and silicon-based defences without affecting herbivores. Funct. Ecol. 2020, 34, 993–1002. [Google Scholar] [CrossRef]
- Tay, W.T.; Soria, M.F.; Walsh, T.; Thomazoni, D.; Silvie, P.; Behere, G.T.; Anderson, C.; Downes, S. A brave new world for an old world pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS ONE 2013, 8, e80134. [Google Scholar] [CrossRef]
- Clissold, F.J.; Sanson, G.D.; Read, J. Indigestibility of plant cell wall by the Australian plague locust, Chortoicetes terminifera. Entomol. Exp. Appl. 2004, 112, 159–168. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Isman, M.B. Herbivory in holometabolous and hemimetabolous insects: Contrasts between Orthoptera and Lepidoptera. Experientia 1989, 45, 229–236. [Google Scholar] [CrossRef]
- Caldwell, E.; Read, J.; Sanson, G.D. Which leaf mechanical traits correlate with insect herbivory among feeding guilds? Ann. Bot. 2016, 117, 349–361. [Google Scholar] [CrossRef]
- Hanley, M.E.; Lamont, B.B.; Fairbanks, M.M.; Rafferty, C.M. Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 157–178. [Google Scholar] [CrossRef]
- Vandegeer, R.K.; Zhao, C.; Hall, C.R.; Tissue, D.T.; Hartley, S.E.; Johnson, S.N. Silicon increases stomatal sensitivity and guard cell K+ efflux of tall fescue in response to abscisic acid. Physiol. Plant. under review.
- Elger, A.; Willby, N.J. Leaf dry matter content as an integrative expression of plant palatability: The case of freshwater macrophytes. Funct. Ecol. 2003, 17, 58–65. [Google Scholar] [CrossRef]
- Pakeman, R.J. Leaf dry matter content predicts herbivore productivity, but its functional diversity is positively related to resilience in grasslands. PLoS ONE 2014, 9, e101876. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, N.C.; Valim, J.O.S.; Oliveira, M.G.A.; Campos, W.G. Combined effects of soil silicon and drought stress on host plant chemical and ultrastructural quality for leaf-chewing and sap-sucking insects. J. Agron. Crop Sci. 2020, 206, 187–201. [Google Scholar] [CrossRef]
- Garbuzov, M.; Reidinger, S.; Hartley, S.E. Interactive effects of plant-available soil silicon and herbivory on competition between two grass species. Ann. Bot. 2011, 108, 1355–1363. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Milstein, Y.; Brami, Y.; Elbaum, M.; Elbaum, R. Mechanism of silica deposition in sorghum silica cells. New Phytol. 2017, 213, 791–798. [Google Scholar] [CrossRef]
- Markovich, O.; Kumar, S.; Cohen, D.; Addadi, S.; Fridman, E.; Elbaum, R. Silicification in Leaves of Sorghum Mutant with Low Silicon Accumulation. Silicon 2019, 11, 2385–2391. [Google Scholar] [CrossRef]
- Dabney, C.; Ostergaard, J.; Watkins, E.; Chen, C. A novel method to characterize silica bodies in grasses. Plant Methods 2016, 12, 3. [Google Scholar] [CrossRef]
- Sakai, W.S.; Sanford, W.G. A developmental study of silicification in the abaxial epidermal cells of sugarcane leaf blades using scanning electron microscopy and energy dispersive X-ray analysis. Am. J. Bot. 1984, 71, 1315–1322. [Google Scholar] [CrossRef]
- Jung, H.; Yan, J.; Zhai, Z.; Vatamaniuk, O.K. Gene Functional Analysis Using Protoplast Transient Assays. In Plant Functional Genomics: Methods and Protocols; Alonso, J.M., Stepanova, A.N., Eds.; Springer: New York, NY, USA, 2015; pp. 433–452. ISBN 978-1-4939-2444-8. [Google Scholar]
- Muiruri, E.W.; Barantal, S.; Iason, G.R.; Salminen, J.; Perez-Fernandez, E.; Koricheva, J. Forest diversity effects on insect herbivores: Do leaf traits matter? New Phytol. 2019, 221, 2250–2260. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Yang, H.; Li, Y. Kinetics of relative electrical conductivity and correlation with gas composition in modified atmosphere packaged bayberries (Myrica rubra Siebold and Zuccarini). LWT Food Sci. Technol. 2005, 38, 249–254. [Google Scholar] [CrossRef]
- Slansky, F. Food utilization by insects: Interpretation of observed differences between dry weight and energy efficiencies. Entomol. Exp. Appl. 1985, 39, 47–60. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Torchiano, M. effsize: Efficient Effect Size Computation. 2017. Available online: http://github.com/mtorchiano/effsize/ (accessed on 30 March 2020).
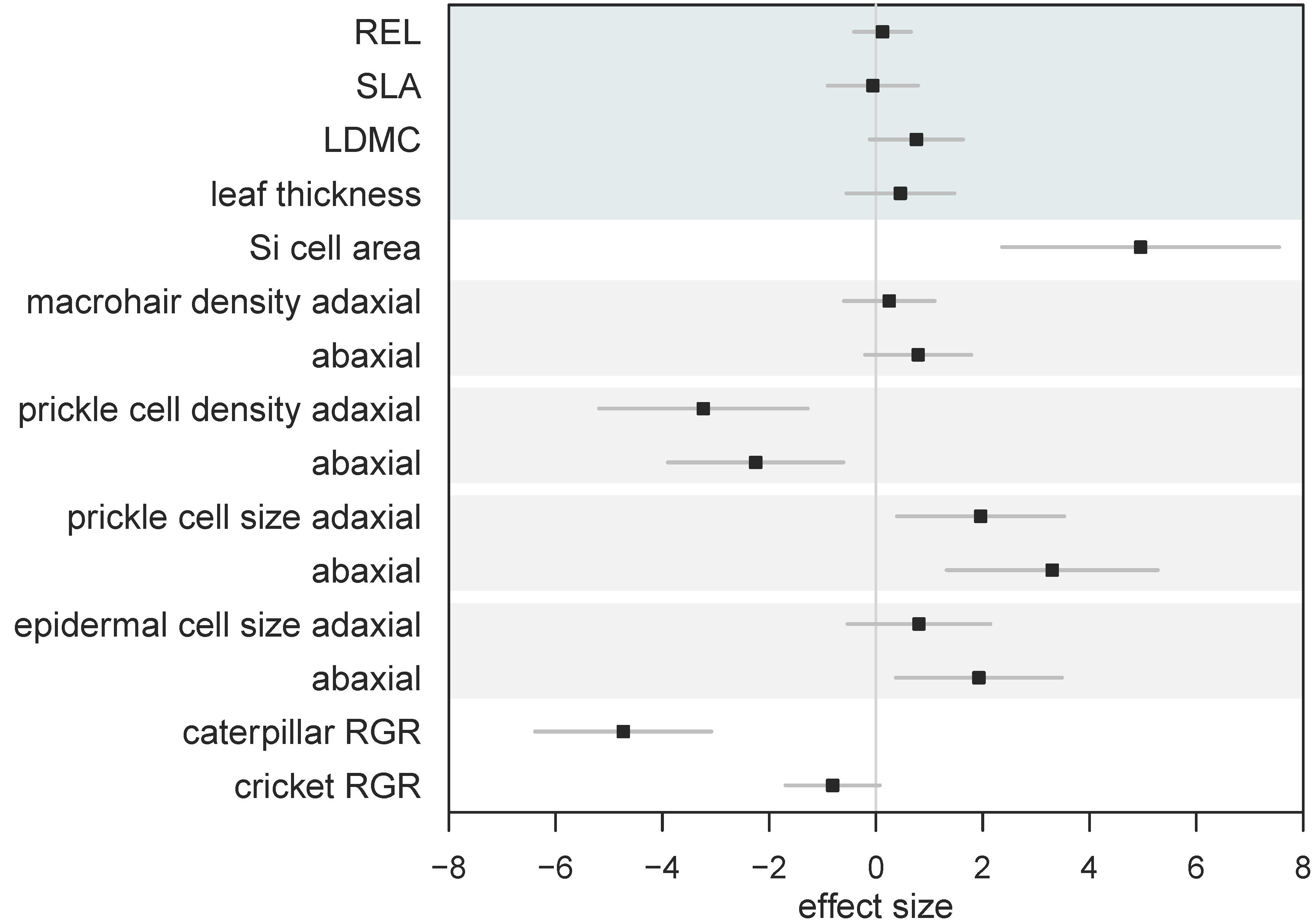
| Si- | Si+ | W | p | |
|---|---|---|---|---|
| (a) Leaf Mechanical Traits | ||||
| Si (% DW) | ND (<0.3) | 1.54 ± 0.14 | - | - |
| Mean relative electrolyte leakage | 0.10 ± 0.01 | 0.11 ± 0.01 | - | - |
| Specific leaf area (mm2 mg−1) | 3.6 ± 0.1 | 3.6 ± 0.2 | 85 | 0.47 |
| Leaf thickness (mm) | 0.15 ± 0.004 | 0.16 ± 0.004 | 28 | 0.29 |
| Dry matter content (%) | 33.7 ± 1.28 | 36.6 ± 0.92 | 38.5 | 0.055 |
| (b) Surface Morphology | ||||
| Macrohair density (mm2) adaxial | 2.15 ± 0.27 | 2.34 ± 0.16 | 48 | 0.17 |
| abaxial | 0.44 ± 0.12 | 0.68 ± 0.09 | 24 | 0.068 |
| Si cell area (μm2) | 287.2 ± 22.2 | 651.6 ± 36.2 | 0 | 0.002 * |
| Prickle cell density (0.1 mm−2) adaxial | 70.0 ± 1.6 | 53.7 ± 2.5 | 36 | 0.005 * |
| abaxial | 121.7 ± 4.6 | 99.5 ± 3.3 | 35 | 0.004 * |
| Prickle cell size (μm2) adaxial | 122.1 ± 7.2 | 162.2 ± 9.3 | 2 | 0.009 * |
| abaxial | 176.0 ± 6.9 | 246 ± 10.1 | 0 | 0.002 * |
| Prickle cell coverage (%) adaxial | 8.6 ± 0.6 | 8.6 ± 0.3 | 16 | 0.82 |
| abaxial | 21.4 ± 1.1 | 24.5 ± 1.4 | 7 | 0.093 |
| Epidermal cell size (μm2) adaxial | 771.3 ± 30.7 | 841.5 ± 39.8 | 10 | 0.240 |
| abaxial | 773.6 ± 44.9 | 1011.3 ± 55.2 | 3 | 0.015 * |
| (c) Herbivore Response | ||||
| H. armigera RGR (mg mg−1) | −26.8 ± 8.7 | −149.5 ± 6.1 | 144 | <0.001 * |
| A. domesticus RGR (mg mg−1) | 1.3 ± 3.7 | −6.4 ± 1.1 | 125 | 0.002 * |
| H. armigera frass (mg) | 28.5 ± 3.7 | 1.3 ± 0.4 | 144 | <0.001 * |
| A. domesticus frass (mg) | 3.5 ± 0.5 | 0.8 ± 0.2 | 129 | 0.001 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hall, C.R.; Dagg, V.; Waterman, J.M.; Johnson, S.N. Silicon Alters Leaf Surface Morphology and Suppresses Insect Herbivory in a Model Grass Species. Plants 2020, 9, 643. https://doi.org/10.3390/plants9050643
Hall CR, Dagg V, Waterman JM, Johnson SN. Silicon Alters Leaf Surface Morphology and Suppresses Insect Herbivory in a Model Grass Species. Plants. 2020; 9(5):643. https://doi.org/10.3390/plants9050643
Chicago/Turabian StyleHall, Casey R., Vaibhav Dagg, Jamie M. Waterman, and Scott N. Johnson. 2020. "Silicon Alters Leaf Surface Morphology and Suppresses Insect Herbivory in a Model Grass Species" Plants 9, no. 5: 643. https://doi.org/10.3390/plants9050643
APA StyleHall, C. R., Dagg, V., Waterman, J. M., & Johnson, S. N. (2020). Silicon Alters Leaf Surface Morphology and Suppresses Insect Herbivory in a Model Grass Species. Plants, 9(5), 643. https://doi.org/10.3390/plants9050643






