Contrasting Leaf Trait Responses of Conifer and Broadleaved Seedlings to Altered Resource Availability Are Linked to Resource Strategies
Abstract
1. Introduction
2. Results
2.1. Evergreen Conifers
2.2. Deciduous Broadleaves
3. Discussion
3.1. Foliar Responses of Evergreen Conifers and Deciduous Broadleaves
3.2. Life-History Strategies as the Key to Foliar Responses to Environmental Changes
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Foliar Traits
4.3. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanewinkel, M.; Cullmann, D.A.; Schelhaas, M.-J.; Nabuurs, G.-J.; Zimmermann, N.E. Climate change may cause severe loss in the economic value of European forest land. Nat. Clim. Chang. 2013, 3, 203–207. [Google Scholar] [CrossRef]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van der Maaten, E.; Schelhaas, M.-J.; Lasch, P.; Eggers, J.; van der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef]
- Buras, A.; Menzel, A. Projecting tree species composition changes of european forests for 2061–2090 under RCP 4.5 and RCP 8.5 scenarios. Front. Plant Sci. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef] [PubMed]
- Rosas, T.; Mencuccini, M.; Barba, J.; Cochard, H.; Saura-Mas, S.; Martínez-Vilalta, J. Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytol. 2019, 223, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Gessler, A.; Keitel, C.; Nahm, M.; Rennenberg, H. Water shortage affects the water and nitrogen balance in Central European beech forests. Plant Biol. 2004, 6, 289–298. [Google Scholar] [PubMed]
- Oren, R.; Ellsworth, D.S.; Johnsen, K.H.; Phillips, N.; Ewers, B.E.; Maier, C.; Schäfer, K.V.R.; McCarthy, H.; Hendrey, G.; McNulty, S.G.; et al. Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 2001, 411, 469–472. [Google Scholar] [CrossRef]
- Fisher, J.B.; Badgley, G.; Blyth, E. Global nutrient limitation in terrestrial vegetation. Glob. Biogeochem. Cycles 2012, 26, 1–9. [Google Scholar] [CrossRef]
- Van Houtven, G.; Phelan, J.; Clark, C.; Sabo, R.D.; Buckley, J.; Thomas, R.Q.; Horn, K.; LeDuc, S.D. Nitrogen deposition and climate change effects on tree species composition and ecosystem services for a forest cohort. Ecol. Monogr. 2019, 89, e01345. [Google Scholar] [CrossRef]
- Gessler, A.; Schaub, M.; Mcdowell, N.G. The role of nutrients in drought-induced tree mortality and recovery. New Phytol. 2017, 214, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kreuzwieser, J.; Gessler, A. Global climate change and tree nutrition: Influence of water availability. Tree Physiol. 2010, 30, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, L.; Gessler, A.; Schaub, M.; Rigling, A.; Hoch, G.; Kahmen, A.; Li, M. Soil nutrients and lowered source: Sink ratio mitigate effects of mild but not of extreme drought in trees. Environ. Exp. Bot. 2020, 169, 103905. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef] [PubMed]
- Maire, V.; Martre, P.; Kattge, J.; Gastal, F.; Esser, G.; Fontaine, S.; Soussana, J.F. The coordination of leaf photosynthesis links C and N fluxes in C3 plant species. PLoS ONE 2012, 7, e38345. [Google Scholar] [CrossRef]
- Buckley, T.N.; Cescatti, A.; Farquhar, G.D. What does optimization theory actually predict about crown profiles of photosynthetic capacity when models incorporate greater realism? Plant Cell Environ. 2013, 36, 1547–1563. [Google Scholar] [CrossRef]
- Oldham, A.R.; Sillett, S.C.; Tomescu, A.M.F.; Koch, G.W. The hydrostatic gradient, not light availability, drives height-related variation in Sequoia sempervirens (Cupressaceae) leaf anatomy. Am. J. Bot. 2010, 97, 1087–1097. [Google Scholar] [CrossRef]
- Bachofen, C.; D’Odorico, P.; Buchmann, N. Light and VPD gradients drive foliar nitrogen partitioning and photosynthesis in the canopy of European beech and silver fir. Oecologia 2020. [Google Scholar] [CrossRef]
- Bachofen, C.; Wohlgemuth, T.; Moser, B. Biomass partitioning in a future dry and CO2 enriched climate: Shading aggravates drought effects in Scots pine but not European black pine seedlings. J. Appl. Ecol. 2019, 56, 1–14. [Google Scholar] [CrossRef]
- Danyagri, G.; Dang, Q.L. Effects of Elevated [CO2] and Low Soil Moisture on the Physiological Responses of Mountain Maple (Acer spicatum L.) Seedlings to Light. PLoS ONE 2013, 8, e76586. [Google Scholar] [CrossRef]
- Pardos, M.; Puértolas, J.; Aranda, I.; Pardos, J.A. Can CO2 enrichment modify the effect of water and high light stress on biomass allocation and relative growth rate of cork oak seedlings? Trees Struct. Funct. 2006, 20, 713–724. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide “fast-slow” plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Chapin, E.S. The Mineral Nutrition of Wild Plants. Annu. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Westoby, M.; Wright, I.J. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 2006, 21, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hu, H.; Zhao, M.; Li, H.; Liu, S.; Fang, J. Latitudinal pattern and the driving factors of leaf functional traits in 185 shrub species across eastern China. J. Plant Ecol. 2017, 12, 67–77. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Osnas, J.L.D.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global Leaf Trait Relationships: Mass, Area, and the Leaf Economics Spectrum. Science 2014, 340, 741–745. [Google Scholar] [CrossRef]
- Reich, A.P.B.; Wright, I.J.; Bares, J.C.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The Evolution of Plant Functional Variation: Traits, Spectra, and Strategies. Int. J. Plant Sci. 2003, 164, 143–164. [Google Scholar] [CrossRef]
- Reich, P.B.; Ellsworth, D.S.; Walters, M.B.; Vose, J.M.; Gresham, C.; Volin, J.C.; Bowman, W.D. Generality of Leaf Trait Relationships: A Test Across Six Biomes. Ecology 1999, 80, 1955–1969. [Google Scholar] [CrossRef]
- Damián, X.; Ochoa-López, S.; Gaxiola, A.; Fornoni, J.; Domínguez, C.A.; Boege, K. Natural selection acting on integrated phenotypes: Covariance among functional leaf traits increases plant fitness. New Phytol. 2020, 225, 546–557. [Google Scholar] [CrossRef]
- Bontemps, A.; Davi, H.; Lefèvre, F.; Rozenberg, P.; Oddou-muratorio, S. How do functional traits syndromes covary with growth and reproductive performance in a water-stressed population of Fagus sylvatica? Oikos 2017, 126, 1472–1483. [Google Scholar] [CrossRef]
- Salguero-Gómez, R.; Jones, O.R.; Jongejans, E.; Blomberg, S.P.; Hodgson, D.J.; Mbeau-Ache, C.; Zuidema, P.A.; De Kroon, H.; Buckley, Y.M. Fast-slow continuum and reproductive strategies structure plant life-history variation worldwide. Proc. Natl. Acad. Sci. USA 2016, 113, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.B.; Reich, P.B. Are Shade Tolerance, Survival, and Growth Linked? Ecology 1996, 77, 841–853. [Google Scholar] [CrossRef]
- Craine, J.M. Resource Strategies of Wild Plants; Princeton University Press: Princeton, NJ, USA, 2009; ISBN 9780691139128. [Google Scholar]
- Walters, M.; Reich, P. Low-light carbon balance and shade tolerance in the seedlings of woody plants: Do winter deciduous and broad-leaved evergreen species differ? New Phytol. 1999, 143, 143–154. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 1–17. [Google Scholar] [CrossRef]
- Grime, J.P. Plant Strategies and Vegetation Processes; John Wiley and Sons: New York, NY, USA, 1979. [Google Scholar]
- Alberto, F.J.; Aitken, S.N.; Alía, R.; González-Martínez, S.C.; Hänninen, H.; Kremer, A.; Lefèvre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change—Evidence from tree populations. Glob. Chang. Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 4–8. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Cochard, H.; Mencuccini, M.; Sterck, F.; Herrero, A.; Korhonen, J.F.J.; Llorens, P.; Nikinmaa, E.; Nolè, A.; Poyatos, R.; et al. Hydraulic adjustment of Scots pine across Europe. New Phytol. 2009, 184, 353–364. [Google Scholar] [CrossRef]
- Walters, M.B.; Gerlach, J.P. Intraspecific growth and functional leaf trait responses to natural soil resource gradients for conifer species with contrasting leaf habit. Tree Physiol. 2013, 33, 297–310. [Google Scholar] [CrossRef]
- Lilles, E.B.; Astrup, R.; Lefranc¸ois, M.L.; Coates, K.D. Sapling leaf trait responses to light, tree height and soil nutrients for three conifer species of contrasting shade tolerance. Tree Physiol. 2014, 34, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Salguero-Gómez, R. Applications of the fast–slow continuum and reproductive strategy framework of plant life histories. New Phytol. 2017, 213, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Lusk, C.H.; Reich, P.B.; Montgomery, R.A.; Ackerly, D.D.; Cavender-Bares, J. Why are evergreen leaves so contrary about shade? Trends Ecol. Evol. 2008, 23, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Hallik, L.; Niinemets, Ü.; Wright, I.J. Are species shade and drought tolerance reflected in leaf-level structural and functional differentiation in Northern Hemisphere temperate woody flora? New Phytol. 2009, 184, 257–274. [Google Scholar] [CrossRef]
- Withington, J.; Reich, P.B.; Oleksyn, J.; Eissenstat, D. Comparisons of structure and life span in roots and leaves. Ecol. Monogr. 2006, 76, 381–397. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Plants—An Economic Analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Reich, P.B. Leaf mass per area, nitrogen content and photosynthetic carbon gain in Acer saccharum seedlings in contrasting forest light environments. Funct. Ecol. 1992, 6, 423–435. [Google Scholar] [CrossRef]
- Coley, P.D. Effects of plant growth rate and leaf lifetime on the amount and type of anti-herbivore defense. Oecologia 1988, 74, 531–536. [Google Scholar] [CrossRef]
- Khan, A. Leaf Anatomy. In Plant Anatomy and Physiology; Kalpaz Publications: New Delhi, India, 2002; pp. 165–188. [Google Scholar]
- Pflug, E.E.; Buchmann, N.; Siegwolf, R.T.W.; Schaub, M.; Rigling, A.; Arend, M. Resilient Leaf Physiological Response of European Beech (Fagus sylvatica L.) to Summer Drought and Drought Release. Front. Plant Sci. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Niinemets, U.; Kull, O.; Tenhunen, J.D. An analysis of light effects on foliar morphology, physiology, and light interception in temperate deciduous woody species of contrasting shade tolerance. Tree Physiol. 1998, 18, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.P.; Beckerman, A.P.; Gu, L.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The relationship of leaf photosynthetic traits—Vcmax and Jmax—To leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef] [PubMed]
- Bachofen, C.; Wohlgemuth, T.; Moser, B.; Hoch, G.; Ghazoul, J. No carbon “bet hedging” in pine seedlings under prolonged summer drought and elevated CO2. J. Ecol. 2018, 106, 31–46. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Aranda, I.; Pardos, M.; Puértolas, J.; Jiménez, M.D.; Pardos, J.A. Water-use efficiency in cork oak (Quercus suber) is modified by the interaction of water and light availabilities. Tree Physiol. 2007, 27, 671–677. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.; Sevanto, S. The mechanisms of carbon starvation: How, when, or does it even occur at all? New Phytol. 2010, 186, 263–264. [Google Scholar] [CrossRef]
- Körner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological mechanisms of drought-induced tree mortality are far from being resolved. New Phytol. 2010, 186, 274. [Google Scholar] [CrossRef]
- Muller, B.; Pantin, F.; Génard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef]
- Antúnez, I.; Retamosa, E.C.; Villar, R. Relative growth rate in phylogenetically related deciduous and evergreen woody species. Oecologia 2001, 128, 172–180. [Google Scholar] [CrossRef]
- Ruiz-Robleto, J.; Villar, R. Relative growth rate and biomass allocation in ten woody species with different leaf longevity using phylogenetic independent contrasts (PICs). Plant Biol. 2005, 7, 484–494. [Google Scholar] [CrossRef]
- Tomlinson, K.W.; Poorter, L.; Sterck, F.J.; Borghetti, F.; Ward, D.; de Bie, S.; van Langevelde, F. Leaf adaptations of evergreen and deciduous trees of semi-arid and humid savannas on three continents. J. Ecol. 2013, 101, 430–440. [Google Scholar] [CrossRef]
- Ackerly, D. Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol. Monogr. 2004, 74, 25–44. [Google Scholar] [CrossRef]
- Kursar, T.A.; Engelbrecht, B.M.J.; Burke, A.; Tyree, M.T.; El Omari, B.; Giraldo, J.P. Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Funct. Ecol. 2009, 23, 93–102. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L. Seedling root morphology and biomass allocation of 62 tropical tree species in relation to drought- and shade-tolerance. J. Ecol. 2009, 97, 311–325. [Google Scholar] [CrossRef]
- Reich, P.B.; Tjoelker, M.G.; Walters, M.B.; Vanderklein, D.W.; Buschena, C. Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Funct. Ecol. 1998, 12, 327–338. [Google Scholar] [CrossRef]
- Niinemets, U. The controversy over traits conferring shade-tolerance in trees: Ontogenetic changes revisited. J. Ecol. 2006, 94, 464–470. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Goude, M.; Nilsson, U.; Holmström, E. Comparing direct and indirect leaf area measurements for Scots pine and Norway spruce plantations in Sweden. Eur. J. For. Res. 2019, 138, 1033–1047. [Google Scholar] [CrossRef]
- Adler, P.B.; Salguero-Gómez, R.; Compagnoni, A.; Hsu, J.S.; Ray-Mukherjee, J.; Mbeau-Ache, C.; Franco, M. Functional traits explain variation in plant lifehistory strategies. Proc. Natl. Acad. Sci. USA 2014, 111, 740–745. [Google Scholar] [CrossRef]
- Valladares, F.; Pearcy, R.W. Drought can be more critical in the shade than in the sun: A field study of carbon gain and photo-inhibition in a Californian shrub during a dry El Nino year. Plant Cell Environ. 2002, 25, 749–759. [Google Scholar] [CrossRef]
- Reyer, C.P.O.; Leuzinger, S.; Rammig, A.; Wolf, A.; Bartholomeus, R.P.; Bonfante, A.; de Lorenzi, F.; Dury, M.; Gloning, P.; Abou Jaoudé, R.; et al. A plant’s perspective of extremes: Terrestrial plant responses to changing climatic variability. Glob. Chang. Biol. 2013, 19, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Ferrini, F.; Pollastrini, M.; Fini, A. The challenge of Mediterranean sclerophyllous vegetation under climate change: From acclimation to adaptation. Environ. Exp. Bot. 2014, 103, 80–98. [Google Scholar] [CrossRef]
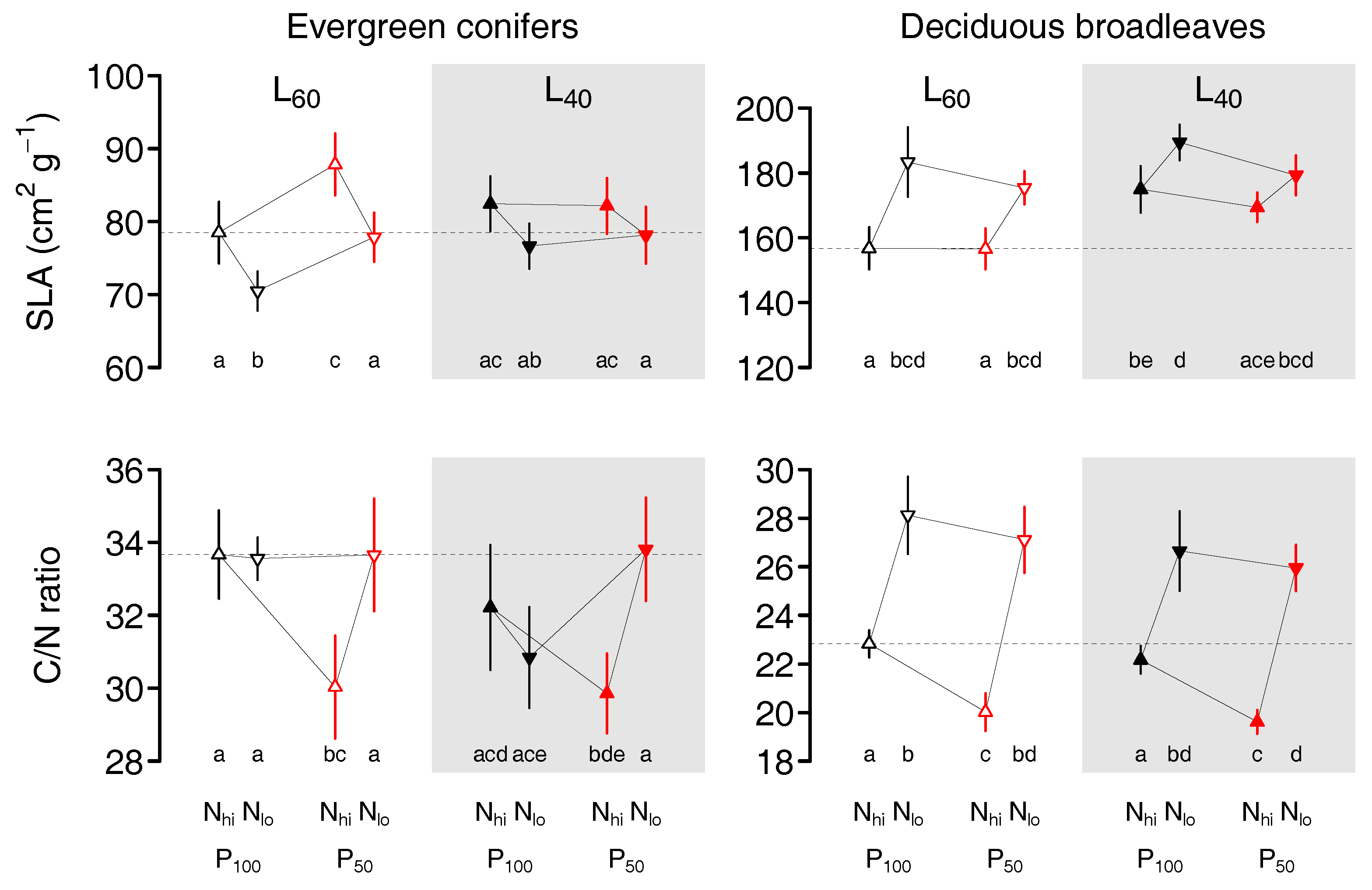
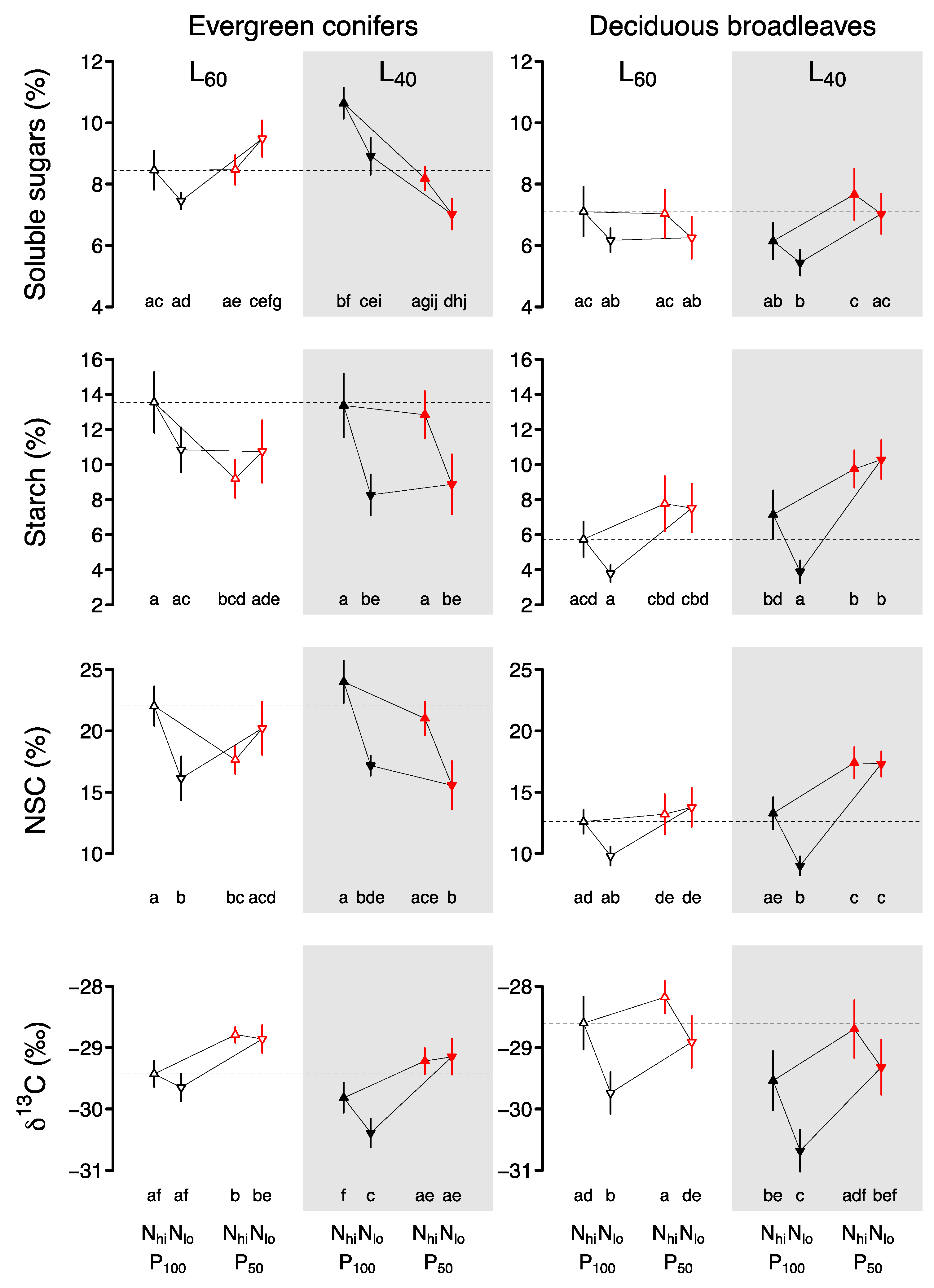
| Conifers | SLA | C/N | Sugars | Starch | NSC | δ13C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | |
| Species (S) | 54.20 | <0.001 | 14.59 | <0.001 | 5.57 | 0.0018 | 23.98 | 0.0010 | 10.59 | <0.001 | 13.86 | 0.0041 |
| Nutrients (N) | 7.85 | 0.0057 | 3.80 | 0.0559 | 4.57 | 0.0363 | 13.70 | <0.001 | 16.29 | <0.001 | 2.29 | 0.1362 |
| Precipitation (P) | 6.74 | 0.0103 | 0.88 | 0.3525 | 2.87 | 0.0952 | 2.95 | 0.0917 | 1.58 | 0.2130 | 40.32 | <0.001 |
| Light (L) | 0.72 | 0.3972 | 1.79 | 0.1861 | 0.43 | 0.5147 | 0.43 | 0.5143 | 0.20 | 0.6538 | 12.80 | <0.001 |
| S × N | 0.13 | 0.9391 | 0.27 | 0.8475 | 0.39 | 0.7635 | 3.31 | 0.0266 | 1.06 | 0.3735 | 2.24 | 0.0932 |
| S × P | 0.24 | 0.8689 | 0.98 | 0.4096 | 1.35 | 0.2658 | 1.26 | 0.2960 | 0.97 | 0.4132 | 1.31 | 0.2808 |
| N × P | 0.45 | 0.5044 | 8.39 | 0.0052 | 3.62 | 0.0615 | 0.69 | 0.4090 | 6.50 | 0.0132 | 2.33 | 0.1329 |
| S × L | 0.46 | 0.7110 | 0.75 | 0.5287 | 1.85 | 0.1467 | 0.81 | 0.4923 | 0.66 | 0.5798 | 1.27 | 0.2942 |
| N × L | 2.51 | 0.1149 | 0.09 | 0.7666 | 4.63 | 0.0353 | 8.97 | 0.0041 | 5.38 | 0.0237 | 0.19 | 0.6606 |
| P × L | 4.45 | 0.0366 | 1.76 | 0.1901 | 22.53 | <0.001 | 2.25 | 0.1392 | 1.22 | 0.2743 | 0.62 | 0.4362 |
| S × N × P | 1.70 | 0.1700 | 0.86 | 0.4650 | 0.87 | 0.4610 | 0.55 | 0.6502 | 1.60 | 0.1979 | 0.29 | 0.8347 |
| S × N × L | 2.10 | 0.1025 | 1.25 | 0.3001 | 0.96 | 0.4173 | 0.59 | 0.6247 | 0.82 | 0.4874 | 0.34 | 0.7994 |
| S × P × L | 0.04 | 0.9898 | 0.13 | 0.9414 | 0.51 | 0.6769 | 0.29 | 0.8292 | 0.95 | 0.4241 | 1.44 | 0.2407 |
| N × P × L | 0.34 | 0.5627 | 0.26 | 0.6113 | 1.16 | 0.2862 | 1.03 | 0.3147 | 3.34 | 0.0723 | 0.86 | 0.3581 |
| S × N × P × L | 1.42 | 0.2401 | 2.65 | 0.0568 | 0.33 | 0.8057 | 0.02 | 0.9948 | 0.33 | 0.8054 | 0.60 | 0.6173 |
| Broadleaves | SLA | C/N | Sugars | Starch | NSC | δ13C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | |
| Species (S) | 7.81 | 0.0167 | 19.47 | 0.0018 | 15.74 | 0.0011 | 2.20 | 0.1927 | 0.70 | 0.5573 | 59.43 | <0.001 |
| Nutrients (N) | 20.50 | <0.001 | 140.01 | <0.001 | 5.41 | 0.0236 | 2.83 | 0.0979 | 3.65 | 0.0608 | 30.49 | <0.001 |
| Precipitation (P) | 1.81 | 0.1805 | 13.23 | <0.001 | 6.30 | 0.0150 | 27.53 | <0.001 | 27.90 | <0.001 | 27.73 | <0.001 |
| Light (L) | 8.41 | 0.0043 | 3.44 | 0.0689 | 0.06 | 0.8130 | 4.74 | 0.0337 | 5.42 | 0.0232 | 18.33 | <0.001 |
| S × N | 2.56 | 0.0569 | 14.64 | <0.001 | 5.33 | 0.0026 | 0.71 | 0.5472 | 0.40 | 0.7565 | 1.29 | 0.2872 |
| S × P | 0.02 | 0.9968 | 5.63 | 0.0019 | 5.06 | 0.0036 | 5.05 | 0.0036 | 3.45 | 0.0219 | 1.70 | 0.1755 |
| N × P | 0.43 | 0.5122 | 3.59 | 0.0632 | 0.03 | 0.8727 | 3.66 | 0.0610 | 5.23 | 0.0256 | 1.95 | 0.1680 |
| S × L | 1.25 | 0.2952 | 1.63 | 0.1926 | 0.64 | 0.5947 | 0.93 | 0.4317 | 0.58 | 0.6313 | 1.39 | 0.2548 |
| N × L | 1.96 | 0.1636 | 0.69 | 0.4107 | 0.11 | 0.7421 | 0.02 | 0.8763 | 0.39 | 0.5367 | 0.03 | 0.8674 |
| P × L | 0.33 | 0.5674 | 0.09 | 0.7592 | 6.55 | 0.0132 | 1.46 | 0.2323 | 6.54 | 0.0130 | 2.07 | 0.1552 |
| S × N × P | 0.61 | 0.6107 | 2.19 | 0.0989 | 1.37 | 0.2617 | 1.60 | 0.2006 | 0.13 | 0.9450 | 1.59 | 0.2015 |
| S × N × L | 1.67 | 0.1765 | 1.24 | 0.3029 | 1.94 | 0.1331 | 0.71 | 0.5496 | 0.66 | 0.5791 | 1.21 | 0.3148 |
| S × P × L | 1.03 | 0.3825 | 0.41 | 0.7436 | 0.19 | 0.9055 | 0.49 | 0.6905 | 0.50 | 0.6808 | 1.19 | 0.3215 |
| N × P × L | 0.03 | 0.8570 | 0.00 | 0.9889 | 0.02 | 0.8761 | 0.47 | 0.4978 | 0.02 | 0.8992 | 0.02 | 0.8763 |
| S × N × P × L | 3.00 | 0.0327 | 1.21 | 0.3150 | 1.89 | 0.1416 | 1.46 | 0.2342 | 0.06 | 0.9794 | 0.12 | 0.9502 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-L.; Moser, B.; Li, M.-H.; Wohlgemuth, T.; Lei, J.-P.; Bachofen, C. Contrasting Leaf Trait Responses of Conifer and Broadleaved Seedlings to Altered Resource Availability Are Linked to Resource Strategies. Plants 2020, 9, 621. https://doi.org/10.3390/plants9050621
Zhang Y-L, Moser B, Li M-H, Wohlgemuth T, Lei J-P, Bachofen C. Contrasting Leaf Trait Responses of Conifer and Broadleaved Seedlings to Altered Resource Availability Are Linked to Resource Strategies. Plants. 2020; 9(5):621. https://doi.org/10.3390/plants9050621
Chicago/Turabian StyleZhang, Yan-Li, Barbara Moser, Mai-He Li, Thomas Wohlgemuth, Jing-Pin Lei, and Christoph Bachofen. 2020. "Contrasting Leaf Trait Responses of Conifer and Broadleaved Seedlings to Altered Resource Availability Are Linked to Resource Strategies" Plants 9, no. 5: 621. https://doi.org/10.3390/plants9050621
APA StyleZhang, Y.-L., Moser, B., Li, M.-H., Wohlgemuth, T., Lei, J.-P., & Bachofen, C. (2020). Contrasting Leaf Trait Responses of Conifer and Broadleaved Seedlings to Altered Resource Availability Are Linked to Resource Strategies. Plants, 9(5), 621. https://doi.org/10.3390/plants9050621





