Ethylene is Involved in Symptom Development and Ribosomal Stress of Tomato Plants upon Citrus Exocortis Viroid Infection
Abstract
1. Introduction
2. Results
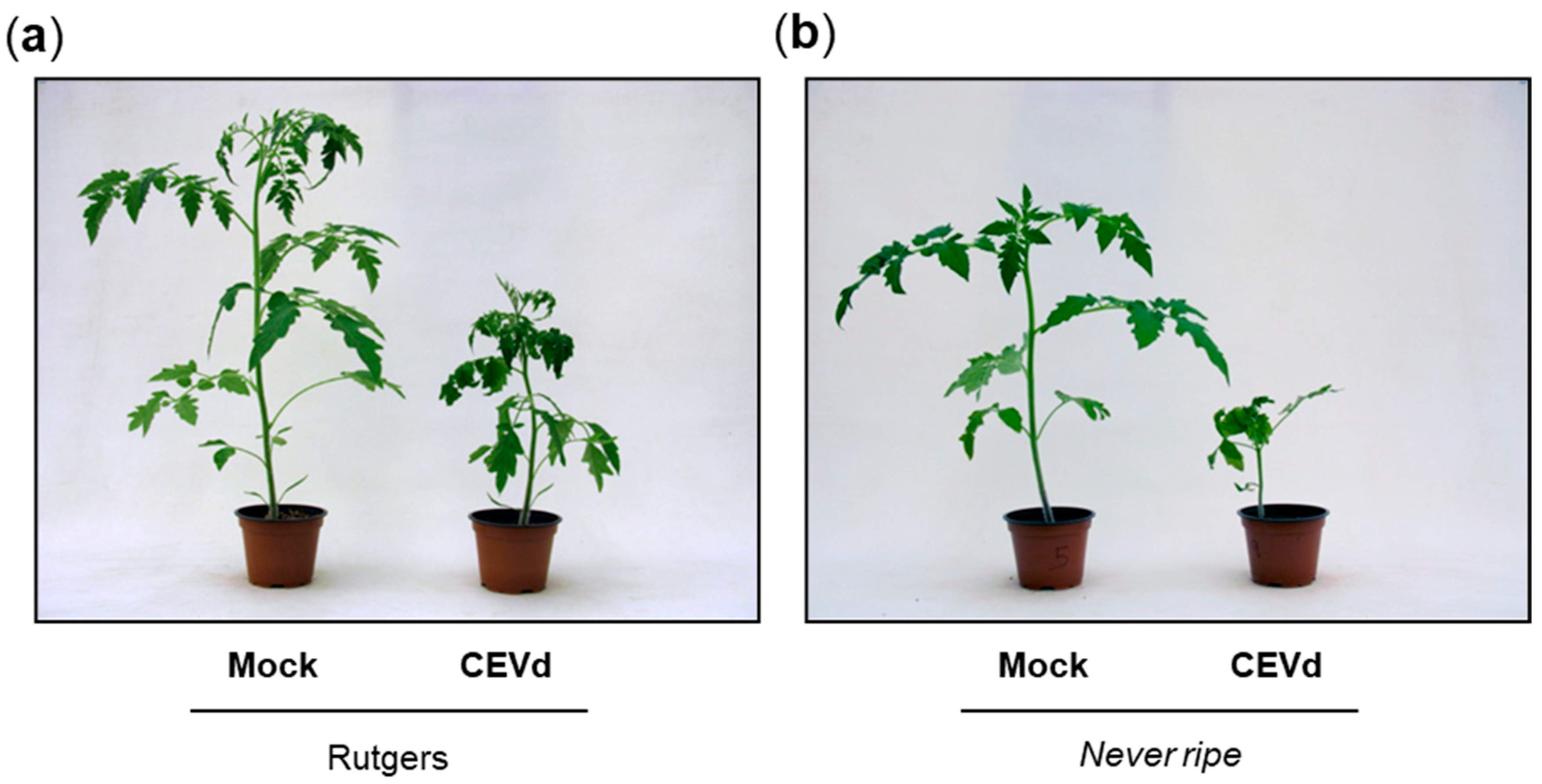
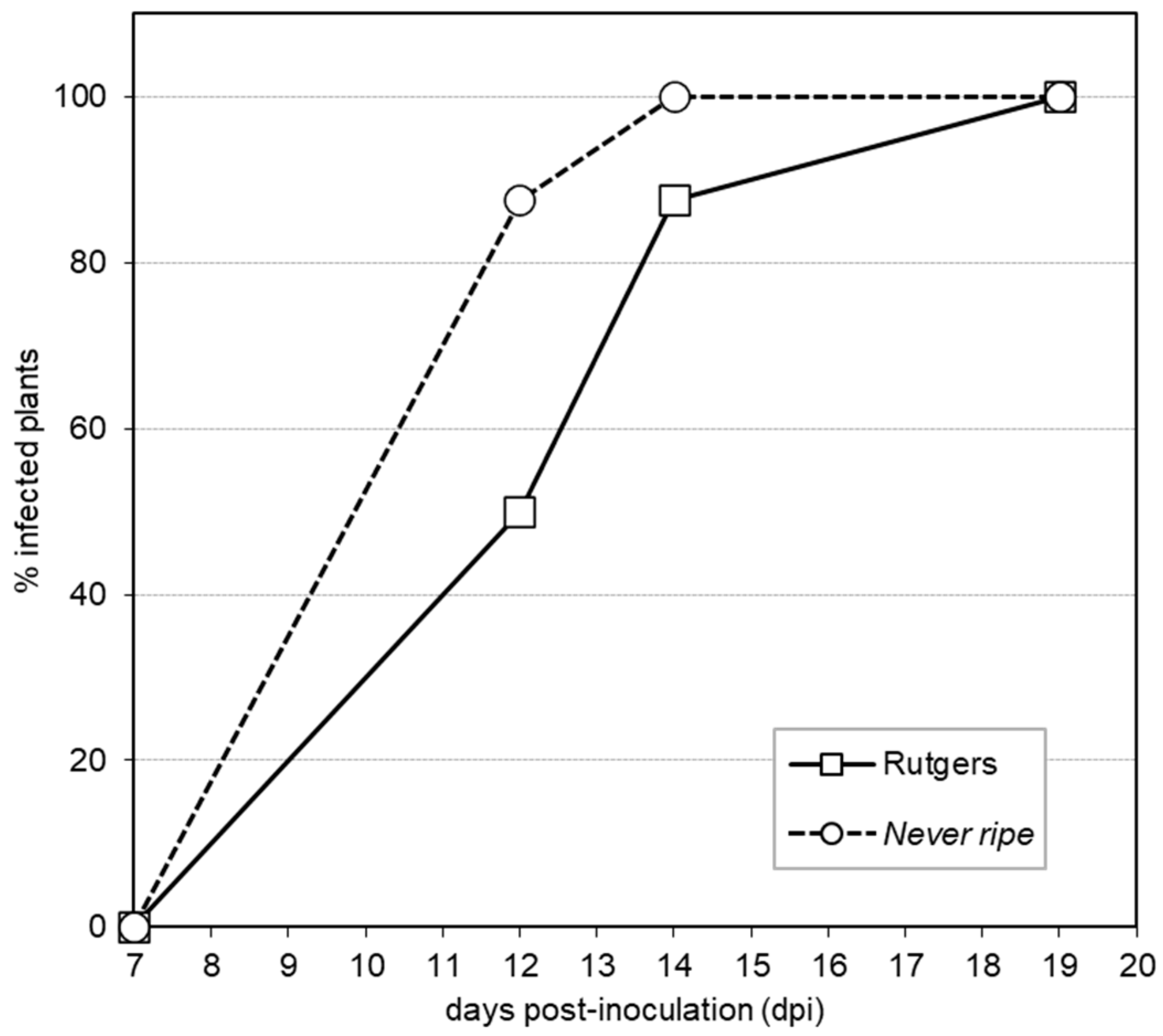
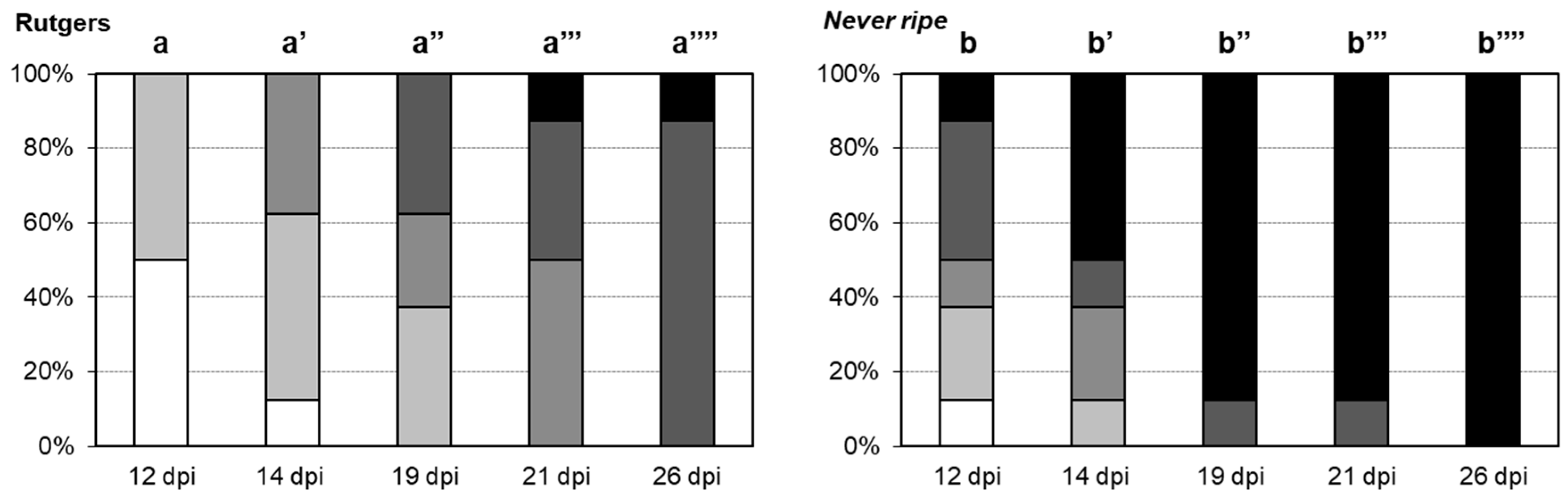
2.1. Never Ripe Tomato Mutants Are Hyper-Susceptible to CEVd Infection
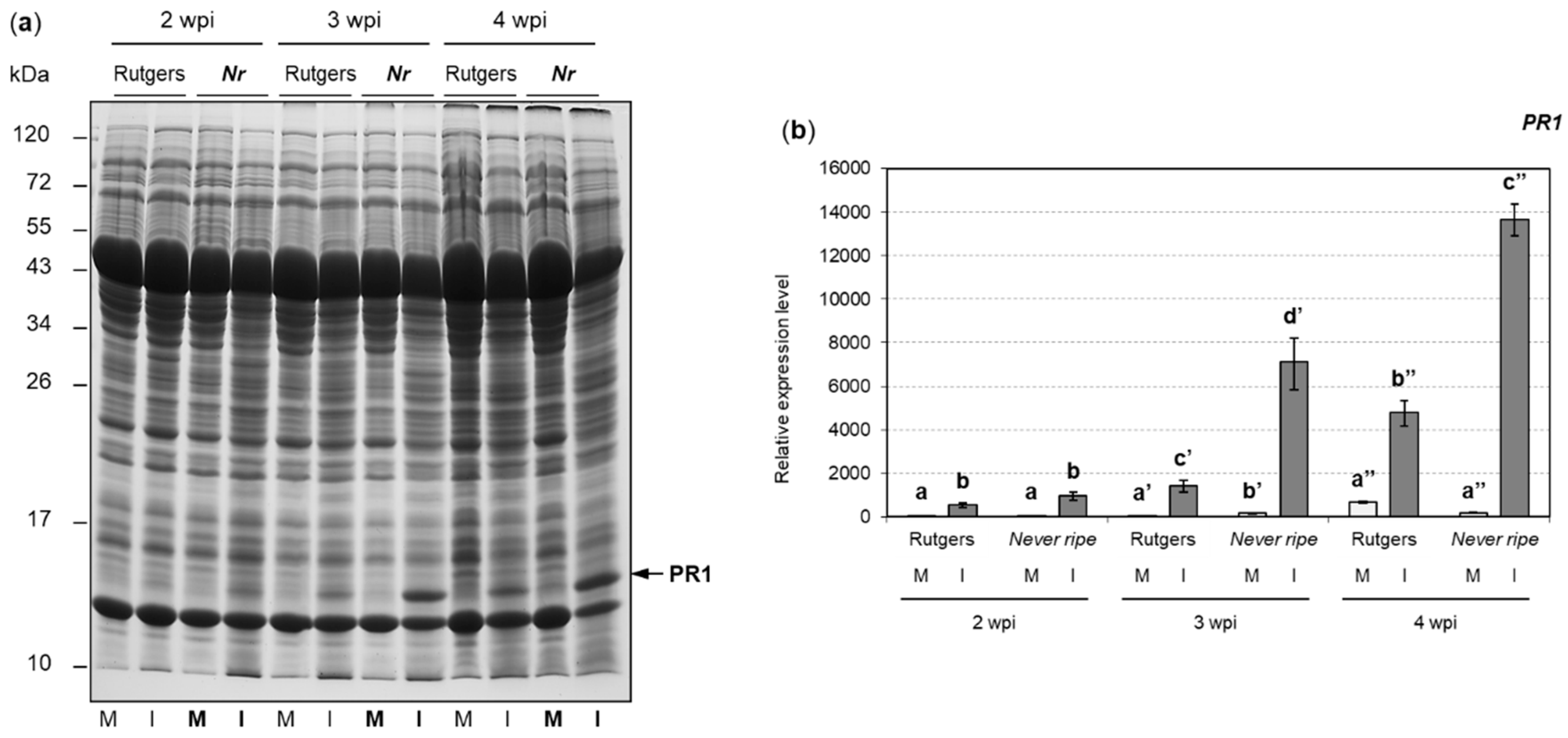
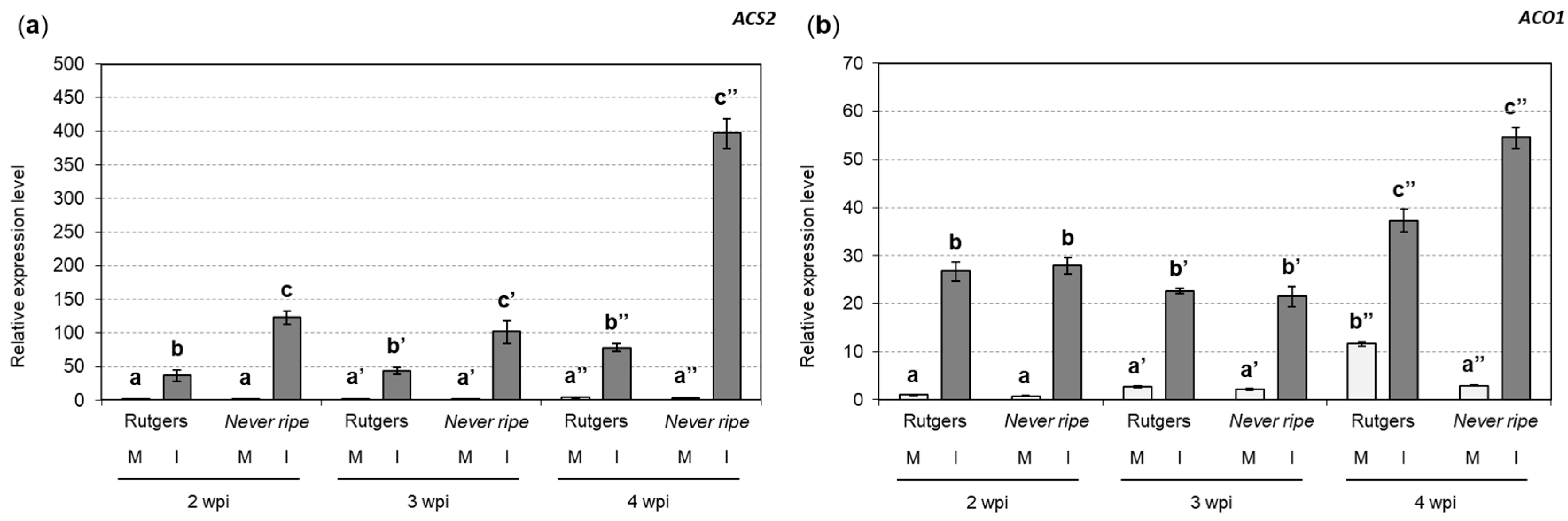
2.2. PR1 and ACS2 Are Highly Induced in Infected Nr Tomato Plants
2.3. Ethylene Production Is Increased in Nr Tomato Mutants upon CEVd Infection
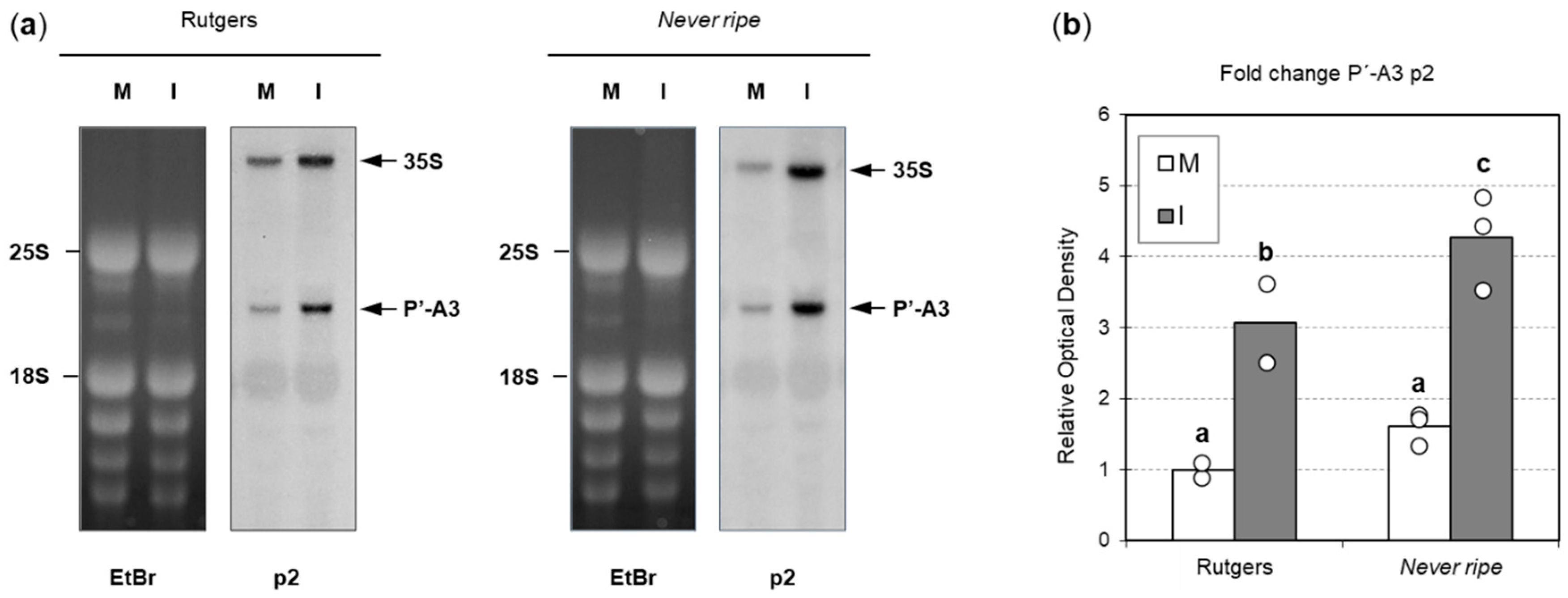
2.4. Ribosomal Stress Is Enhanced in Never Ripe Tomato Mutants upon CEVd Infection
3. Discussion
4. Materials and Methods
4.1. Plant Material and Viroid Inoculation
4.2. Ethylene Measurements
4.3. RNA Preparation
4.4. RT-PCR and qRT-PCR
4.5. Northern Blot Hybridization
4.6. Protein Extraction and Electrophoresis Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flores, R.; Hernández, C.; Martínez de Alba, A.E.; Daròs, J.-A.; Di Serio, F. Viroids and Viroid-Host Interactions. Annu. Rev. Phytopathol. 2005, 43, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Adkar-Purushothama, C.R.; Perreault, J. Current overview on viroid-host interactions. Wiley Interdiscip. Rev. RNA 2019, 11, e1570. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, F.; Flores, R.; Verhoeven, J.T.J.; Li, S.F.; Pallás, V.; Randles, J.W.; Sano, T.; Vidalakis, G.; Owens, R.A. Current status of viroid taxonomy. Arch. Virol. 2014, 159, 3467–3478. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.T.J.; Jansen, C.C.C.; Willemen, T.M.; Kox, L.F.F.; Owens, R.A.; Roenhorst, J.W. Natural infections of tomato by Citrus exocortis viroid, Columnea latent viroid, Potato spindle tuber viroid and Tomato chlorotic dwarf viroid. Eur. J. Plant Pathol. 2004, 110, 823–831. [Google Scholar] [CrossRef]
- López-Gresa, M.P.; Lisón, P.; Yenush, L.; Conejero, V.; Rodrigo, I.; Bellés, J.M. Salicylic acid is involved in the basal resistance of tomato plants to citrus exocortis viroid and tomato spotted wilt virus. PLoS ONE 2016, 11, e0166938. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Qiu, Y.; Atta, S.; Zhou, C.; Cao, M. Global transcriptomic analysis reveals insights into the response of ‘etrog’ citron (Citrus medica L.) to Citrus Exocortis viroid infection. Viruses 2019, 11, 453. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, L.; Liu, L.; Wang, J.; Li, C.; Wang, Q. Multiple phytohormone signalling pathways modulate susceptibility of tomato plants to Alternaria alternata f. sp. lycopersici. J. Exp. Bot. 2013, 64, 637–650. [Google Scholar] [CrossRef]
- van Loon, L.C.; Geraats, B.P.J.; Linthorst, H.J.M. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef]
- Bellés, J.M.; Conejero, V. Ethylene Mediation of the Viroid-Like Syndrome Induced by Ag+ Ions in Gynura aurantiaca DC Plants. J. Phytopathol. 1989, 124, 275–284. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Yang, S.F.; Hoffman, N.E. Ethylene Biosynthesis and its Regulation in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Wang, K.L.-C.; Li, H.; Ecker, J.R. Ethylene Biosynthesis and Signaling Networks. Plant Cell 2002, 14, S131–S151. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, G.-J.; Yang, K.-Y.; Mao, G.; Wang, R.; Liu, Y.; Zhang, S. Mitogen-activated protein kinase 3 and 6 regulate Botrytis cinerea-induced ethylene production in Arabidopsis. Plant J. 2010, 64, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Bellés, J.M.; Granell, A.; Durán-vila, N.; Conejero, V. ACC Synthesis as the Activated Step Responsible for the Rise of Ethylene Production Accompanying Citrus Exocortis Viroid Infection in Tomato Plants. J. Phytopathol. 1989, 125, 198–208. [Google Scholar] [CrossRef]
- Bellés, J.M.; Vera, P.; Durán-Vila, N.; Conejero, V. Ethylene production in tomato cultures infected with citrus exocortis viroid (CEV). Can. J. Plant Pathol. 1989, 11, 256–262. [Google Scholar] [CrossRef]
- Bellés, J.M.; Conejero, V. Evolution of Ethylene Production, ACC and Conjugated ACC Levels Accompanying Symptom Development in Tomato and Gynura aurantiaca DC Leaves Infected with Citrus Exocortis Viroid (CEV). J. Phytopathol. 1989, 127, 81–85. [Google Scholar] [CrossRef]
- Bellés, J.M.; Conejero, V. Suppression by Citrus Exocortis Viroid Infection of the Naturally Occurring Inhibitor of the Conversion of 1-aminocyclopropane-1-carboxylic Acid to Ethylene by Tomato Microsomes. J. Phytopathol. 1991, 132, 245–250. [Google Scholar] [CrossRef]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Groth, G.; Tucker, M.L.; et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef]
- Aloni, R.; Wolf, A.; Feigenbaum, P.; Avni, A.; Klee, H.J. The Never ripe Mutant Provides Evidence That Tumor-Induced Ethylene Controls the Morphogenesis of Agrobacterium tumefaciens -Induced Crown Galls on Tomato Stems. Plant Physiol. 1998, 117, 841–849. [Google Scholar] [CrossRef]
- Klee, H.J. Ethylene signal transduction. Moving beyond arabidopsis. Plant Physiol. 2004, 135, 660–667. [Google Scholar] [CrossRef]
- Chen, Y.; Rofidal, V.; Hem, S.; Gil, J.; Nosarzewska, J.; Berger, N.; Demolombe, V.; Bouzayen, M.; Azhar, B.J.; Shakeel, S.N.; et al. Targeted Proteomics Allows Quantification of Ethylene Receptors and Reveals SlETR3 Accumulation in Never-Ripe Tomatoes. Front. Plant Sci. 2019, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Nie, X.; Song, Y.; Xiong, X.; Tai, H. Ethylene is Involved but Plays a Limited Role in Tomato Chlorotic Dwarf Viroid-Induced Symptom Development in Tomato. Agric. Sci. China 2011, 10, 544–552. [Google Scholar] [CrossRef]
- Díaz, J.; ten Have, A.; van Kan, J.A.L. The Role of Ethylene and Wound Signaling in Resistance of Tomato to Botrytis cinerea. Plant Physiol. 2002, 129, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.T.; Stall, R.E.; Klee, H.J. Ethylene Regulates the Susceptible Response to Pathogen Infection in Tomato. Plant Cell 1998, 10, 371–382. [Google Scholar] [CrossRef]
- Tsolakidou, M.-D.; Pantelides, L.S.; Tzima, A.K.; Kang, S.; Paplomatas, E.J.; Tsaltas, D. Disruption and Overexpression of the Gene Encoding ACC (1-Aminocyclopropane-1-Carboxylic Acid) Deaminase in Soil-Borne Fungal Pathogen Verticillium dahliae Revealed the Role of ACC as a Potential Regulator of Virulence and Plant Defense. Mol. Plant-Microbe Interact. 2019, 32, 639–653. [Google Scholar] [CrossRef]
- Więsyk, A.; Iwanicka-Nowicka, R.; Fogtman, A.; Zagórski-Ostoja, W.; Góra-Sochacka, A. Time-course microarray analysis reveals differences between transcriptional changes in tomato leaves triggered by mild and severe variants of potato spindle tuber viroid. Viruses 2018, 10, 257. [Google Scholar] [CrossRef]
- Eiras, M.; Nohales, M.A.; Kitajima, E.W.; Flores, R.; Daròs, J.A. Ribosomal protein L5 and transcription factor IIIA from Arabidopsis thaliana bind in vitro specifically Potato spindle tuber viroid RNA. Arch. Virol. 2011, 156, 529–533. [Google Scholar] [CrossRef]
- Dube, A.; Bisaillon, M.; Perreault, J.-P. Identification of Proteins from Prunus persica That Interact with Peach Latent Mosaic Viroid. J. Virol. 2009, 83, 12057–12067. [Google Scholar] [CrossRef]
- Lisón, P.; Tárraga, S.; López-Gresa, P.; Saurí, A.; Torres, C.; Campos, L.; Bellés, J.M.; Conejero, V.; Rodrigo, I. A noncoding plant pathogen provokes both transcriptional and posttranscriptional alterations in tomato. Proteomics 2013, 13, 833–844. [Google Scholar] [CrossRef]
- Cottilli, P.; Belda-Palazón, B.; Adkar-Purushothama, C.R.; Perreault, J.-P.; Schleiff, E.; Rodrigo, I.; Ferrando, A.; Lisón, P. Citrus exocortis viroid causes ribosomal stress in tomato plants. Nucleic Acids Res. 2019, 47, 8649–8661. [Google Scholar] [CrossRef]
- Ohbayashi, I.; Lin, C.-Y.; Shinohara, N.; Matsumura, Y.; Machida, Y.; Horiguchi, G.; Tsukaya, H.; Sugiyama, M. Evidence for a Role of ANAC082 as a Ribosomal Stress Response Mediator Leading to Growth Defects and Developmental Alterations in Arabidopsis. Plant Cell 2017, 29, 2644–2660. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Grummt, I. Cellular Stress and Nucleolar Function. Cell Cycle 2005, 4, 1036–1038. [Google Scholar] [CrossRef] [PubMed]
- Weis, B.L.; Kovacevic, J.; Missbach, S.; Schleiff, E. Plant-Specific Features of Ribosome Biogenesis. Trends Plant Sci. 2015, 20, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Palm, D.; Streit, D.; Shanmugam, T.; Weis, B.L.; Ruprecht, M.; Simm, S.; Schleiff, E. Plant-specific ribosome biogenesis factors in Arabidopsis thaliana with essential function in rRNA processing. Nucleic Acids Res. 2019, 47, 1880–1895. [Google Scholar] [CrossRef]
- Christoffersen, R.E.; Laties, G.G. Ethylene regulation of gene expression in carrots. Proc. Natl. Acad. Sci. USA 1982, 79, 4060–4063. [Google Scholar] [CrossRef]
- Marei, N.; Romani, R. Ethylene-stimulated Synthesis of Ribosomes, Ribonucleic Acid, and Protein in Developing Fig Fruits. Plant Physiol. 1971, 48, 806–808. [Google Scholar] [CrossRef][Green Version]
- Spiers, J.; Brady, C.; Grierson, D.; Lee, E. Changes in Ribosome Organization and Messenger RNA Abundance in Ripening Tomato Fruits. Funct. Plant Biol. 1984, 11, 225. [Google Scholar] [CrossRef]
- Merchante, C.; Brumos, J.; Yun, J.; Hu, Q.; Spencer, K.R.; Enríquez, P.; Binder, B.M.; Heber, S.; Stepanova, A.N.; Alonso, J.M. Gene-Specific Translation Regulation Mediated by the Hormone-Signaling Molecule EIN2. Cell 2015, 163, 684–697. [Google Scholar] [CrossRef]
- Tornero, P.; Rodrigo, I.; Conejero, V.; Vera, P. Nucleotide Sequence of a cDNA Encoding a Pathogenesis-Related Protein, P1-p14, from Tomato (Lycopersicon esculentum). Plant Physiol. 1993, 102, 325. [Google Scholar] [CrossRef][Green Version]
- Conejero, V.; Bellés, J.M.; García-Breijo, F.; Garro, R.; Hernández-Yago, J.; Rodrigo, I.; Vera, P. Signalling in Viroid Pathogenesis. In Recognition and Response in Plant-Virus Interactions; Springer: Berlin/Heidelberg, Germany, 1990; pp. 233–261. [Google Scholar]
- Granell, A.; Bellés, J.M.; Conejero, V. Induction of pathogenesis-related proteins in tomato by citrus exocortis viroid, silver ion and ethephon. Physiol. Mol. Plant Pathol. 1987, 31, 83–90. [Google Scholar] [CrossRef]
- Mehari, Z.H.; Elad, Y.; Rav-David, D.; Graber, E.R.; Meller Harel, Y. Induced systemic resistance in tomato (Solanum lycopersicum) against Botrytis cinerea by biochar amendment involves jasmonic acid signaling. Plant Soil 2015, 395, 31–44. [Google Scholar] [CrossRef]
- Nakatsuka, A.; Murachi, S.; Okunishi, H.; Shiomi, S.; Nakano, R.; Kubo, Y.; Inaba, A. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening. Plant Physiol. 1998, 118, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, K.; Wu, Y.; Zhang, R.; Bonar, N.; Morris, J.; Hedley, P.E.; Bryan, G.J.; Kalantidis, K.; Hornyik, C. Insight on genes affecting tuber development in potato upon potato spindle tuber viroid (PSTVd) infection. PLoS ONE 2016, 11, e0150711. [Google Scholar] [CrossRef] [PubMed]
- Bellés, J.M.; Carbonell, J.; Conejero, V. Polyamines in Plants Infected by Citrus Exocortis Viroid or Treated with Silver Ions and Ethephon. Plant Physiol. 1991, 96, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, P.J.; Jones, J.B.; Antoine, F.R.; Ciardi, J.; Klee, H.J. Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J. 2001, 25, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Gómez, G.; Martínez, G.; Pallás, V. Viroid-Induced Symptoms in Nicotiana benthamiana Plants Are Dependent on RDR6 Activity. Plant Physiol. 2008, 148, 414–423. [Google Scholar] [CrossRef]
- Li, G.; Meng, X.; Wang, R.; Mao, G.; Han, L.; Liu, Y.; Zhang, S. Dual-Level Regulation of ACC Synthase Activity by MPK3/MPK6 Cascade and Its Downstream WRKY Transcription Factor during Ethylene Induction in Arabidopsis. PLoS Genet. 2012, 8, e1002767. [Google Scholar] [CrossRef]
- Berrocal-Lobo, M.; Molina, A.; Solano, R. Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 2002, 29, 23–32. [Google Scholar] [CrossRef]
- Chowdhury, S.; Basu, A.; Kundu, S. Biotrophy-necrotrophy switch in pathogen evoke differential response in resistant and susceptible sesame involving multiple signaling pathways at different phases. Sci. Rep. 2017, 7, 17251. [Google Scholar] [CrossRef]
- Shin, S.; Lv, J.; Fazio, G.; Mazzola, M.; Zhu, Y. Transcriptional regulation of ethylene and jasmonate mediated defense response in apple (Malus domestica) root during Pythium ultimum infection. Hortic. Res. 2014, 1, 14053. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting Mechanisms of Defense Against Biotrophic and Necrotrophic Pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef] [PubMed]
- McDowell, J.M.; Dangl, J.L. Signal transduction in the plant immune response. Trends Biochem. Sci. 2000, 25, 79–82. [Google Scholar] [CrossRef]
- Heck, S.; Grau, T.; Buchala, A.; Metraux, J.-P.; Nawrath, C. Genetic evidence that expression of NahG modifies defence pathways independent of salicylic acid biosynthesis in the Arabidopsis-Pseudomonas syringae pv. tomato interaction. Plant J. 2003, 36, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Conejero, V.; Granell, A. Stimulation of a viroid-like syndrome and the impairment of viroid infection in Gynura aurantiaca plants by treatment with silver ions. Physiol. Mol. Plant Pathol. 1986, 29, 317–323. [Google Scholar] [CrossRef]
- Yan, S.; Dong, X. Perception of the plant immune signal salicylic acid. Curr. Opin. Plant Biol. 2014, 20, 64–68. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef]
- Schott-Verdugo, S.; Müller, L.; Classen, E.; Gohlke, H.; Groth, G. Structural Model of the ETR1 Ethylene Receptor Transmembrane Sensor Domain. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef]
- Lanahan, M.B.; Yen, H.C.; Giovannoni, J.J.; Klee, H.J. The never ripe mutation blocks ethylene perception in tomato. Plant Cell 1994, 6, 521–530. [Google Scholar]
- Clark, D.G.; Gubrium, E.K.; Barrett, J.E.; Nell, T.A.; Klee, H.J. Root Formation in Ethylene-Insensitive Plants. Plant Physiol. 1999, 121, 53–60. [Google Scholar] [CrossRef]
- Rodriguez, F.I.; Esch, J.J.; Hall, A.E.; Binder, B.M.; Schaller, G.E.; Bleecker, A.B. A Copper Cofactor for the Ethylene Receptor ETR1 from Arabidopsis. Science 1999, 283, 996–998. [Google Scholar] [CrossRef]
- Schaller, G.E.; Ladd, A.N.; Lanahan, M.B.; Spanbauer, J.M.; Bleecker, A.B. The Ethylene Response Mediator ETR1 from Arabidopsis Forms a Disulfide-linked Dimer. J. Biol. Chem. 1995, 270, 12526–12530. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Schaller, G.E. The role of receptor interactions in regulating ethylene signal transduction. Plant Signal. Behav. 2009, 4, 1152–1153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, Z.; Wen, C.-K.; Binder, B.M.; Chen, Y.-F.; Chang, J.; Chiang, Y.-H.; Kerris, R.J.; Chang, C.; Schaller, G.E. Heteromeric Interactions among Ethylene Receptors Mediate Signaling in Arabidopsis. J. Biol. Chem. 2008, 283, 23801–23810. [Google Scholar] [CrossRef] [PubMed]
- Grefen, C.; Städele, K.; Růžička, K.; Obrdlik, P.; Harter, K.; Horák, J. Subcellular Localization and In Vivo Interactions of the Arabidopsis thaliana Ethylene Receptor Family Members. Mol. Plant 2008, 1, 308–320. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.H.; Kim, J.; Kim, J.J.; Hong, S.; Kim, J.; Kim, J.H.; Woo, H.R.; Hyeon, C.; Lim, P.O.; et al. Time-evolving genetic networks reveal a nac troika that negatively regulates leaf senescence in arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, E4930–E4939. [Google Scholar] [CrossRef]
- Semancik, J.S.; Roistacher, C.N.; Rivera-Bustamante, R.; Duran-Vila, N. Citrus Cachexia Viroid, a New Viroid of Citrus: Relationship to Viroids of the Exocortis Disease Complex. J. Gen. Virol. 1988, 69, 3059–3068. [Google Scholar] [CrossRef]
- Campos, L.; Granell, P.; Tárraga, S.; López-Gresa, P.; Conejero, V.; Bellés, J.M.; Rodrigo, I.; Lisón, P. Salicylic acid and gentisic acid induce RNA silencing-related genes and plant resistance to RNA pathogens. Plant Physiol. Biochem. 2014, 77, 35–43. [Google Scholar] [CrossRef]
- Adkar-Purushothama, C.R.; Brosseau, C.; Giguère, T.; Sano, T.; Moffett, P.; Perreault, J.-P. Small RNA Derived from the Virulence Modulating Region of the Potato spindle tuber viroid Silences callose synthase Genes of Tomato Plants. Plant Cell 2015, 27, 2178–2194. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]



| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Actin | CTAGGGTGGGTTCGCAGGAGATGATGC | GTCTTTTTGACCCATACCCACCATCACAC |
| PR1 | ACTCAAGTAGTCTGGCGCAACTCA | AGTAAGGACGTTGTCCGATCGAGT |
| ACS2 | GATGGATTTGCGTCCACTTT | GATCCAGGCGAGACGTTAAG |
| ACO1 | TGTCCTAAGCCCGATTTGAT | TTGAGGAGTTGAAGGCCACT |
| CEVd | AGGAGCTCGTCTCCTTCCTT | CACCGGGTAGTAGCCAGAAG |
| SlNAC082 | TGCTGAAACCATTGGAACTG | CCAAGGAATTGCTTCCAAAA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez Prol, F.; López-Gresa, M.P.; Rodrigo, I.; Bellés, J.M.; Lisón, P. Ethylene is Involved in Symptom Development and Ribosomal Stress of Tomato Plants upon Citrus Exocortis Viroid Infection. Plants 2020, 9, 582. https://doi.org/10.3390/plants9050582
Vázquez Prol F, López-Gresa MP, Rodrigo I, Bellés JM, Lisón P. Ethylene is Involved in Symptom Development and Ribosomal Stress of Tomato Plants upon Citrus Exocortis Viroid Infection. Plants. 2020; 9(5):582. https://doi.org/10.3390/plants9050582
Chicago/Turabian StyleVázquez Prol, Francisco, M. Pilar López-Gresa, Ismael Rodrigo, José María Bellés, and Purificación Lisón. 2020. "Ethylene is Involved in Symptom Development and Ribosomal Stress of Tomato Plants upon Citrus Exocortis Viroid Infection" Plants 9, no. 5: 582. https://doi.org/10.3390/plants9050582
APA StyleVázquez Prol, F., López-Gresa, M. P., Rodrigo, I., Bellés, J. M., & Lisón, P. (2020). Ethylene is Involved in Symptom Development and Ribosomal Stress of Tomato Plants upon Citrus Exocortis Viroid Infection. Plants, 9(5), 582. https://doi.org/10.3390/plants9050582







