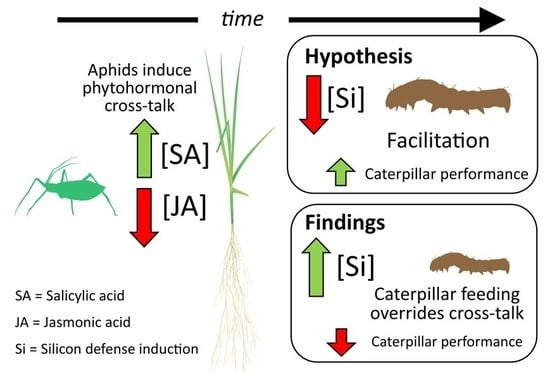
Aphid Feeding Induces Phytohormonal Cross-Talk without Affecting Silicon Defense against Subsequent Chewing Herbivores
Abstract
1. Introduction
2. Results
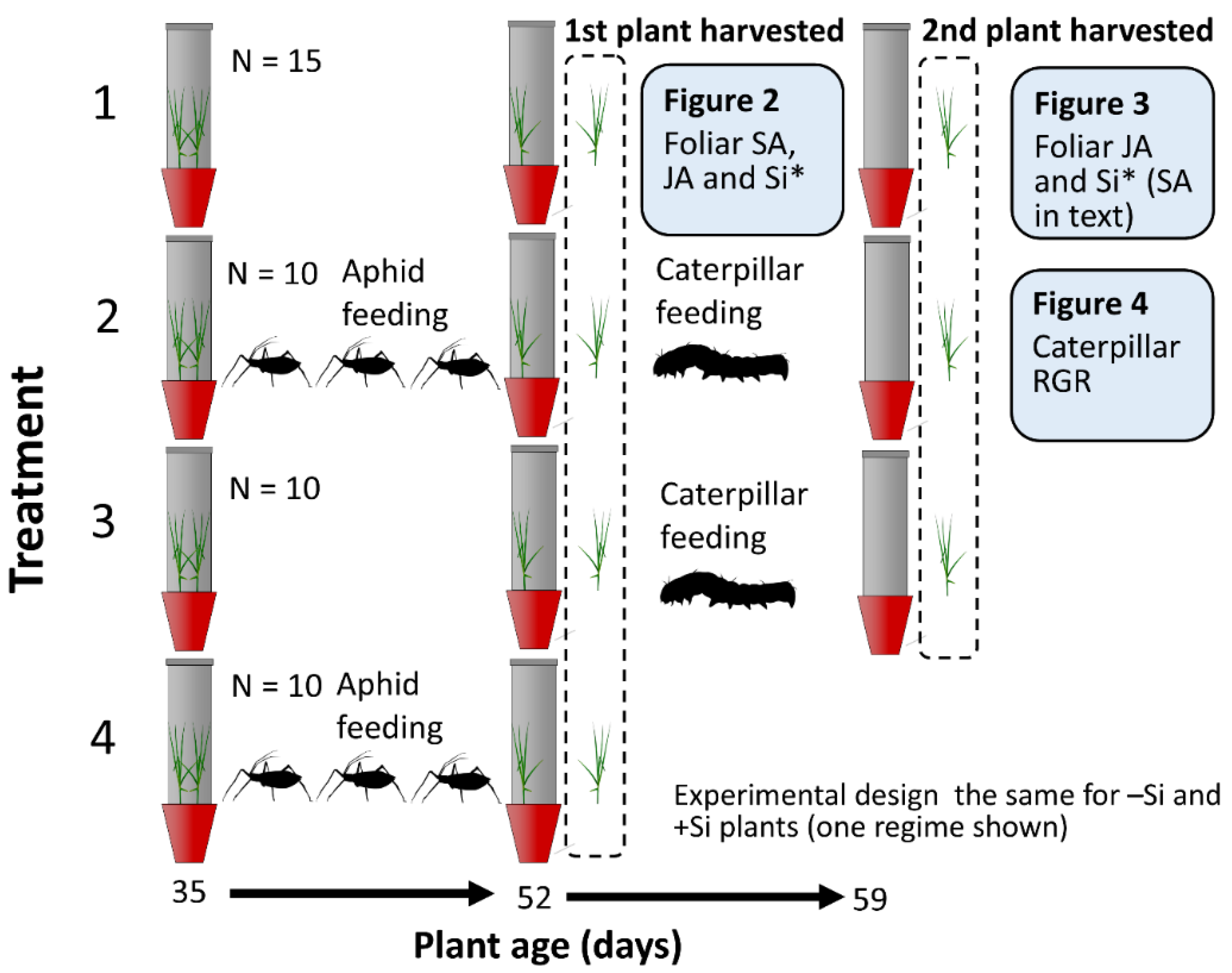
2.1. Experiment Summary
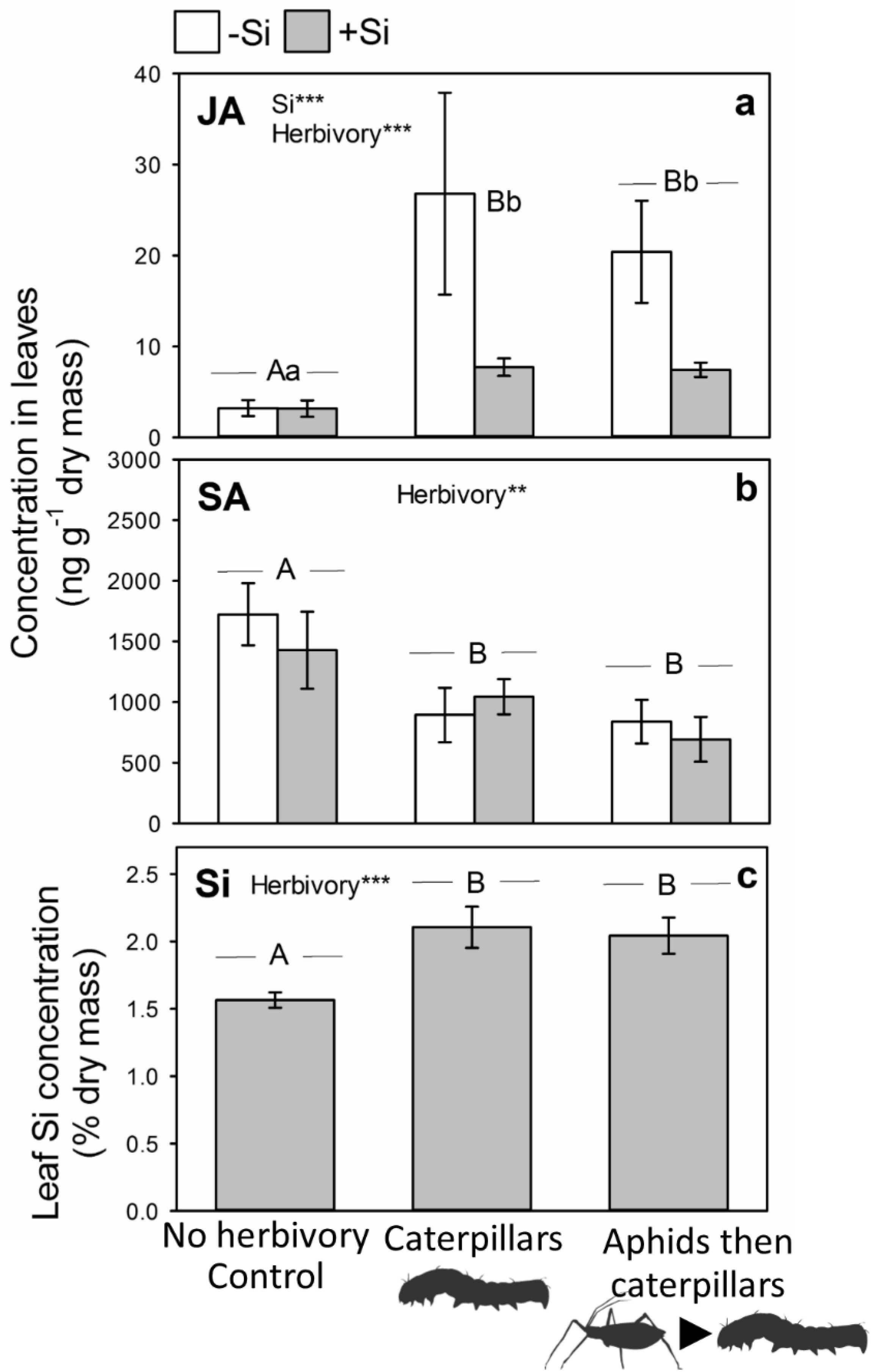
2.2. Impacts of Aphid Feeding on Phytohormones and Si
2.3. Subsequent Changes in JA and Si When Attacked by Caterpillars
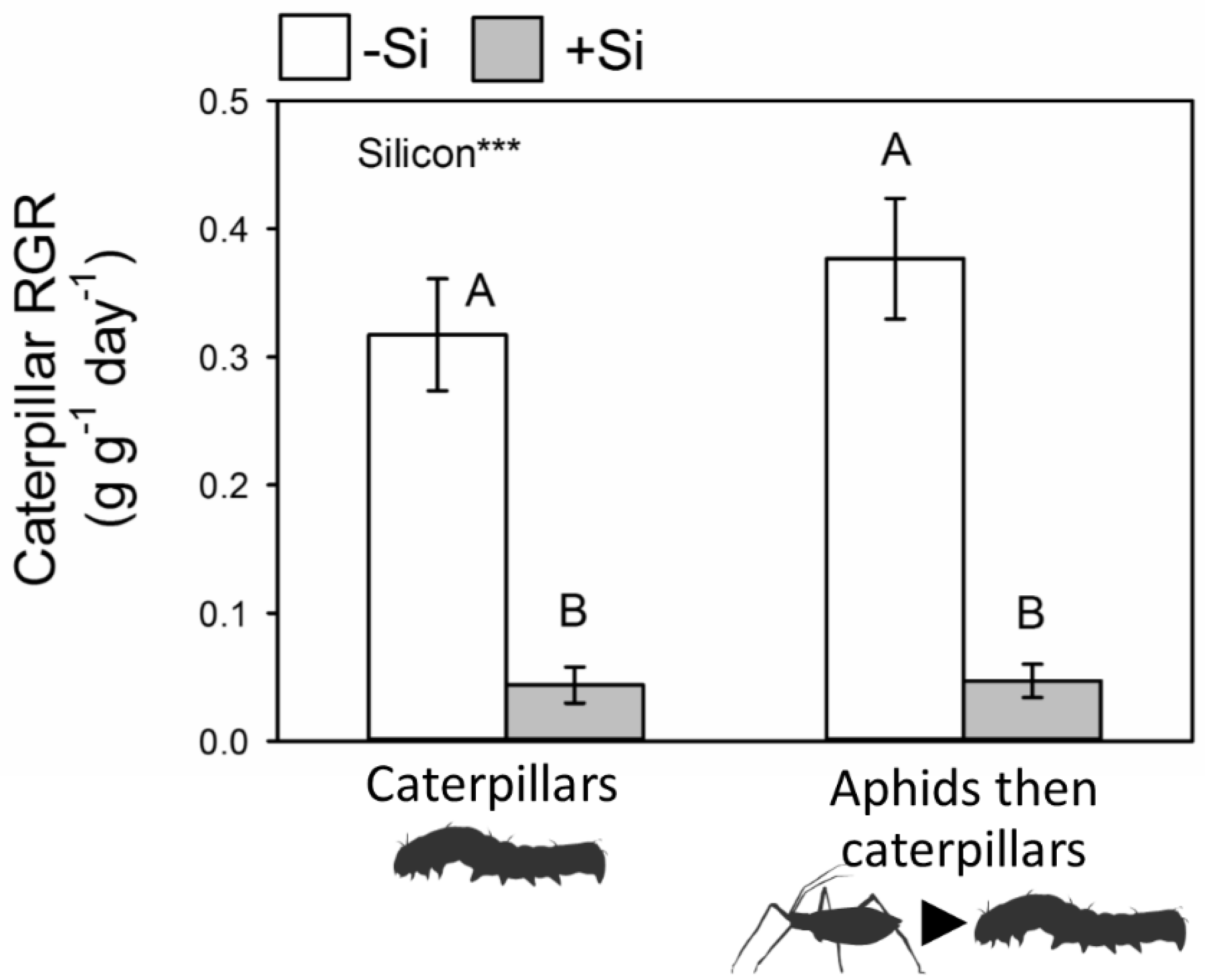
2.4. Caterpillar Performance
3. Discussion
4. Materials and Methods
4.1. Plants and Herbivores
4.2. Experimental Procedure
4.3. Si Analysis
4.4. JA and SA Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Denno, R.F.; McClure, M.S.; Ott, J.R. Interspecific interactions in phytophagous insects-competition reexamined and resurrected. Annu. Rev. Entomol. 1995, 40, 297–331. [Google Scholar] [CrossRef]
- Kaplan, I.; Denno, R.F. Interspecific interactions in phytophagous insects revisited: A quantitative assessment of competition theory. Ecol. Lett. 2007, 10, 977–994. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Badenes-Pérez, F.R.; Broekgaarden, C.; Zheng, S.J.; David, A.; Boland, W.; Dicke, M. Plant-mediated facilitation between a leaf-feeding and a phloem-feeding insect in a brassicaceous plant: From insect performance to gene transcription. Funct. Ecol. 2012, 26, 156–166. [Google Scholar] [CrossRef]
- Li, Y.H.; Dicke, M.; Kroes, A.; Liu, W.; Gols, R. Interactive effects of cabbage aphid and caterpillar herbivory on transcription of plant genes associated with phytohormonal signalling in wild cabbage. J. Chem. Ecol. 2016, 42, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Stout, M.J.; Fidantsef, A.L.; Duffey, S.S.; Bostock, R.M. Signal interactions in pathogen and insect attack: Systemic plant-mediated interactions between pathogens and herbivores of the tomato, Lycopersicon esculentum. Physiol. Mol. Plant Pathol. 1999, 54, 115–130. [Google Scholar] [CrossRef]
- Bertini, L.; Palazzi, L.; Proietti, S.; Pollastri, S.; Arrigoni, G.; de Laureto, P.; Caruso, C. Proteomic analysis of MeJa-induced defense responses in rice against wounding. Int. J. Mol. Sci. 2019, 20, 2525. [Google Scholar] [CrossRef]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 15206. [Google Scholar] [CrossRef] [PubMed]
- Bostock, R.M.; Karban, R.; Thaler, J.S.; Weyman, P.D.; Gilchrist, D. Signal interactions in induced resistance to pathogens and insect herbivores. Eur. J. Plant Pathol. 2001, 107, 103–111. [Google Scholar] [CrossRef]
- Schweiger, R.; Heise, A.M.; Persicke, M.; Müller, C. Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant Cell Environ. 2014, 37, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.G.; Agrawal, A.A. Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct. Ecol. 2014, 28, 1404–1412. [Google Scholar] [CrossRef]
- Li, Y.H.; Dicke, M.; Harvey, J.A.; Gols, R. Intra-specific variation in wild Brassica oleracea for aphid-induced plant responses and consequences for caterpillar-parasitoid interactions. Oecologia 2014, 174, 853–862. [Google Scholar] [CrossRef]
- Vicari, M.; Bazely, D.R. Do grasses fight back - the case for antherbivore defences. Trends Ecol. Evol. 1993, 8, 137–141. [Google Scholar] [CrossRef]
- Moore, B.D.; Johnson, S.N. Get tough, get toxic, or get a bodyguard: Identifying candidate traits conferring belowground resistance to herbivores in grasses. Front. Plant Sci. 2017, 7, 1925. [Google Scholar] [CrossRef]
- Reynolds, O.L.; Padula, M.P.; Zeng, R.S.; Gurr, G.M. Silicon: Potential to promote direct and indirect effects on plant defense against arthropod pests in agriculture. Front. Plant Sci. 2016, 7, 744. [Google Scholar] [CrossRef]
- Alhousari, F.; Greger, M. Silicon and mechanisms of plant resistance to insect pests. Plants 2018, 7, 33. [Google Scholar] [CrossRef]
- Kvedaras, O.L.; An, M.; Choi, Y.S.; Gurr, G.M. Silicon enhances natural enemy attraction and biological control through induced plant defences. Bull. Ent. Res. 2010, 100, 367–371. [Google Scholar] [CrossRef]
- Leroy, N.; de Tombeur, F.; Walgraffe, Y.; Cornélis, J.-T.; Verheggen, F.J. Silicon and plant natural defenses against insect pests: Impact on plant volatile organic compounds and cascade effects on multitrophic interactions. Plants 2019, 8, 444. [Google Scholar] [CrossRef] [PubMed]
- Clissold, F.J. The biomechanics of chewing and plant fracture: Mechanisms and implications. Adv. Insect Physiol. 2007, 34, 317–372. [Google Scholar] [CrossRef]
- Hunt, J.W.; Dean, A.P.; Webster, R.E.; Johnson, G.N.; Ennos, A.R. A novel mechanism by which silica defends grasses against herbivory. Ann. Bot. 2008, 102, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.E.; Fitt, R.N.; McLarnon, E.L.; Wade, R.N. Defending the leaf surface: Intra- and inter-specific differences in silicon deposition in grasses in response to damage and silicon supply. Front. Plant Sci. 2015, 6, 35. [Google Scholar] [CrossRef]
- Hall, C.R.; Dagg, V.; Waterman, J.M.; Johnson, S.N. Silicon alters leaf surface morphology and suppresses insect herbivory in a model grass species. Plants 2020, 9, 643. [Google Scholar] [CrossRef]
- Massey, F.P.; Ennos, A.R.; Hartley, S.E. Silica in grasses as a defence against insect herbivores: Contrasting effects on folivores and a phloem feeder. J. Anim. Ecol. 2006, 75, 595–603. [Google Scholar] [CrossRef]
- Massey, F.P.; Hartley, S.E. Physical defences wear you down: Progressive and irreversible impacts of silica on insect herbivores. J. Anim. Ecol. 2009, 78, 281–291. [Google Scholar] [CrossRef]
- Kvedaras, O.L.; Byrne, M.J.; Coombes, N.E.; Keeping, M.G. Influence of plant silicon and sugarcane cultivar on mandibular wear in the stalk borer Eldana saccharina. Agric. For. Entomol. 2009, 11, 301–306. [Google Scholar] [CrossRef]
- Massey, F.P.; Ennos, A.R.; Hartley, S.E. Herbivore specific induction of silica-based plant defences. Oecologia 2007, 152, 677–683. [Google Scholar] [CrossRef]
- Johnson, S.N.; Reynolds, O.L.; Gurr, G.M.; Esveld, J.L.; Moore, B.D.; Tory, G.J.; Gherlenda, A.N. When resistance is futile, tolerate instead: Silicon promotes plant compensatory growth when attacked by above- and belowground herbivores. Biol. Letts. 2019, 15, 20190361. [Google Scholar] [CrossRef]
- Hartley, S.E.; DeGabriel, J.L. The ecology of herbivore-induced silicon defences in grasses. Funct. Ecol. 2016, 30, 1311–1322. [Google Scholar] [CrossRef]
- Johnson, S.N.; Rowe, R.C.; Hall, C.R. Silicon is an inducible and effective herbivore defence against Helicoverpa punctigera (Lepidoptera: Noctuidae) in soybean. Bull. Entomol. Res. 2020, 110, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.R.; Mikhael, M.; Hartley, S.E.; Johnson, S.N. Elevated atmospheric CO2 suppresses jasmonate and silicon-based defences without affecting herbivores. Funct. Ecol. 2020, 34, 993–1002. [Google Scholar] [CrossRef]
- Hall, C.R.; Waterman, J.M.; Vandegeer, R.K.; Hartley, S.E.; Johnson, S.N. The role of silicon in phytohormonal signalling. Front. Plant Sci. 2019, 10, 1132. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.; Zhang, P.; Han, L.; Reynolds, O.L.; Zeng, R.S.; Wu, J.; Shao, Y.; You, M.; Gurr, G.M. Silicon supplementation alters the composition of herbivore induced plant volatiles and enhances attraction of parasitoids to infested rice plants. Front. Plant Sci. 2017, 8, 1265. [Google Scholar] [CrossRef]
- Ye, M.; Song, Y.Y.; Long, J.; Wang, R.L.; Baerson, S.R.; Pan, Z.Q.; Zhu-Salzman, K.; Xie, J.F.; Cai, K.Z.; Luo, S.M.; et al. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Waqas, M.; Jeong, H.J.; Kim, D.H.; Shin, J.S.; Kim, J.G.; Yeon, M.H.; Lee, I.J. Regulation of jasmonic acid biosynthesis by silicon application during physical injury to Oryza sativa L. J. Plant Res. 2014, 127, 525–532. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.R.; Musser, R.O.; Vogel, H.; Hum-Musser, S.M.; Thaler, J.S. Molecular, biochemical, and organismal analyses of tomato plants simultaneously attacked by herbivores from two feeding guilds. J. Chem. Ecol. 2010, 36, 1043–1057. [Google Scholar] [CrossRef]
- Eisenring, M.; Glauser, G.; Meissle, M.; Romeis, J. Differential impact of herbivores from three feeding guilds on systemic secondary metabolite induction, phytohormone levels and plant-mediated herbivore interactions. J. Chem. Ecol. 2018, 44, 1178–1189. [Google Scholar] [CrossRef]
- Li, Y.H.; Meijer, D.; Dicke, M.; Gols, R. Oviposition preference of three lepidopteran species is not affected by previous aphid infestation in wild cabbage. Entomol. Exp. Appl. 2018, 166, 402–411. [Google Scholar] [CrossRef]
- Kroes, A.; Stam, J.M.; David, A.; Boland, W.; van Loon, J.J.A.; Dicke, M.; Poelman, E.H. Plant-mediated interactions between two herbivores differentially affect a subsequently arriving third herbivore in populations of wild cabbage. Plant Biol. 2016, 18, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Davidson-Lowe, E.; Szendrei, Z.; Ali, J.G. Asymmetric effects of a leaf-chewing herbivore on aphid population growth. Ecol. Entomol. 2019, 44, 81–92. [Google Scholar] [CrossRef]
- Stout, M.J.; Workman, K.V.; Bostock, R.M.; Duffey, S.S. Specificity of induced resistance in the tomato, Lycopersicon esculentum. Oecologia 1997, 113, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C. Does Silicon Supplementation Affect Plant Traits That Impact the Performance and Feeding Behaviour of Cereal Aphids? Master’s Thesis, Western Sydney University, Richmond, Australia, 2019. [Google Scholar]
- Rowe, R.C.; Trębicki, P.; Gherlenda, A.; Johnson, S.N. Cereal aphid performance and feeding behaviour largely unaffected by silicon enrichment of host plants. J. Pest Sci. 2020, 93, 41–48. [Google Scholar] [CrossRef]
- Sanchez-Arcos, C.; Reichelt, M.; Gershenzon, J.; Kunert, G. Modulation of legume defense signaling pathways by native and non-native pea aphid clones. Front. Plant Sci. 2016, 7, 1872. [Google Scholar] [CrossRef]
- Johnson, S.N.; Hartley, S.E. Elevated carbon dioxide and warming impact silicon and phenolic-based defences differently in native and exotic grasses. Glob. Chang. Biol. 2018, 24, 3886–3896. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Tradeoffs between foliar silicon and carbon-based defences: Evidence from vegetation communities of contrasting soil types. Oikos 2012, 121, 2052–2060. [Google Scholar] [CrossRef]
- Frew, A.; Powell, J.R.; Sallam, N.; Allsopp, P.G.; Johnson, S.N. Trade-offs between silicon and phenolic defences may explain enhanced performance of root herbivores on phenolic-rich plants. J. Chem Ecol. 2016, 42, 768–771. [Google Scholar] [CrossRef]
- Moles, A.T.; Peco, B.; Wallis, I.R.; Foley, W.J.; Poore, A.G.B.; Seabloom, E.W.; Vesk, P.A.; Bisigato, A.J.; Cella-Pizarro, L.; Clark, C.J.; et al. Correlations between physical and chemical defences in plants: Tradeoffs, syndromes, or just many different ways to skin a herbivorous cat? New Phytol. 2013, 198, 252–263. [Google Scholar] [CrossRef]
- Johnson, S.N.; Benefer, C.M.; Frew, A.; Griffiths, B.S.; Hartley, S.E.; Karley, A.J.; Rasmann, S.; Schumann, M.; Sonnemann, I.; Robert, C.A.M. New frontiers in belowground ecology for plant protection from root-feeding insects. Appl. Soil Ecol. 2016, 108, 96–107. [Google Scholar] [CrossRef]
- FAOSTAT. FAOSTAT Database Collections. Available online: www.fao.org/faostat/en (accessed on 12 February 2020).
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot.-London 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Teakle, R.E.; Jensen, J.M. Heliothis punctiger. In Handbook of Insect Rearing, Vol. 2; Singh, R., Moore, R.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; pp. 312–322. [Google Scholar]
- Reidinger, S.; Ramsey, M.H.; Hartley, S.E. Rapid and accurate analyses of silicon and phosphorus in plants using a portable X-ray fluorescence spectrometer. New Phytol. 2012, 195, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Hiltpold, I.; Demarta, L.; Johnson, S.N.; Moore, B.D.; Power, S.A.; Mitchell, C. Silicon and other essential element composition in roots using X-ray fluorescence spectroscopy: A high throughput approach. In Invertebrate Ecology of Australasian Grasslands, Proceedings of the Ninth ACGIE, Hawkesbury, NSW, Australia, 4–7 April 2016; pp. 191–196.
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, S.N.; Rowe, R.C.; Hall, C.R. Aphid Feeding Induces Phytohormonal Cross-Talk without Affecting Silicon Defense against Subsequent Chewing Herbivores. Plants 2020, 9, 1009. https://doi.org/10.3390/plants9081009
Johnson SN, Rowe RC, Hall CR. Aphid Feeding Induces Phytohormonal Cross-Talk without Affecting Silicon Defense against Subsequent Chewing Herbivores. Plants. 2020; 9(8):1009. https://doi.org/10.3390/plants9081009
Chicago/Turabian StyleJohnson, Scott N., Rhiannon C. Rowe, and Casey R. Hall. 2020. "Aphid Feeding Induces Phytohormonal Cross-Talk without Affecting Silicon Defense against Subsequent Chewing Herbivores" Plants 9, no. 8: 1009. https://doi.org/10.3390/plants9081009
APA StyleJohnson, S. N., Rowe, R. C., & Hall, C. R. (2020). Aphid Feeding Induces Phytohormonal Cross-Talk without Affecting Silicon Defense against Subsequent Chewing Herbivores. Plants, 9(8), 1009. https://doi.org/10.3390/plants9081009